A Case of Mistaken Identity: Doubled Superior Cervical Ganglia
Received: 20-Mar-2021 Accepted Date: Apr 05, 2021; Published: 12-Apr-2021, DOI: 10.37532/1308-4038.14(4).188-190
Citation: Mansour Y, Kulesza R. A case of mistaken identity: doubled superior cervical ganglia. Int J Anat Var. 2021;14(4):74-76.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The sympathetic chain serves as a major hub for distribution of both sensory and motor innervation of smooth muscle, cardiac muscle and glands throughout the entire body. The sympathetic chain, along its course from the base of the skull to the tip of the coccyx, includes numerous ganglia that house cell bodies of postsynaptic neurons. The cervical portion of the sympathetic chain includes superior, middle, inferior/stellate and vertebral ganglia. As a group, these ganglia are subject to significant anatomical variation in their location and even presence. While the superior cervical ganglion appears to be the most consistent of these ganglia, there remains a discrepancy in the literature.
Specifically, the superior cervical ganglion has been reported to be doubled but this appears to have been perpetuated without clear documentation. This discrepancy was brought to our attention during a detailed dissection of the retropharyngeal region that exposed the superior cervical ganglion and structures at the skull base, including what appeared to be a ganglion anterior to the carotid sheath. This dissection matched exactly the earliest report of a doubled superior cervical ganglion. However, histological examination revealed the mass anterior to the carotid sheath to be a retropharyngeal lymph node. Based on this finding, consistent with large-scale studies of the cervical sympathetic chain, we posit the original observation was of a lymph node and not a doubled superior cervical ganglion.
Keywords
Sympathetic chain; Retropharyngeal; Lymph node; Variation
Introduction
The autonomic nervous system (ANS) includes efferent and afferent circuits among both the central and peripheral nervous systems. The ANS utilizes two major pathways, sympathetic and parasympathetic, to monitor and regulate the viscera (smooth muscle, cardiac muscle and glands). A notable feature of the efferent circuits of these systems is the utilization of two neurons between the central nervous system and peripheral targets. For the sympathetic pathway, this includes preganglionic neurons in the interomediolateral cell column of the spinal cord, extending from the first thoracic segment to about the second lumbar segment (i.e. T1-L2). Axons of preganglionic neurons leave the spinal cord and enter the sympathetic chain via white rami. The sympathetic chain extends from the base of the skull to the tip of the coccyx and includes numerous ganglia along its course that contain the cell bodies of many postganglionic neurons. While axons of preganglionic neurons may synapse within the chain or pass through to reach other (pre-aortic) ganglia, the sympathetic chain serves as an important hub to distribute both efferent and afferent axons of the sympathetic pathway to viscera throughout the entire body.
However, there is some discrepancy between the literature and textbooks of anatomy on duplication of the superior cervical ganglion (SCG). Specifically, in Morris’ Human Anatomy (12) it is noted that “Occasionally the ganglion [SCG] is double, with an anterior portion lying superficial to the carotid sheath and a posterior portion deep to the sheath”. However, no further description or documentation of this finding is provided, and no reference is cited. Further, reference to a duplicated SCG is made in texts of anatomical variation (13; 14). In the latter, a citation is made to Lang (15). Specifically, that “the superior cervical ganglion can be duplicated”. However, this statement cannot be found in Lang’s book. Additionally, a doubled SCG is included as an anatomical variant on radiopedia.org (16). Taken together, it appears that the observation of a duplicated SCG is not substantiated by any photographic evidence or histological confirmation. Further, a number of large-scale studies of the cervical sympathetic chain suggest that such a claim may have been made in error (4; 2).
