3D carotid ultrasound in patients with a negative exercise stress test: A practical risk stratification tool
2 Division of Cardiology, Department of Medicine, University of Alberta, Edmonton, Canada
3 Alberta Cardiovascular and Stroke Research Centre, Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Canada
Received: 09-Feb-2021 Accepted Date: Mar 09, 2021; Published: 16-Mar-2021
Citation: Bainey KR, Thomas J, Alkurtass S, et al. 3D carotid ultrasound in patients with a negative exercise stress test: A practical risk stratification tool. Clin Cardiol J. 2021;5(2):11-13.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Exercise stress tests (EST) are frequently used to evaluate chest pain. While a negative EST is reassuring, further risk stratification for coronary artery disease (CAD) may be warranted. Three-dimensional (3D) carotid ultrasound is a non-invasive tool which can be used to identify subclinical atherosclerotic disease. We investigated the prevalence and extent of subclinical atherosclerotic lesions using 3D carotid ultrasound in patients with chest pain and a negative EST, with the aim of understanding the changes in atherosclerotic lesions with statin therapy at 1 year.
Methods: 80 patients were prospectively enrolled and all received additional vascular assessment with 3D carotid ultrasound. If subclinical atherosclerosis was identified, high-dose statin therapy was recommended. A 3D carotid ultrasound scan was repeated at 12 months.
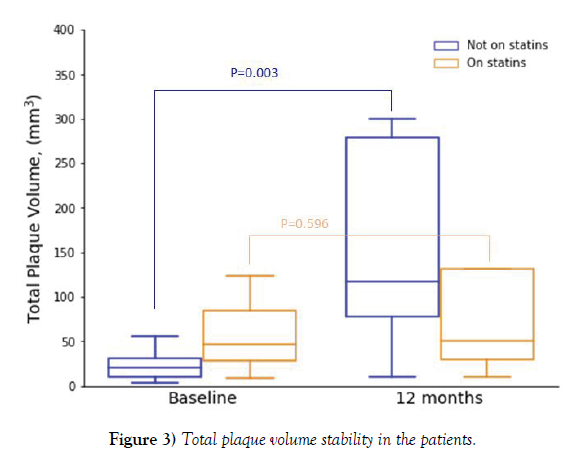
Results: Of our cohort, 37 patients (46.3%) had carotid plaque. At baseline, median plaque volume (IQR) (left carotid, right carotid, or both) was 32.0 mm3 (17-123). At 12-month follow-up (n=22), median plaque volume was 116.0 mm3 (46-279) (p=0.004). Of these, only 40.9% were on high-dose statin therapy. Total plaque volume remained stable in subjects on statins (46.5 mm3 [25-97.5] at baseline; 50 mm3 [29-131] at 12 months, p=0.596) while it increased in those without statins (20 mm3 [10-31] at baseline; 117 mm3 [78-279] at 12 months, p=0.003).
Conclusion: In patients with chest pain and a negative EST, roughly one-half have subclinical atherosclerosis as detected with 3D carotid ultrasound. Plaque progression commonly occurs at 12 months, but is less likely with statin therapy. A further randomized study is required to confirm our findings.
Keywords
Exercise stress test; 3D carotid ultrasound; Atherosclerosis
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the commonest cause of morbidity and mortality worldwide. Within the western world, cardiovascular disease accounts for roughly 30% of all-cause deaths, of which one-half are due to ischemic heart disease. Coronary atherosclerotic disease spans an entire spectrum of latent asymptomatic development to the progression of fibro-fatty plaque vulnerable to rupture [1]. Moreover, more than one-half of acute coronary syndrome (ACS) events result from coronary lesions that are subclinical (i.e., not flow-limiting) [2,3]. Multiple scoring systems have been developed to help predict the risk of coronary events earlier with variable success [4-9]. More recently, other non-invasive markers of atherosclerosis have been utilized to assess cardiovascular risk [10].
We investigated the prevalence and extent of subclinical atherosclerotic lesions using three-dimensional (3D) carotid ultrasound in patients with chest pain and a negative exercise stress test (EST). In those with subclinical atherosclerosis, primary prevention strategies were encouraged and changes in atherosclerotic lesions were assessed at 1 year.
