A multifactorial approach to understanding andropause
Gold Cross Medical Centre, London, England
- *Corresponding Author:
- Dr M Carruthers
Suite 20 Harmont House, 20 Harley Street, London W1N 1AL England.
Telephone: +44-0-20-8636-8223 Fax +44-0-20-8636-8292
E-mail: carruthers@goldcrossmedical.com
Citation: M Carruthers. A multifactorial approach to understanding andropause. J Sex Reprod Med 2001;1(2):69-74.
[ft_below_content] =>Keywords
Diet profile; Familial history; Lifestyle; Medical problems; Sedentary status; Stress factors
The best current definition of the term ‘andropause’ probably is that proposed by Tremblay and Morales [1]: “when men exhibit several of the symptoms and/or clinical features of reduced testosterone availability to various systems or organ functions”. Tremblay and Morales’ key article [1] provides a detailed list of symptoms that are strikingly similar both in content and frequency to those originally listed by Werner [2] and other authors over the past 60 years, as shown in the comparison of andropausal symptoms in Table 1 [1,3,4,5].
The characteristic ‘identikit’ pattern of andropausal symptoms presented in Table 1 is the same as that generally seen in androgen-deficient adult males, regardless of whether it is caused by testicular damage, suppression of testosterone by a prolactinoma, antiandrogens or increases in sex hormone-binding globulin (SHBG).
| Author (reference) | |||||||
|---|---|---|---|---|---|---|---|
| Werner (2) | Heller andMeyer (3) | Reiter (4) | Carruthers* | Carruthers (5) | Tremblay and Morales (1) | Heineman et al (14) |
|
| Year | 1946 | 1944 | 1963 | 1989 | 1996 | 1998 | 1999 |
| Number of patients in study Symptoms | 273 | 23 | 100 | 990 | 1533 | about 300 | 116 |
| Erectile dysfunction | 95 | † | † | 84 | 83 | † | 88 |
| Libido, sex drive or desire | 90 | † | † | 82 | 87 | † | 84 |
| Reduced fatigue or energy | 76 | † | ‡ | 76 | 94 | ‡ | 80 |
| Depression | 89 | ‡ | † | 60 | 88 | ‡ | 75 |
| Anxiety or nervousness | 100 | † | † | † | 85 | ‡ | 69 |
| Memory or concentration | 87 | ‡ | ‡ | 37 | 90 | ‡ | N/A |
| Irritability or anger | 59 | ‡ | ‡ | 54 | 85 | ‡ | 72 |
| Aches or pains in joints | 75 | ‡ | N/A | 55 | 83 | N/A | 77 |
| Sweating, especially at night | 35 | ‡ | N/A | 49 | 63 | ‡ | 66 |
| Vasomotor or flushes | 46 | ‡ | N/A | 27 | N/A | ‡ | N/A |
| Age older than 50 years | N/A | N/A | N/A | N/A | 40 | 55 | 59 |
| Dry or thinning skin | 30 | ‡ | N/A | 39 | 63 | N/A | N/A |
Table 1: Comparison of andropausal symptoms reported by various authors
It is important to distinguish the symptoms of andropause from those of a male mid-life crisis. While the former most commonly present in the 45- to 55-year age group and are brought on by androgen deficiency, the latter typically occur from ages 35 to 45 years and constitute a psychological, existential crisis. Although andropause and midlife crisis are often confused by lay people and professionals, to the detriment of the diagnosis and treatment of both conditions, they have widely different clinical pictures, which can and should be distinguished [5].
Having defined the syndrome of andropause, the present paper considers the many factors that may contribute to its causation. These factors can conveniently be considered by looking at every level of the sequence of events that regulates the production and action of androgens (Table 2).
| Cerebral cortex |
| Stress (overload and underload) |
| Hypothalamus and pituitary disorders |
| Gonadotrophin-releasing hormone (decreased release) |
| Luteinizing hormone and follicle-stimulating hormone |
| (increased but insufficient) |
| Testes |
| Damage and aging (infections, warming, trauma) |
| Testosterone |
| Decreased synthesis and action of sex hormone-binding globulin, |
| estrogens and antiandrogens |
| Target organs |
| Decrease in androgen and receptor content; decrease in 17-beta-estradiol |
Table 2: Factors contributing to andropause
The High Testosterone Male
Testosterone has been called ‘the hormone of kings – the king of hormones’ [5], and the level to which a man becomes accustomed during puberty and youth may determine the level of androgens at which a man develops andropausal symptoms later in life. An age-related decline in total testosterone (TT) levels and, to an even greater extent, in free or biologically active testosterone levels, has been documented in even the healthiest men as they age [6]; however, the threshold at which the onset of symptoms occurs shows great variability.
Dominance among men has throughout recorded history been decided by a combination of predominantly testosterone- related physical and mental attributes. The anabolic action of testosterone, particularly when combined with the physical activity of sports, hunting or fighting, builds the muscular physique; however, in large amounts, it may limit height by causing early closure of the epiphyses. Sexual dominance was highly prized among leaders, and its loss often signalled the end of power for a potentate.