Case Report
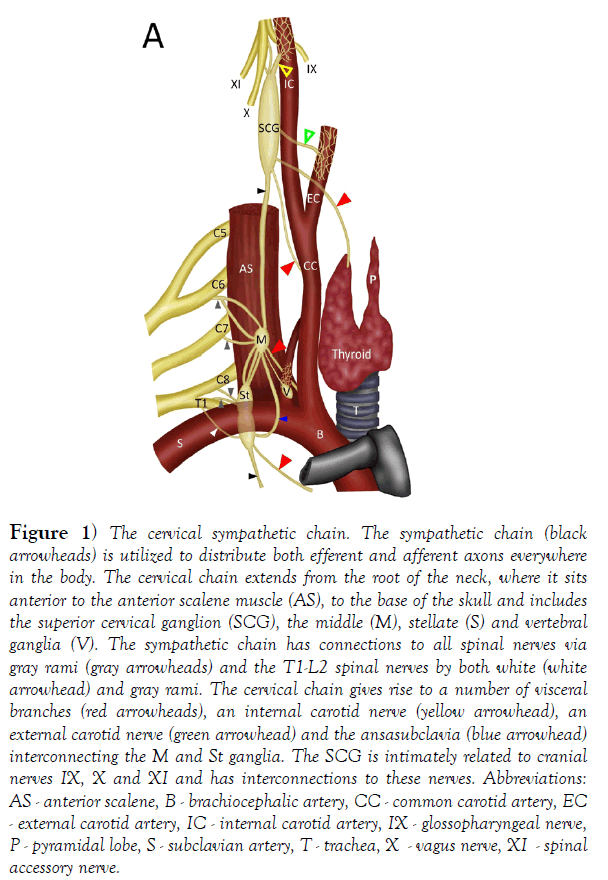
In humans, the sympathetic chain typically includes between 20 and 23 ganglia [1]. Developmentally, the sympathetic chain begins as a continuous column of ganglia with neurons present at every spinal level. However, these ganglia fuse unevenly, and this leads to the absence of ganglia at certain spinal levels. This is most notable along the cervical portion of the sympathetic chain: while there are eight cervical nerves/segments there are typically only three or four cervical chain ganglia. At the root of the neck there is an inferior cervical ganglion, composed of fused ganglia of C7-8 [2] (Figure 1). This ganglion is often fused with the T1 ganglion (40-80% of subjects), forming a stellate ganglion. Additionally, in most subjects there is a vertebral ganglion. This ganglion sits anterior or medial to the vertebral artery [2] (Figure 1). The middle cervical ganglion is composed of fused ganglia for C5-6 and is typically found at the level of the arch of the inferior thyroid artery. This ganglion is frequently absent (absent in ~37% of subjects - 2; absent in 26% of subjects [3], but ganglion cells may be displaced inferiorly in the sympathetic chain or to lower ganglia [4]. The superior cervical ganglion (SCG) is composed of the fused ganglia of C1-4 [1] (Figure 1). The SCG is the largest of the cervical ganglia and includes over 1 million neurons [5]. The SCG is the most consistent of the cervical ganglia - it is typically located posterior to the carotid sheath and internal carotid artery at the level of CV2-3 and is present in 100% of subjects [2,6-10]. The consistency of the SCG is emphasized by two larger studies of the cervical sympathetic chain. In dissection of 100 cervical sympathetic trunks, the SCG was present in all subjects as a single oval-shaped mass [4]. In a further study of 114 cervical chains, the SCG was identified in all subjects and never found as a multiple structure [2]. We interpret this observation to indicate that no duplication of the SCG was found in these specimens. However, there is a report of bilateral ectopic SCG. In this case, the SCG was situated more inferiorly in the neck at the level of the carotid bifurcation on the right side and at the level of the thyroid gland on the left [11-15].