Research Methodology
Study population
Eighty healthy adult subjects without a history of coronary or peripheral arterial disease and negative exercise stress tests (EST) were prospectively enrolled. Included subjects were those referred to Chest Pain Program Clinic at the Mazankowski Alberta Heart Institute, University of Alberta Hospital with a negative EST; age 40-60 years for males, age 50-60 years for females, and agreed to participate following informed consent. Excluded subjects were those who had a history of obstructive coronary artery disease (CAD) (previous myocardial infarction, coronary revascularization procedure, angina pectoris with documented ischemia, coronary angiogram documenting obstructive CAD); history of cerebrovascular disease (previous stroke, transient ischemic attack, or carotid revascularization procedure); history of peripheral arterial disease (claudication with abnormal ankle-brachial indices or previous revascularization procedure); unstable life-threatening or severe medical conditions; refused to be involved in the study or were unable to give valid consent. The study protocol was approved by the University of Alberta Research Ethics Board. Written informed consent was obtained for all subjects.
Study protocol
After enrollment, all subjects underwent an additional vascular assessment with 3D carotid ultrasound to detect subclinical atherosclerosis. If subclinical atherosclerosis was identified, medical treatment with high-dose statin therapy and physical activity/risk factor modification (referral to a cardiac rehabilitation program) was encouraged. Given the controversial benefits of aspirin for primary prevention, this was not mandated. A letter was sent to the primary care physician informing participation in the study. The primary care physician was encouraged to promote adherence to statins and risk factor modification. A 3D carotid ultrasound scan was repeated at 12-month follow-up.
3D carotid ultrasound
Technique: Carotid scans were performed using a standard ultrasound system (Philips IU22; Philips Medical Systems) equipped with highfrequency VL 13-5 transducer. Patients were placed in a supine position with slight hyperextension and rotation of the neck in the direction opposite to the probe. The proximal, mid, and distal common carotid artery (CCA), bifurcation (BIF) of the CCA (segment between flow divider and increase in diameter of CCA), and proximal portion of the internal and external carotid arteries were systematically interrogated in long-axis and short-axis views. The mechanical single sweep 3D imaging method was used to acquire 3D volumetric data of carotid arteries. The transducer automatically moves the ultrasound beam through a pre-defined area, obtaining data that is then reconstructed into multiplanar and volume views.
Measurements
Carotid plaque was defined as a focal structure that protrudes into the arterial lumen of the carotid artery at least 0.5 mm, or a presence of focal wall thickening that is at least 50% greater than that of the surrounding vessel wall, or as a focal region with carotid intima-media thickness (CIMT) greater than 1.5 mm that protrudes into the lumen that is distinct from the adjacent boundary [11].
The 3D ultrasound images of plaque volumes were measured by manual planimetry method using the Q-Lab software. Plaque segmentation was performed manually by tracing the outline of the plaque contour on each slice. The vascular sonographer and or the reading cardiologist sliced the 3D image with an Interslice Distance (ISD) of 1.0 mm from one end of the plaque to the other (along with the vessel axis in the scan direction) and traced the individual plaque boundaries of the image plane by using a mousedriven cross-hair cursor. As contours were manually outlined, the software (Q-Lab) calculates the contour area automatically. The areas measured in each slice were summed and multiplied by the ISD to calculate total plaque volume. After measuring the plaque, the experienced research reading physician reviewed the 3D image to ensure that the set of measured plaque boundaries match the plaque volume. Mismatches were adjusted by dragging the boundaries to fit the correct contour.
CIMT measurements were taken at the far wall of CCA and BIF at enddiastole using a semiautomated edge detection algorithm (QLAB version; Philips Medical Systems). A region of interest, 1cm in length, was placed parallel to the vessel wall, allowing the software to detect the luminal-intimal and medial-adventitial interfaces, enabling determination of the CIMT. When carotid plaque was verified in a segment, care was taken not to include the plaque in the CIMT measurement. The 3D carotid ultrasound was reported as: (1) normal CIMT, no plaque; (2) abnormal CIMT, no plaque; (3) abnormal CIMT, plaque present, plaque volume, degree of stenosis, degree of obstruction, and; (4) equivocal, poor image quality, non-diagnostic.
