A case report of Klinefelter’s syndrome hiding behind pulmonary embolism
2 Department of Cardiology, First Affiliated Hospital, Nanjing Medical University, Jiangsu 210029, P.R. China
Received: 10-Aug-2017 Accepted Date: Sep 05, 2017; Published: 07-Sep-2017, DOI: 10.4172/2368-0512.1000091
Citation: Xiao P, Nabeel DM, Zhang K, et al. A case report of Klinefelter’s syndrome hiding behind pulmonary embolism. Curr Res Cardiol 2017;4(3):45-47.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Klinefelter’s syndrome, characterized by a karyotype of XXY, is characterized by a series of clinical manifestation such as hypogonadism, small testes, gynecomastia and infertility. This condition promotes hypofribrinolysis due to hormonal imbalances and thrombophillic factors resulting in a hypercoagulability state. Rare complications include thromboembolic events and deep vein thrombosis. Here is a typical case of a 51-year-old man diagnosed with Klinefelter’s syndrome after being admitted and treated for pulmonary embolism.
Keywords
Klinefelter’s syndrome; Pulmonary embolism; Hypogonadism; Deep vein thrombosis
Klinefelter 's syndrome (KS), one of the most common chromosomal disorders with a karyotype of 47XXY [1], is associated with male infertility along with a series of clinical manifestations such as hypogonadism, feminization, microorchidism and gynaecomastia. Because the clinical features are usually manifested after puberty, thus the diagnosis of KS is often delayed and most patients have already been adult when diagnosed. Due to the coexistence of other disturbances such as glucose tolerance, obesity and other cardiovascular diseases [2], KS has been reported with a higher mortality and morbidity as compared to general population [3]. These features may be accounted from endocrine dysfunction particularly increased level of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and low serum level of testosterone. Moreover, deep vein thrombosis and thromboembolic events are complications that have been reported in this condition. [4]We herein report a classical case of KS, with a first clinicalimpression “pulmonary embolism”.
Case Report
A 51-year-old male was admitted with main complain of "repeated shortness of breath". One month ago, he started to experience repeated chest tightness and shortness of breath, which was aggravated after physical activity. Rest could not alleviate the symptoms. A week ago, the pain worsened and an ECG done in local hospital showed a sinus rhythm with inversed T wave in V1-V4 lead, and markers of myocardial injury, such as troponin I, creatinine phosphokinase and Heart-type fatty acid binding protein (H-FABP) were all negative.
Coagulation function test revealed the following: Plasma prothrombin time was 13.4 sec, PT-INR was 1.16, PT activity was 67.5%, APTT was 28.7 seconds, D-dimer was 0.28 ug/ml. Erythropoietin, folic acid, vitamin B12, ferritin was all normal. All the serum markers of rheumatoid immune system were negative. Blood gas analysis results were also normal.
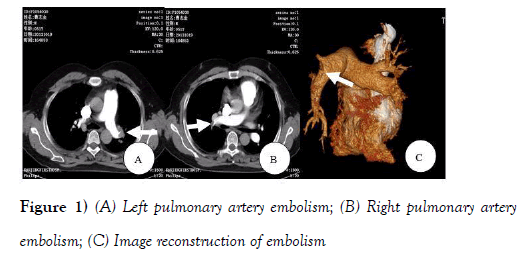
Chest X-ray showed an increased heart shadow with a thickened lung marking. Echocardiography examination results were: aortic diameter 38 mm, left atrial diameter 47 mm, left ventricular end-diastolic diameter 55 mm, left ventricular end-systolic diameter 35 mm, ventricular septal thickness 9 mm, left ventricular wall thickness 8 mm.Atrial septal and ventricular septal was continuous withoutinterruption. There was a mild mitral regurgitation, a mild tricuspid regurgitation, pulmonary artery systolic pressure (PASP) of 35 mmHg and ejection fraction of 65%. Enhanced CT scan confirmed scattered embolism in bilateral pulmonary artery (Figure 1).
