A case of asymptomatic primary biliary cholangitis accompanied by drug-induced liver injury caused by bicalutamide
2 Department of Urology, Harasanshin Hospital, Fukuoka, Japan, Email: HaruhikoIchikura90@yahoo.com
3 Department of Pathology, Harasanshin Hospital, Fukuoka, Japan, Email: ShinjiKono90@yahoo.com
4 Department of Hepatology, Saiseikai Fukuoka General Hospital, Fukuoka, Japan, Email: ShusukeMorizono@hotmail.com
Received: 01-Oct-2018 Accepted Date: Oct 09, 2018; Published: 15-Oct-2018
Citation: Kotoh K, Noguchi K, Ichikura H, et al. A case of asymptomatic primary biliary cholangitis accompanied by drug-induced liver injury caused by bicalutamide. J Hepato Gastroenterol. 2018;2(2):37-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
A 74-year-old man was admitted to our department suffering from liver injury sustained during treatment for advanced prostate carcinoma. He had been diagnosed as having asymptomatic primary biliary cholangitis 6 years earlier. Three months before admission, he began anti-androgen treatment with abiraterone acetate. Because of mildly elevated serum transaminases, abiraterone acetate was changed to bicalutamide. Although liver injury improved shortly after changing medications, severe liver injury occurred acutely 6 weeks from beginning bicalutamide, which worsened until after bicalutamide was discontinued for 11 days. Pathological findings indicated that pre-existing asymptomatic primary biliary cholangitis may worsen druginduced liver injury. This is the first report indicating the potential risk for drug-induced liver injury in the patients with asymptomatic primary biliary cholangitis despite their normalized results of serum laboratory examination.
Keywords
Asymptomatic primary biliary cholangitis; Drug-induced liver injury; Bicalutamide
Introduction
Primary biliary cholangitis (PBC) is a relatively rare autoimmune liver disease characterized by progressive destruction of intrahepatic bile ducts. Classically, the typical form appeared as progressive liver fibrosis with pruritus, jaundice, and xanthomatosis. However, the majority of patients are now diagnosed and maintained in the early asymptomatic stage, especially in developed countries [1]. In this report, we describe a patient with druginduced liver injury (DILI) who was diagnosed as having asymptomatic PBC (aPBC) several years earlier. To the best of our knowledge, no previous studies have focused on patients with PBC complicated with DILI. There are two likely reasons for this lack. First, patients with PBC and DILI show similar results for serum biochemical markers including elevated serum levels of alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase(GGT). In patients with PBC complicated with DILI, elevated serum ALP and GGT levels may be considered a flare as part of the natural clinical course of PBC. Second, drugs with a high risk of inducing DILI should be avoided in patients with PBC, but this is not always so. Many patients with aPBC treated with ursodeoxycholic acid (UDCA) have serum laboratory data within normal ranges including bilirubin and hepatobiliary enzymes [2]. To our knowledge, no studies have evaluated the possibility that aPBC could affect the development of DILI. If a cholestatic tendency remains in patients with aPBC even during periods of normal-range serum data, the clinical course of DILI caused by bile-excretion drugs could worsen or be prolonged.
Case Report
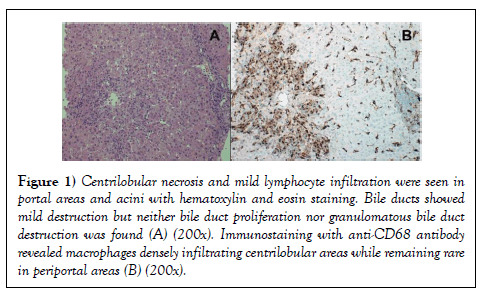