Total plaque volume was calculated as sum of left carotid artery (LCA) plaque volume if plaque was seen at left side, right carotid artery (RCA) plaque volume if plaque was seen at right side, and combined LCA plaque volume and RCA plaque volume if plaque was seen at both sides. Total maximum area of vessel reduction was calculated as sum of LCA maximum area of vessel reduction if plaque was seen at left side, RCA maximum area of vessel reduction if plaque was seen at right side, and combined LCA and RCA maximum area of vessel reduction if plaque was seen at both sides.
Data processing
The 3D carotid ultrasound measurements were performed using the Q-Lab software which is available on the IU22 scanner and the results were transferred into an Excel spreadsheet with random numbers for the patient (research coordinator). The datasets were stored in the clinical cardiovascular database (Xcelera), which guaranteed safe storage of the clinical data. All images, measurements and interpretations made for each subject were performed in a blinded fashion (unaware of current therapy) by an experienced research reading physician and confirmed by a research cardiologist (also blinded to ongoing treatment). Corrections, modifications, and reporting of incidental findings were provided to the subject’s primary care physician.
Statistical Analysis
Participant characteristics were summarized according to the absence or presence of plaque in the carotid arteries. Participants identified with plaque at baseline were followed at 12 months. Categorical variables were reported as counts and percentages, whereas continuous variables were presented as medians with 25th and 75th percentiles. Differences between groups were tested using the χ2 test or Fisher’s exact test when cell number is less than 5 for categorical variables and using t-test or Wilcoxon rank-sum test when normality assumption is violated for continuous variables.
Mixed model for repeated measurement was used to obtain interaction between time and whether a participant is using cholesterol lowering medication by considering the participant as a random effect.
All statistical tests were two-sided with a p-value <0.05 considered as statistically significant. Statistical analyses were performed using SAS (version 9.4; Cary, NC). No correction was made for multiple comparisons.
Results
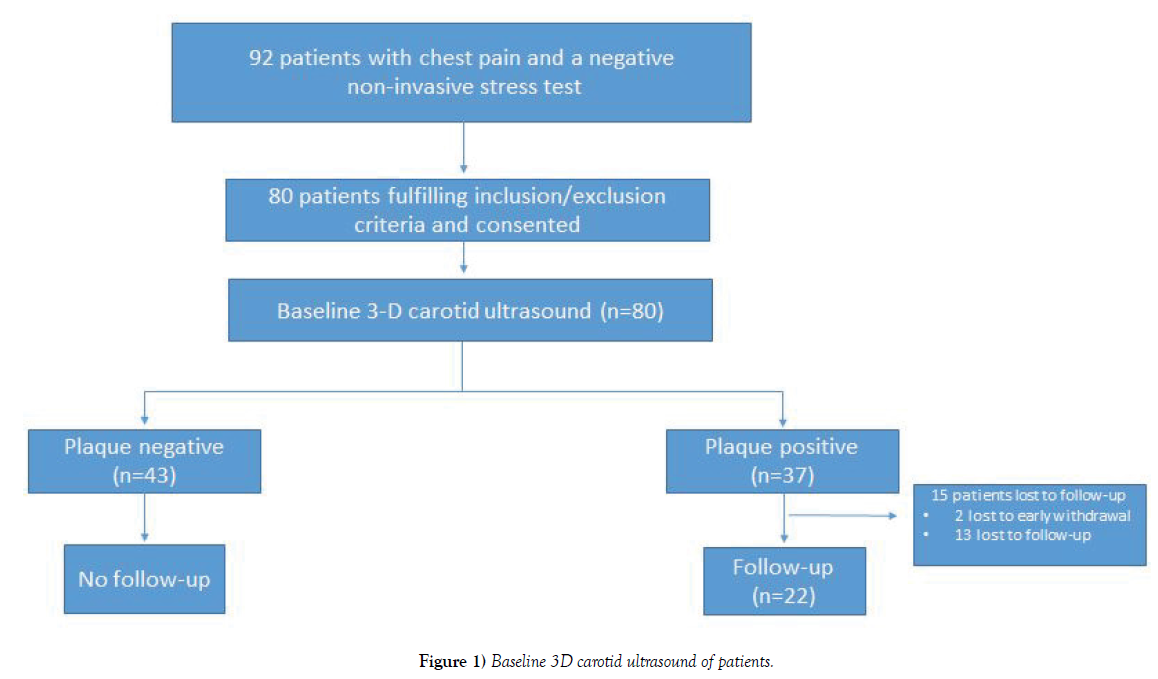
In our cohort, 92 consecutive subjects with chest pain and a negative EST were reviewed. Of these, 80 subjects fulfilled the criteria and consented to participate in the study. All patients received a baseline 3D carotid ultrasound, with 37 patients (46.3%) showing evidence of carotid plaque (Figure 1).