Figure 1) The cervical sympathetic chain. The sympathetic chain (black arrowheads) is utilized to distribute both efferent and afferent axons everywhere in the body. The cervical chain extends from the root of the neck, where it sits anterior to the anterior scalene muscle (AS), to the base of the skull and includes the superior cervical ganglion (SCG), the middle (M), stellate (S) and vertebral ganglia (V). The sympathetic chain has connections to all spinal nerves via gray rami (gray arrowheads) and the T1-L2 spinal nerves by both white (white arrowhead) and gray rami. The cervical chain gives rise to a number of visceral branches (red arrowheads), an internal carotid nerve (yellow arrowhead), an external carotid nerve (green arrowhead) and the ansasubclavia (blue arrowhead) interconnecting the M and St ganglia. The SCG is intimately related to cranial nerves IX, X and XI and has interconnections to these nerves. Abbreviations: AS - anterior scalene, B - brachiocephalic artery, CC - common carotid artery, EC - external carotid artery, IC - internal carotid artery, IX - glossopharyngeal nerve, P - pyramidal lobe, S - subclavian artery, T - trachea, X - vagus nerve, XI - spinal accessory nerve.
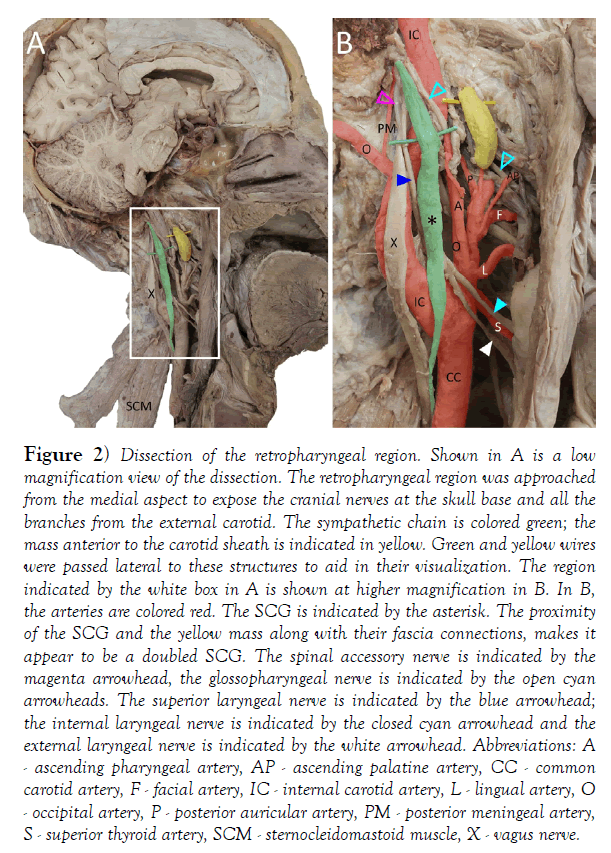
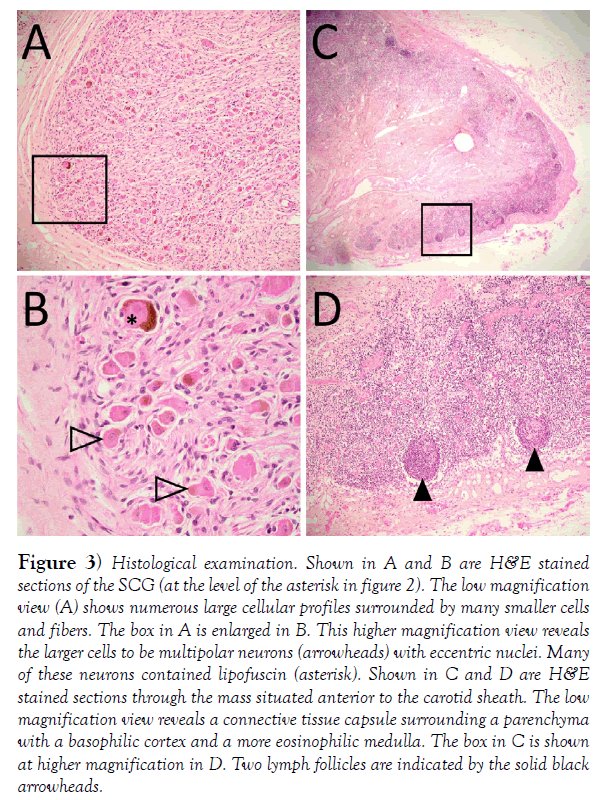
The aforementioned discrepancy in duplication of the SCG between texts and quantitative studies was brought to our attention during dissection of the retropharyngeal area in a 60-year-old male (cause of death listed as cardiopulmonary arrest) revealing what we initially identified as a doubled SCG (Figure 2). In this specimen, we identified the sympathetic chain and SCG posterior to the carotid sheath and internal carotid artery (Figure 2); green, asterisk). Additionally, there was another mass situated anterior to the carotid sheath that appeared to be connected to the sympathetic chain via 1 or 2 thin branches (Figure 2); yellow. Interestingly, our observations appeared to match exactly the description in Morris’ textbook. We hoped to confirm this observation and the SCG and the anterior mass were excised and processed for histological study. Microscopic examination of hematoxylin and eosinstained tissues sections [17] from these masses revealed the mass posterior to the carotid sheath to include numerous neuronal profiles, characterized by large irregular somata, eccentric nuclei and prominent nucleoli (Figure 3). Many neurons had cytoplasmic accumulation of lipofuscin (asterisk in Figure 3). There were intermingled axonal profiles and numerous satellite cells. Together, these features are consistent with an autonomic ganglion. The mass anterior to the carotid sheath had a connective tissue capsule forming septa that could be seen to penetrate the parenchyma (Figure 3). The parenchyma had a darker staining cortical zone with compact lymphocytes and occasional nodules (Figure 3, arrowheads). There was also a deeper, medullary region that contained only diffuse lymphocytes. Based on these histological features and location, this is most likely a retropharyngeal lymph node. Indeed, confusion of SCG with retropharyngeal lymph nodes is recognized [18-20].