A 74-year-old man was admitted to the Department of Hepatology of our hospital suffering from liver injury without clinical symptoms. He had been diagnosed as having PBC 6 years before admission, at a previous hospital, based on liver biopsy, which showed chronic nonsuppurative destructive cholangitis without fibrosis. The results of serum laboratory examination on the date of the biopsy were: aspartate aminotransferase (AST): 28 U/L, alanine aminotransferase (ALT): 56 U/L, ALP: 389 U/L, GGT: 139 U/L, total bilirubin: 0.9 mg/dL, and positive anti-mitochondrial antibody status. Administration of 900 mg/day of UDCA was started immediately after serum examinations, which quickly normalized all serum hepatobiliary enzyme levels. The patient had been continuously receiving the same dose of UDCA for 6 years to control the aPBC. Three months before admission, he visited the Department of Urology of our hospital for a general health examination, which revealed elevated prostate-specific antigen levels. Computed tomography revealed advanced prostate carcinoma with bone, lung, and lymph node metastasis, and invasion to the rectum and bladder. Serum tests showed completely normal hepatic function (albumin: 4.0 g/ dL, total bilirubin: 1.0 mg/dL, AST: 19 U/L, ALT: 12 U/L, ALP: 305 U/L, and GGT: 27 U/L). Based on cancer stage cT4N1M1, anti-androgen therapy with daily abiraterone acetate (1000 mg/day) with prednisolone (5 mg/day) and monthly degarelix acetate was initiated (240 mg in the first month, and 80 mg in following months). On the 22nd day of abiraterone acetate, mildly elevated serum hepatobiliary enzyme levels were discovered (AST: 102 U/L, ALT: 113 U/L, ALP: 648 U/L, and GGT: 110 U/L). Based on recommendations for the use of abiraterone acetate, the drug was stopped for 1 week. After confirming improvement of the liver injury, abiraterone acetate was restarted at 750 mg/day. However, 5 days after the restart, serum hepatobiliary enzyme levels increased again. Abirateron acetate was immediately discontinued, and 80 mg/day bicalutamide was started. Although serum hepatobiliary enzyme levels decreased steadily until the 14th day of bicalutamide therapy, levels increased abruptly on the 42nd day (AST: 231 U/L, ALT: 346 U/L, ALP: 445 U/L, and GGT: 77 U/L). All markers for acute hepatitis viral infection, cytomegalovirus, and Epsteinâ€ÂBarr virus were negative. Positive anti-mitochondrial antibody and a high level of serum IgM (260 mg/dL) indicated PBC, but antinuclear antibody was negative. Also, the patient showed no clinical symptoms including icterus. Bicalutamide was discontinued on the 42nd day, but blood laboratory examination revealed that hepatobiliary enzyme levels had increased further, and the patient was admitted to the Department of Hepatology 8 days after discontinuation of bicalutamide. Two days later, we performed a liver biopsy, and hematoxylin and eosin staining showed centrilobular necrosis and mild lymphocyte infiltration into portal areas and acini (Figure 1A). Immunostaining with anti-CD68 antibody, which stains positive in affected monocytes/macrophages, showed CD68-positive cells densely populating the centrilobular areas (Figure 1B).
Figure 1: Centrilobular necrosis and mild lymphocyte infiltration were seen in portal areas and acini with hematoxylin and eosin staining. Bile ducts showed mild destruction but neither bile duct proliferation nor granulomatous bile duct destruction was found (A) (200x). Immunostaining with anti-CD68 antibody revealed macrophages densely infiltrating centrilobular areas while remaining rare in periportal areas (B) (200x).
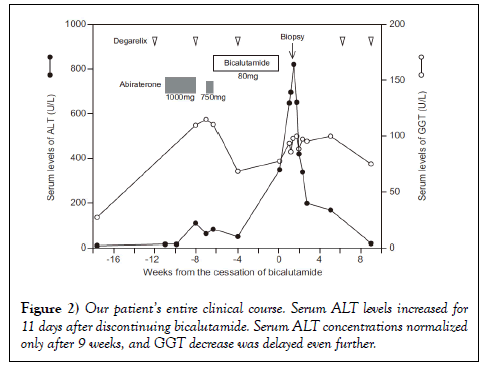
Serum hepatobiliary enzyme levels increased until the 3rd day in the Department of Hepatology, but began to decrease the next day without treatment. The patient was discharged on the 13th day after admission to the Department of Hepatology. Although follow-up examinations showed continuously decreasing serum AST and ALT levels, which normalized 9 weeks after discontinuing bicalutamide (23 U/L and 16 U/L, respectively), ALP and GGT levels remained high (325 U/L and 74 U/L, respectively). The patient’s clinical course is shown in Figure 2.