Subject characteristics
Baseline characteristics in subjects with and without carotid plaque are outlined in Table 1. Median age was 53 years (IQR: 50-58 years) and 47.5% were female with the majority being Caucasian. As expected, the majority of subjects presented with chest pain prompting an EST. The majority of subjects had an intermediate pre-test probability of CAD (Duke Treadmill Score). Plaque positive subjects more commonly had a history of dyslipidemia (Framingham Risk Score). None of these subjects were on lipid lowering therapy prior to enrollment.
| Variables | Overall (n=80) |
Plaque Negative (n=43) |
Plaque Positive (n=37) |
p-value |
|---|---|---|---|---|
| Demographics, n (%) | ||||
| Age, yrs | 53 (50- 58) | 55 (49- 58) | 52 (50- 56) | 0.442 |
| Female | 38 (47.5%) | 20 (46.5%) | 18 (48.6%) | 0.849 |
| Race | ||||
| Caucasian | 68 (85.0%) | 35 (81.4%) | 33 (89.2%) | 0.904 |
| Black or African Americans | 2 (2.5%) | 1 (2.3%) | 1 (2.7%) | |
| Hispanic or Latino | 3 (3.8%) | 2 (4.7%) | 1 (2.7%) | |
| Asian | 6 (7.5%) | 4 (9.3%) | 2 (5.4%) | |
| Other | 1 (1.3%) | 1 (2.3%) | 0 (0.0%) | |
| Medical History, n (%) | ||||
| Chest pain | 74 (92.5%) | 41 (95.3%) | 33 (89.2%) | 0.407 |
| Shortness of breath | 7 (8.8%) | 3 (7.0%) | 4 (10.8%) | 0.698 |
| Palpitation | 7 (8.8%) | 3 (7.0%) | 4 (10.8%) | 0.698 |
| Diabetes Type 1 | 2 (2.5%) | 2 (4.7%) | 0 (0.0%) | 0.497 |
| Diabetes Type 2 | 12 (15.0%) | 5 (11.6%) | 7 (18.9%) | 0.363 |
| Dyslipidemia | 44 (55.0%) | 16 (37.2%) | 28 (75.7%) | <.001 |
| Hypertension | 35 (43.8%) | 16 (37.2%) | 19 (51.4%) | 0.204 |
| Current smoker | 14 (17.5%) | 5 (11.6%) | 9 (24.3%) | 0.136 |
| Family history of CAD | 41 (51.3%) | 21 (48.8%) | 20 (54.1%) | 0.214 |
| Sedentary lifestyle | 45 (56.3%) | 23 (53.5%) | 22 (59.5%) | 0.591 |
| Obesity | 39 (48.8%) | 22 (51.2%) | 17 (45.9%) | 0.642 |
| Overweight | 27 (33.8%) | 14 (32.6%) | 13 (35.1%) | 0.808 |
| Pre-test probability for CAD (Duke Treadmill Score) | ||||
| Low | 2 (2.5%) | 2 (4.7%) | 0 (0.0%) | 0.495 |
| Low-intermediate | 2 (2.5%) | 2 (4.7%) | 0 (0.0%) | |
| Intermediate | 74 (92.5%) | 38 (88.4%) | 36 (97.3%) | |
| High | 2 (2.5%) | 1 (2.3%) | 1 (2.7%) | |
| Physical Exam | ||||
| Systolic blood pressure, mmHg | 120 (110- 131) | 118 (110- 137) | 120 (113- 128) | 0.585 |
| Diastolic blood pressure, mmHg | 72 (70- 80) | 72 (70- 80) | 71 (70- 79) | 0.344 |
| BMI kg/m2 | 30.1 (26.7- 35.0) | 30.5 (26.4- 35.0) | 29.4 (27.0- 35.1) | 0.901 |
| Waist circumference, cm | 101 (94- 114) | 103 (94- 114) | 99 (94- 112) | 0.94 |
| Lipid Lab | ||||
| Total cholesterol, mmol/L | 5.1 (4.4- 5.7) | 5.1 (4.4- 5.7) | 5.2 (4.3- 5.8) | 0.76 |
| HDL cholesterol, mmol/L | 1.3 (1.1- 1.6) | 1.3 (1.1- 1.7) | 1.3 (1.1- 1.6) | 0.827 |
| LDL cholesterol, mmol/L | 2.9 (2.4- 3.5) | 2.7 (2.6- 3.7) | 3.0 (2.1- 3.3) | 0.377 |
| Triglycerides, mmol/L | 1.5 (1.0- 2.1) | 1.4 (0.9- 2.4) | 1.6 (1.2- 1.9) | 0.89 |
Table 1: Baseline characteristics of participants, stratified by plaque.