Upon admission, the patient was treated accordingly with measures including that of oxygen inhalation, anticoagulation, vasodilator to reduce the pulmonary artery pressure and diuretic to decrease cardiac preload. Because of a history of more than one month, the best time window of thrombolysis has been missed and the patient were prescribed with warfarin, 2.5 mg, once daily while monitoring the INR adjusted between 2.1-2.5.
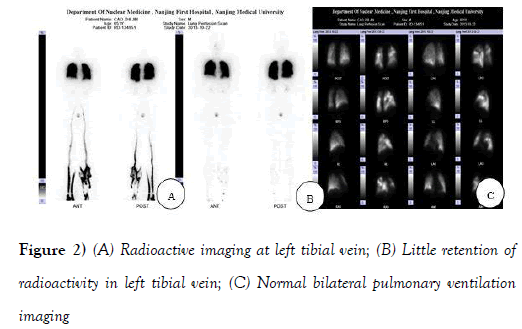
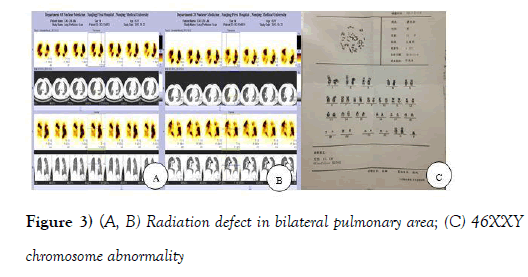
In order to explore the origin of the pulmonary embolism, A 99 mTC-DTPA was used for pulmonary ventilation imaging, and a 99 mTC-MAA for pulmonary perfusion imaging as well as dynamic and delayed imaging of bilateral lower limb. Multiple mismatches between ventilation and perfusion was found by lung ventilation and perfusion scan (Figures 2A-2C and Figures 3A-3C).
Figure 3 (A, B) Radiation defect in bilateral pulmonary area; (C) 46XXY chromosome abnormality
The lower limbs’ radionuclide imaging revealed an embolization in left tibial vein (Figures 2A and 2B), suggesting the origin of the pulmonary embolism. Chest and abdominal CT for the screening of cancer was performed, but these were normal with no lymph node enlargement.
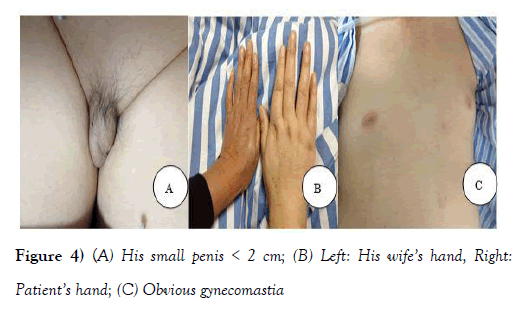
During physical examination, no pathological murmur in valvular areas but a loud second heart sound (P2) was heard upon auscultation, which was consistent with pulmonary hypertension. The patient’s skin was white and tender with obvious gynecomastia and long limbs which was very feminine in nature, along with a small penis and atrophic testes (Figure 4). His wife also revealed that he has low sexual appetite and infertility.
A series of screening tests such as semen and sperm routine examination were carried out and anti-sperm antibody were negative. Endocrine studies revealed that estradiol level was 14 pg/ml (reference value range: 20 to 47 pg/ml) and testosterone level was 0.01 ng/ml (reference value range: 1.75 to 7.81 ng/ml). Thus, the patient was diagnosed as hypogonadism and azoospermia. Low testosterone level might lead to thrombophilia. The whole clinical picture associated with the patient drew our attention towards Klinefelter’s syndrome.
With the patient's consent, a chromosomal karyotype analysis was done and the result confirmed that he did have Klinefelter 's syndrome, with a 46XXY chromosomal abnormality (Figure 3C).
Finally, it was concluded that the pulmonary embolism was due to DVT because of thrombophilic tendencies, which may be explained by low level of testosterone. Hypogonadism was due to KS.