Figure 2) Dissection of the retropharyngeal region. Shown in A is a low magnification view of the dissection. The retropharyngeal region was approached from the medial aspect to expose the cranial nerves at the skull base and all the branches from the external carotid. The sympathetic chain is colored green; the mass anterior to the carotid sheath is indicated in yellow. Green and yellow wires were passed lateral to these structures to aid in their visualization. The region indicated by the white box in A is shown at higher magnification in B. In B, the arteries are colored red. The SCG is indicated by the asterisk. The proximity of the SCG and the yellow mass along with their fascia connections, makes it appear to be a doubled SCG. The spinal accessory nerve is indicated by the magenta arrowhead, the glossopharyngeal nerve is indicated by the open cyan arrowheads. The superior laryngeal nerve is indicated by the blue arrowhead; the internal laryngeal nerve is indicated by the closed cyan arrowhead and the external laryngeal nerve is indicated by the white arrowhead. Abbreviations: A - ascending pharyngeal artery, AP - ascending palatine artery, CC - common carotid artery, F - facial artery, IC - internal carotid artery, L - lingual artery, O - occipital artery, P - posterior auricular artery, PM - posterior meningeal artery, S - superior thyroid artery, SCM - sternocleidomastoid muscle, X - vagus nerve.
Figure 3) Histological examination. Shown in A and B are H&E stained sections of the SCG (at the level of the asterisk in figure 2). The low magnification view (A) shows numerous large cellular profiles surrounded by many smaller cells and fibers. The box in A is enlarged in B. This higher magnification view reveals the larger cells to be multipolar neurons (arrowheads) with eccentric nuclei. Many of these neurons contained lipofuscin (asterisk). Shown in C and D are H&E stained sections through the mass situated anterior to the carotid sheath. The low magnification view reveals a connective tissue capsule surrounding a parenchyma with a basophilic cortex and a more eosinophilic medulla. The box in C is shown at higher magnification in D. Two lymph follicles are indicated by the solid black arrowheads.
Conclusion
The burden of proof for a doubled SCG would require both gross anatomical and histological confirmation. The gross anatomical proof should include location and continuity with the sympathetic chain through gray rami. The histological proof should include clear identification of neuronal profiles and organization consistent with an autonomic ganglion. This burden has not been met by the texts noted previously; the claim of a doubled SCG is unsubstantiated. Given the lack of descriptions and photographic evidence for doubled SCG, we call into question the statement that doubled SCG has been identified and documented. Instead, we believe the original statement was based on the scenario we have described above: a SCG and a retropharyngeal lymph node. The authors hope that future texts of anatomical variation can correct this unsubstantiated claim that has been perpetuated since at least 1942.