Baseline 3D carotid ultrasound
Carotid ultrasound characteristics for subjects with plaque are identified in Table 2. Median plaque volume was 32.0 mm3 (IQR: 17.0-123.0 mm3) with the majority (97.3%) being smooth in morphology. Of these plaques, 51.4% were homogeneous isoechoic and immobile. For those subjects without evidence of 3D plaque, CIMT was performed. Figures 2A and 2B shows an example of baseline 3D carotid ultrasound measurement.
| Variables | Plaque Negative (n=43) |
Plaque Positive (n=37) |
|---|---|---|
| Plaque Characteristics for Plaque Seen (n=37) | ||
| Total plaque volume, mm3 | - | 32.0 (17.0- 123.0) |
| Maximum area of vessel reduction, % | - | 10.0 (10.0- 31.0) |
| Plaque surface morphology | ||
| Smooth | - | 36 (97.3%) |
| Irregular (defect< 2 mm) | - | 1 (2.7%) |
| Plaque internal properties | ||
| Homogeneous hyperechoic | - | 4 (10.8%) |
| Homogeneous isoechoic | - | 19 (51.4%) |
| Homogeneous hypoechoic | - | 3 (8.1%) |
| Heterogeneous hyperechoic | - | 6 (16.2%) |
| Heterogeneous isoechoic | - | 3 (8.1%) |
| Heterogeneous hypoechoic | - | 2 (5.4%) |
| Plaque mobility | ||
| Mobile | - | 1 (2.7%) |
| Not mobile | - | 36 (97.3%) |
| CIMT for Plaque Not Seen (n=43) | ||
| LCA Maximum carotid intimae media thickness, mm | 0.9 (0.8- 1.0) | - |
| RCA Maximum carotid intimae media thickness, mm | 0.9 (0.8- 1.0) | - |
Table 2: Baseline 3D carotid ultrasound imaging.
12-month 3D carotid ultrasound in plaque positive subjects
Of the 37 subjects with carotid plaque identified, 22 agreed to receive a followup 3D carotid ultrasound at 12 months (59.5%) (Table 3). The total plaque volume significantly increased at 12 months (p=0.004) with progression in carotid stenosis (p<0.001). No difference in plaque morphology or mobility was noted at 12 months. Follow-up plaque progression can be seen in Figures 2C and 2D.
| Variables | Baseline 3D (n=22) |
12-month 3D (n=22) |
p-value |
|---|---|---|---|
| Total plaque volume, (mm3) | 25.0 (13.1- 48.0) | 116.0 (46.0- 279.0) | 0.004 |
| Maximum area of vessel reduction, (%) | 10.0 (10.0- 30.0) | 50.0 (20.0- 100.0) | <.001 |
| Plaque surface morphology | |||
| Smooth | 22 (100.0%) | 22 (100.0%) | - |
| Irregular (defect< 2 mm) | 0 (0.0%) | 0 (0.0%) | - |
| Plaque internal properties | |||
| Homogeneous hyperechoic | 3 (13.6%) | 0 (0.0%) | 0.308 |
| Homogeneous isoechoic | 10 (45.5%) | 15 (68.2%) | |
| Homogeneous hypoechoic | 2 (9.1%) | 1 (4.5%) | |
| Heterogeneous hyperechoic | 4 (18.2%) | 2 (9.1%) | |
| Heterogeneous isoechoic | 1 (4.5%) | 3 (13.6%) | |
| Heterogeneous hypoechoic | 2 (9.1%) | 1 (4.5%) | |
| Plaque mobility | |||
| Mobile | 0 (0.0%) | 0 (0.0%) | - |
| Not Mobile | 22 (100.0%) | 22 (100.0%) | - |
Table 3: Baseline and follow-up 3D carotid ultrasound imaging in those with plaque.