Discussion
Abiraterone acetate and bicalutamide are used in patients with advanced prostate carcinoma worldwide, and are known to rarely induce severe hepatotoxicity. The rate of serum aminotransferase elevations in patients receiving abiraterone acetate is as high as 13%, but reported abnormalities are mild and transient [3]; no reported patients developed jaundice. In patients treated with bicalutamide, the prevalence of serum aminotransferase elevations is approximately 6% [4]. Although the liver injury caused by bicalutamide is generally asymptomatic and transient [5], five patients with severe liver injury have been reported (Table 1) [6-10].
| Author | Prior treatment | Duration of bicalutamide | Detected liver injury | Outcome | |
|---|---|---|---|---|---|
| T-Bili | ALT | ||||
| Dawson et al. | flutamide | 2 days | 19.47 mg/dL* | 2690 U/L | Improved |
| Castro Beza et al. | none | 3 months | 11.57 mg/dL | 1923 U/L | Died |
| O’Bryant et al. | none | 4 days | N.D. | 111 U/L | Died |
| Hussain et al. | none | 3 weeks | 3.7 mg/dL | 576 U/L | Improved |
| Yun et al. | none | 5 months | 1.62 mg/dL | 677 U/L | Improved |
*333μmol/L in the original paper; N.D.:not described; TBili: total bilirubin; ALT: alanine aminotransferase
Table 1: Reported cases of severe liver injury resulting from bicalutamide treatment
However, liver injury in two of the reported patients was not considered to result from bicalutamide therapy. Dawson et al.’s patient’s liver failure was considered to have resulted from prior treatment with flutamide that was used for 3 months before only two doses of bicalutamide, which is too short a time (2 days) to develop severe jaundice. O’Bryant et al. described a patient who developed fatal acute liver failure after 4 days’ administration of bicalutamide, but the patient was admitted to the hospital with complaints of right lower abdominal pain and distension that are not seen in typical drug-induced liver failure. Laboratory data indicating liver failure in this patient appears to have been part of multiple organ failure caused by etiologies other than drugs. In the other three reported cases, bicalutamideinduced liver injury was found with jaundice of varying degrees. In Castro Beza et al.’s report, the patient’s serum bilirubin level reached 11.57 mg/dL, and bicalutamide was not discontinued until 2 weeks later, ending in patient death; the remaining two reported patients improved after discontinuing bicalutamide. Bicalutamide rarely causes severe liver injury, but a shorter duration from the onset of liver injury to discontinuing bicalutamide could inhibit the progression of hepatotoxicity and lead to faster improvement. Our patient’s clinical course revealed two rare episodes of DILI that appeared to be continuous. Considering the low probability, it is natural to consider that these episodes occurred separately, in a series. Two possible explanations exist for this unusual sequence. First, hepatocytes damaged by abiraterone acetate might lead to delayed washout of the metabolites of bicalutamide. However, serum ALT levels continued to decrease even 2 weeks from the start of bicalutamide, in our patient, which strongly indicates that the liver injury caused by abiraterone acetate did not influence the subsequent bicalutamideinduced liver injury. Another explanation is that the background aPBC in our patient contributed to the two rare DILI episodes. Abiraterone acetate is mainly metabolized in the liver by CYP3A4 and CYP2D6, and >95% of the drug is eliminated in feces [11]. Bicalutamide includes two isomers: the active form, (R)-bicalutamide, and the inactive form, (S)-bicalutamide. The steady-state plasma concentrations of (R)-bicalutamide are 100-fold higher than those of the (S)-enantiomer. The major pathway of (R)-bicalutamide elimination is by hydroxylation in the liver, which is largely mediated by CYP3A4, followed by glucuronidation, whereas (S)-bicalutamide undergoes direct glucuronidation. Bicalutamide metabolites are excreted almost equally in urine and feces [12]. Therefore, decreased liver function delays the elimination of abiraterone acetate and bicalutamide, both of which are prohibited in patients with severe liver impairment [13]. Although earlier reports showed a low risk in patients with mild liver injury, the degree of liver impairment was judged by Child-Pugh score and serum transaminase levels and was not assessed by biopsy. DILI secondary to bile-excretion drug administration could be affected by both hepatocyte destruction and bile duct obstruction. However, no reports have discussed whether patients with aPBC controlled by UDCA have a cholestatic tendency despite their normalized hepatic laboratory data. It is important to note that serum ALT levels in our patient continued to increase for 11 days after discontinuing bicalutamide, which has not previously been found in general liver impairment caused by bicalutamide. This unusual prolonged liver injury and the rare occurrence of DILI indicate that patients with aPBC have a risk for liver injury caused by metabolites that should be eliminated through the bile ducts. Our pathological findings appear to support this hypothesis. Hepatotoxicity caused by bicalutamide metabolites is dominant in zone 3 where CYP enzymes are rich compared with zone 1. Our patient’s findings showed centrilobular necrosis and macrophage accumulation in zone 3 with mild periportal lymphocyte infiltration. Intrahepatic macrophages are activated by damage-associated molecular patterns generated by dead and dying hepatocytes [14].