A CT scan was done after one month of follow-up and only a small range of embolism in left pulmonary artery was identified. The patient was feeling better without shortness of breath. We recommended him to take testosterone propionate injection but he refused. The patient didn’t receive placement of vena caval filter (Figures 5A-5C).
Discussion
Thrombophilia consists of Deep Vein Thrombosis (DVT) and its associated complication, pulmonary embolism. Classic risk factors for venous thromboembolism (VTE) are surgery, cancer, bone fracture, being bed ridden, oral contraceptives use, coagulopathies disorders among others. Recently, an increase incidence of VTE has been reported in patients with Klinefelter’s syndrome. A high risk of VTE has been showed to be associated with KS [4-8]. The exact mechanism behind the association between VTE and KS is still unclear but may be multifactorial. Associated diseases and complications in KS such as metabolic syndrome, diabetes, systemic lupus erythematous among others could increase the risk of VTE [9,10]. Male hypogonadism is associated with a reduced fibrinolytic activity possibly due to the inverse relationship existing between plasminogen activator-1 (PAI-1) synthesis and testosterone levels [11]. The testosterone level of our patient was 0.01 ng/ml suggesting severe hypogonadism which probably reflecting increased fibrinolytic tendencies due to increased PAI-1 synthesis. Generally, plasminogen binds to fibrin and tissue-type plasminogen activator (t-PA) to make complexes. The latter are responsible for the conversion of plasminogen into the active proteolytic plasmin. However, increased synthesis of PAI-1 disturbs the hemostasis by suppressing t-PA and thus inhibiting plasmin formation, which prevent fibrinolysis [12].
Conclusion
It is therefore advisable for those with past medical history of VTE to be screened for endocrine abnormalities and chromosomal karyotype analysis if there is any suspicion of KS.
Conflict of Interest
The Authors declare that they have no conflict of interests.
REFERENCES
- Groth KA, Skakkebæk A, Høst C, et al. Clinical review: Klinefelter syndrome—a clinical update. J Clin Endocrinol Metab. 2013;98:20-30.
- Nieschlag E. Klinefelter syndrome: The commonest form of hypogonadism, but often overlooked or untreated. Dtsch Arztebl Int. 2013;110:347-53.
- Bojesen A, Gravholt CH. Morbidity and mortality in Klinefelter sundrome (47,XXY). Acta Paediatr. 2011;100:807-13.
- Campbell WA, Price WH. Venous thromboembolic disease in Klinefelter’s syndrome. Clin Genet. 1981;19:275-80.
- Swerdlow AJ, Higgins CD, Schoemaker MJ, et al. United Kingdom Clinical Cytogenetics Group. Mortality in patients with Klinefelter syndrome in Britain: A cohort study. J Clin Endocrinol Metab. 2005;90:6516-22.
- Murray FE. Mesenteric vein thrombosis associated with Klinefelters syndrome—A case report. Angiology. 1988;39:45-8.
- Boos CJ, Matfin G. Klinefelter’s syndrome manifesting as an acute pulmonary embolus in a 52-year-old man. Endocr Pract 2002;8:68-9.
- Lapecorella M, Marino R, De Pergola G, et al. Severe venous thromboembolism in a young man with Klinefelter’s syndrome and heterozygosis for both G20210A prothrombin and factor V Leiden mutations. Blood Coagul Fibrinolysis. 2003;14:95-8.
- Nieschlag E, Werler S, Wistuba J, et al. New approaches to the Klinefelter syndrome. Ann Endocrinol (Paris). 2014;75:88-97.
- Abramowitz LK, Olivier-Van Stichelen S, Hanover JA. Chromosome imbalance as a driver of sex disparity in disease. J Genomics 2014;2:77-88.
- Zollner TM, Veraart JC, Wolter M, et al. Leg ulcers in Klinefelter’s syndrome: Further evidence for an involvement of plasminogen activator in- hibitor-1. Br J Dermatol. 1997;136:341-4.
- Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69:381-7.