Primary prevention in plaque positive subjects at 12 months
Of the 37 plaque positive subjects, 21 participants (56.8%) agreed to be referred to cardiac rehabilitation and 12 subjects (33.3%) completed the program. As seen in Table 1, 40.9% were on statin therapy (all were suggested to start high-dose statin therapy following identification of carotid plaque). Antiplatelet therapy was used in 31.8%, angiotensin converting enzyme inhibitors in 22.7%, and beta blockers in 13.6% of subjects in follow-up.
12-month 3D carotid ultrasound in plaque positive subjects stratified by use of cholesterol lowering medication
As seen in Figure 3, total plaque volume remained stable in subjects on statins (46.5 mm3 [25-97.5 mm3] at baseline; 50 mm3 [29-131 mm3] at 12 months, p=0.596), while it increased in those without statin therapy (20 mm3 [10-31 mm3] at baseline; 117 mm3 [78-279 mm3] at 12 months, p=0.003).
Discussion
In a healthy middle-aged cohort of subjects without a history of ASCVD and no evidence of ischemia (negative EST), almost one-half have evidence of subclinical atherosclerosis as detected by 3D carotid ultrasound. Moreover, plaque progression commonly occurs but appears to be attenuated by statins - as observed with 3D carotid ultrasound at 1 year. Use of 3D carotid ultrasound as an additional tool for risk stratification could be considered although further investigation is warranted.
While current vascular disease prediction models are helpful in identifying high-risk individuals where primary prevention would be warranted, there appears to be a false sense of reassurance of those deemed to be intermediate (or low) risk [12,13]. In fact, a high percentage of ASCVD events still occur in this patient population [14]. Further, clinical risk models do not consider lifetime risk (usually limited to 10-year risk), yet a younger population (like our study cohort) may incrementally benefit from aggressive risk factor reduction. Importantly, derivation of risk scores are based on populationbased studies which do not account for ethnicity [15] or socioeconomic status [16] – now considered powerful predictors of ASCVD risk [16]. In a multi-ethnic community-based cohort, the majority of cardiovascular risk assessment scores actually overestimate ASCVD events [17]. Hence, additional risk stratification metrics are required to help mitigate outcomes in a primary prevention population.
In our cohort, a majority had an intermediate pre-test probability of CAD, yet more than one-half did not have subclinical atherosclerosis (as measured by 3D carotid ultrasound). Conversely, a minority of low-risk patients demonstrated evidence of subclinical atherosclerosis. Hence, reclassification measures may be required to help identify appropriate patients for primary prevention [18,19]. In the Multi-Ethnic Study of Atherosclerosis study evaluating additional markers of risk, a coronary calcium score of 0 was the strongest modulator of ASCVD risk and had the greatest impact on reclassification [10]. Acknowledging the importance of this risk stratification test, coronary calcium scores are not universally readily available and subject patients to the detriments of radiation exposure (albeit lower in dose with current technology). Carotid ultrasound is readily available, safe and easy to perform with no risks of radiation exposure. In addition, quantification of carotid plaque (larger plaque burden) has been associated with incremental risk of vascular death, myocardial infarction or stroke at 5 years [20]. Our results suggest this simple and inexpensive test can be used in clinical practice and does help in the reclassification of subjects at risk.
CIMT has been proposed as a potential tool for additional risk stratification as a surrogate measurement. However, in a systematic review and metaanalysis of a one-time CIMT measurement of asymptomatic subjects, CIMT did not predict myocardial infarction or stroke (median of 10.8 years of follow-up) [21]. Similarly, in studies with serial CIMT measurements where yearly progression was measured, no association was demonstrated between CIMT progression and cardiovascular risk [22,23]. In fact, compared to CIMT, carotid plaque area is more strongly associated with traditional risk factors and more commonly predicts myocardial infarction [24,25]. As such, CIMT has not been widely adopted as a reclassification tool.