REFERENCES
- Crosby EC, Humphrey T, Lauer EW. Correlative Anatomy of the Nervous System. New York; The MacMillan Company, First Edition. New York, NY: The Macmillan Company. 1962.
- Becker RF, Grunt JA.The cervical sympathetic ganglia. Anat Rec. 1957;127:1-14.
- Civelek E, Karasu A, Cansever T, et al. Surgical anatomy of the cervical sympathetic trunk during anterolateral approach to cervical spine. Euro spine J. 2008;17:991-5.
- Jamieson RW, Smith DB, Anson BJ. The cervical sympathetic ganglia. An anatomical study of 100cervicothoracic dissections. Q Bull. Northwestern University Medical School. 1952;26:219-27.
- Pearson J, Pytel BA. Quantitative studies of sympathetic ganglia and spinal cord intermedio-lateral gray columns in familial dysautonomia. J Neurol Sci. 1978;39:47-59.
- Kiray A, Arman C, Naderi S, et al. Surgical anatomy of the cervical sympathetic trunk. Clin Anat. 2005;18:179-85.
- Tubbs RS, Salter EG, Oakes WJ. Anatomic landmarks for nerves of the neck: a Vade Mecum for neurosurgeons. Oper Neurosurg. 2005;2:256-60
- Fazliogullari Z, Kilic C, Karabulut AK, et al. A morphometric analysis of the superior cervical ganglion and its surrounding structures. Surg Radiol Anat. 2016;38(3):299-302.
- Stark ME, Safir I, Wisco JJ. Probabilistic mapping of the cervical sympathetic trunk ganglia. Auton Neurosci. 2014;181:79-84.
- Yin Z, Yin J, Cai J, et al. Neuroanatomy and clinical analysis of the cervical sympathetic trunk and longuscolli. J Biomed Res. 2015;29:501-7.
- Bhatnagar KP, Nettleton GS, Kuwabara N, et al. A case of bilateral ectopic superior cervical ganglia in man. Ann Anat. 2003;185:149-52.
- Schaeffer JP. Morris' Human Anatomy: A complete systematic treatise. 10th ed. Philadelphia, PA. The Blakiston Company. 1942.
- Bergman RA, Thompson SA, Afifi AK. Compendium of Human Anatomic Variation: Text, Atlas, and World Literature. Germany: Urban & Schwarzenberg. 1988.
- Tubbs RS, Shoja MM, Loukas M. Bergman's Comprehensive Encyclopedia of Human Anatomic Variation, First Edition. Hoboken, NJ: Wiley-Blackwell. 2016:1456.
- Lang J. Clinical Anatomy of the Nose, Nasal Cavity and Paranasal Sinuses. Thieme Medical Publishers, Inc. New York. 1989.
- https://radiopaedia.org/articles/sympathetic-chain-1?lang=us
- Hegazy R, Hegazy A. Hegazy’ Simplified Method of Tissue Processing (Consuming Less Time and Chemicals). AIMDR. 2015;1:57-61.
- Loke SC, Karandikar A, Ravanelli M. Superior cervical ganglion mimicking retropharyngeal adenopathy in head and neck cancer patients: MRI features with anatomic, histologic, and surgical correlation. Neuroradiology. 2016;58:45-50.
- Moubayed SP, Machado R, Osorio M, et al. Metastatic squamous cell carcinoma to the superior cervical ganglion mimicking a retropharyngeal lymph node. Am J Otolaryngol. 2017;38:720-3.
- Yokota H, Mukai H, Hattori S, et al. MR Imaging of the Superior Cervical Ganglion and Inferior Ganglion of the Vagus Nerve: Structures That Can Mimic Pathologic Retropharyngeal Lymph Nodes. AJNR Am J Neuroradiol. 2018;39:170-6.