In contrast, assessment of carotid arterial plaque offers additional risk stratification given the identification of actual plaque morphology – a more meaningful reflection of atherosclerosis. Moreover, correlation exists with carotid atherosclerosis and disease burden in the coronary vascular bed [26]. Quantification of carotid plaque has now emerged as an important tool for ASCVD risk stratification, enhanced with 3D quantification [27]. With a single sweep full-vessel protocol, 3D datasets are acquired and can be serially tracked with repeated measurements that can be performed offline [28]. Additionally, the volume of all plaque lesions within the carotid can be captured and the total plaque volume measured – as has been demonstrated in our study. We have also established the feasibility of a simple test within our cohort of subjects where reclassification of cardiovascular risk becomes important in an asymptomatic population. The 2016 European Society of Cardiology guidelines on cardiovascular disease prevention now include plaque detection as a tool for refining cardiovascular risk following risk score assessment (Class IIB, LOE B) – analogous to a similar recommendation for coronary calcium scoring (Class IIB, LOE B) [29]. The 2019 American Heart Association/American College of Cardiology guidelines for primary prevention of cardiovascular disease have been silent on the use of coronary plaque imaging [30], however the 2020 American Society of Echocardiography have endorsed the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and assessment of cardiovascular risk [31]. In this context, it is interesting to note the recent findings of a prospective study showing the prognostic value of identifying subclinical carotid atherosclerosis in patients with a negative exercise echocardiogram [32].
In those subjects with established ASCVD, it is interesting to note the increase in total plaque volume and carotid stenosis at 12 months (without a change in plaque morphology) in those receiving a follow-up 3D carotid ultrasound. In a prospective observational study of 715 asymptomatic carotid disease subjects with serial carotid ultrasounds performed biannually, (mean followup of 3.2 years), progression of carotid disease was associated with a higher risk of cerebrovascular events or death [33]. Data from the Asymptomatic Carotid Stenosis and Risk of Stroke study group found 20% of subjects had progression of carotid plaque and twice the risk of ipsilateral stroke [34]. The low use of cardiac rehabilitation and primary prevention recommendations despite evidence of ASCVD was disappointing, prompting further efforts in this vulnerable population.
Still, in subjects with confirmed ASCVD on 3D carotid ultrasound who decided to use statins (as per our recommendations), carotid plaques were stable in volume at 12 months. Similar findings were demonstrated in a small randomized study of high-dose statin therapy on 3D carotid plaque volume at three months [35]. Recognizing this in a small cohort of patients in the current study, it is reassuring to see the benefits of cholesterol lowering therapy in these asymptomatic carotid disease subjects. Moreover, it has been demonstrated that statin therapy in higher-risk subjects reduces coronary events and ischemic strokes long term [36].
Our results are with limitations. This is a prospective observational cohort subject to unmeasured confounders. Roughly one-half of subjects with carotid plaque at baseline had a follow-up carotid ultrasound at 12 months. While strongly encouraged, only a minority of subjects attended (and completed) cardiac rehabilitation. Similarly, a minority of subjects with plaque were on statin medication (although this did allow us to assess the treatment effect on carotid plaque volume). Finally, this was a small cohort of patients; however, to our knowledge, this is the only study assessing 3D carotid ultrasound in an asymptomatic population with a normal EST – a population that is frequently encountered for chest pain assessment.
While functional non-invasive stress tests are important for the assessment of coronary disease, many with negative tests may have evidence of ASCVD, as seen in our study. Patients are often reassured and not provided any additional tests for risk stratification. Direct assessments for atherosclerosis, such as carotid plaque imaging, may be helpful for primary prevention. Those without plaque can be reassured, whereas those with carotid plaque would benefit from primary preventative strategies. While our results are provocative, we propose a further randomized study assessing the efficacy of carotid plaque assessment in subjects with negative ESTs.
Author Contributions
All authors made a substantial contribution to the design of the study; acquisition, analysis and interpretation of the data. All authors contributed to drafting the manuscript and provided important intellectual content.
Acknowledgements
The authors would like to thank Lisa Soulard of the Canadian VIGOUR Centre for her editorial assistance and preparation of the manuscript submission.
Funding
Heart and Stroke Foundation of Canada Operating Grant.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005;111:3481–88.
- Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988;12:56–62.
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–35.
- Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur Heart J 2003;24:987–1003.
- D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart study. Circulation 2008;117:743–53.
- Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ 2008;336:1475–82.
- Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA 2014;311:1406–15.
- Pylypchuk R, Wells S, Kerr A, et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: A derivation and validation study. Lancet 2018;391:1897–1907.
- Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47.
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–58.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular. J Am Soc Echocardiogr 2008;21:93–111.
- Vasan RS, Sullivan LM, Wilson PWF, et al. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann Intern Med 2005;142:393–402.
- Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–98.
- Berry JD, Liu K, Folsom AR, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease. The coronary artery risk development in young adults study and multi-ethnic study of atheroscleros. Circulation 2009;119:382–89.
- Ko DT, Sivaswamy A, Sud M, et al. Calibration and discrimination of the Framingham Risk Score and the Pooled Cohort Equations. CMAJ 2020;192:442–49.
- Dalton JE, Perzynski AT, Zidar DA, et al. Accuracy of cardiovascular risk prediction varies by neighborhood socioeconomic position a retrospective cohort study. Ann Intern Med. 2017;167:456–64.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–75.
- Pasternak RC, Abrams J, Greenland P, et al. 34th Bethesda Conference: Task force #1--Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol 2003;41:1863–74.
- Akosah KO, Schaper A, Cogbill C, et al. Preventing myocardial infarction in the young adult in the first place: How do the national cholesterol education panel III guidelines perform? J Am Coll Cardiol 2003;41:1475–79.
- Spence JD, Eliasziw M, Di-Cicco M, et al. Carotid plaque area: A tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916–22.
- Den Ruijter HM, Peters SAE, Anderson TJ, et al. Common carotid intima-media thickness measurements incardiovascular risk prediction: A meta-analysis. JAMA 2012;308:796–803.
- Lorenz MW, Gao L, Ziegelbauer K, et al. Predictive value for cardiovascular events of common carotid intima media thickness and its rate of change in individuals at high cardiovascular risk – Results from the PROG-IMT collaboration. PLoS One 2018;13:0191172.
- Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (The PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet 2012;379:2053–62.
- Spence JD. Technology insight: Ultrasound measurement of carotid plaque - Patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol 2006;2:611–19.
- Johnsen SH, Mathiesen EB, Joakimsen O, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The Tromsø study. Stroke 2007;38:2873–80.
- Naqvi TZ, Mendoza F, Rafii F, et al. High prevalence of ultrasound detected carotid atherosclerosis in subjects with low framingham risk score: Potential implications for screening for subclinical atherosclerosis. J Am Soc Echocardiogr 2010;23:809–15.
- Johri AM, Chitty DW, Matangi M, et al. Can Carotid bulb plaque assessment rule out significant coronary artery disease? A comparison of plaque quantification by two- and three-dimensional ultrasound. J Am Soc Echocardiogr 2013;26:86–95.
- Kalashyan H, Saqqur M, Shuaib A, et al. Comprehensive and rapid assessment of carotid plaques in acute stroke using a new single sweep method for three-dimensional carotid ultrasound. Echocardiography 2013;30:414–18.
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2016;37:2315–81.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:596–646.
- Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: From the American Society of Echocardiography. J Am Soc Echocardiogr 2020;33:917-33.
- Vidal-Perez R, Franco-Gutiérrez R, Pérez-Pérez AJ, et al. Subclinical carotid atherosclerosis predicts all-cause mortality and cardiovascular events in obese patients with negative exercise echocardiography. World J Cardiol 2019;11:24–37.
- Lewis RF, Abrahamowicz M, Côté R, et al. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997;127:13–20.
- Kakkos SK, Nicolaides AN, Charalambous I, et al. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg 2014;59:956-967.
- Ainsworth CD, Blake CC, Tamayo A, et al. 3D ultrasound measurement of change in carotid plaque volume: A tool for rapid evaluation of new therapies. Stroke 2005;36:1904–09.
- Collins R, Armitage J, Parish S, et al. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757–67.