The above considerations led to the idea that each man may have his own ‘reference range’ for several endocrine variables, including androgens. As well as postural, diurnal and possibly seasonal variations, clinical experience suggests that genetic and behavioural patterns can play a major role in deciding at what level an individual feels he is functioning best in terms of general and sexual drive, energy, enthusiasm and physical activity. Certainly, the author’s 10 years of clinical experience with a range of captains of industry, tycoons, politicians and those who are sometimes called ‘movers and shakers’ shows that they need higher doses of testosterone than less ambitious males to restore their competitive edge.
Testosterone and Stress – The Cortex
A wealth of evidence from both primate and human research shows that a man’s testosterone level changes when his status changes, rising when he achieves or defends a dominant position and falling when he is dominated. Results of studies of men in various competitive and stressful situations suggest that when a man achieves a rise in status through his own efforts and experiences an elated mood over the achievement, he is most likely to have a rise in testosterone levels.
Both excessive and unpleasant physical and mental stress can activate the hypothalamo-pituitary-adrenal axis and reduce either the amount or activity of androgens. For example, extreme endurance training in military cadets that involved psychological stress, and the deprivation of food and sleep resulted in a marked drop in testosterone levels, as have intensive on-duty periods in resident physicians.
Less acute psychological stress, such as financial problems, serious quarrels and the loss of close friends or relatives, also have been shown to lower androgen levels. Physical illnesses, ranging from life-threatening trauma to a variety of chronic diseases, have also been related to reduced testosterone levels, although it is difficult to establish which came first.
Hypothalamus and Pituitary Function
Detailed studies of the gonadal axis in older men have shown the disappearance of nyctohemeral variation in T levels, increased sensitivity of the gonadostat to sex hormone feedback and decreased opioid tonus, which suggests altered regulation at the hypothalamo-pituitary level [6]. The loss of circadian rythmicity of luteinizing hormone (LH) and hence testosterone with age, has been attributed to a reduced number and size of LH pulses.
Although there tends to be higher gonadotrophin levels in older men, this increase is insufficient to maintain declining androgen levels. The rise in follicle-stimulating hormone tends to be greater than that in LH and falls with testosterone treatment. It is known that both of these gonadotrophins are required for the development and maintenance of testicular function, and while LH is the most important hormone for the control of Leydig’s cell function, other hormones and locally produced factors also play a role in testicular function.
Reductions in testosterone production may also occur with increased production of prolactin. This increase may result from stress, but prolactin production rarely goes over 500 pmol/L due to reductions in testosterone. Prolactin may be regarded as ‘nature’s contraceptive’ in both sexes; it suppresses the libido and fertility in both sexes during times when procreation may detract from the immediate survival of the human species, as well as during lactation in women.
Larger increases in prolactin, usually to over 1000 pmol/L, are seen with prolactinomas and can cause marked suppression of testosterone levels. Although prolactinoma tumours are rare, occurring in approximately 0.4% of andropausal patients, they are eminently treatable and should be considered to be diagnostic possibilities in younger patients with very low testosterone levels and no other obvious cause of dysfunction of the hypothalamopituitary axis.
A wide variety of drugs (including dopamine antagonists, such as phenothiazines and imipramine, and those antagonists that interfere with dopamine synthesis [eg, alpha-methyldopa], the depletion of dopamine stores [eg, reserpine] or the direct stimulation of prolactin production [eg, H2-blockers and estrogens]) can also raise prolactin levels. As well as inhibiting gonadotrophin-releasing hormone (GnRH) secretion, chronic renal failure and hypothyroidism can also cause hyperprolactinemia, and, as with other causes, may be associated with gynecomastia. Opiates, such as heroin, morphine and methadone, all markedly suppress LH and testosterone secretion by suppressing GnRH release.
Testes
Impaired development
Men with nondescent or late descent of one or both testes are often hypogonadal throughout their lives, and when testosterone treatment is stopped, they develop typical andropausal symptoms. Even when the defect has been anatomically corrected by orchidopexy, testicular function may still be impaired in terms of both sperm and testosterone production. Sometimes, there is no overt history of testicular problems, but when the patient presents in middle age or later, there may be a life long history of low sex drive and activity, unexplained infertility and poor secondary sexual characteristics. Physical examination may show small, easily retractile testes in a poorly developed scrotum.
Impaired development of the testes is a reminder that testosterone is active in promoting the development of the male urogenital tract from the ninth week of intrauterine life onwards and reaches a peak during the first few weeks after birth, which is not reached again until puberty. If the levels of this hormone are insufficient in utero or if they are opposed by estrogens, then not only may penile development be impaired, with resulting micropenis or hypospadias, but the active part played by the gubernaculums in steering the testes toward the developing scrotum is also retarded, which can impair their function for life. Minor degrees of these developmental disorders can be missed surprisingly often and predispose a patient to hypogonadism in later life.
Infections
Mumps is the classic example of an infection that causes an endocrine disorder. The resultant orchitis, first described by Hippocrates, occurs in 25% of postpubertal cases and, as with many testicular disorders, may affect endocrine function and sperm production. As in women, there is a tendency for puberty to occur earlier in men who have had mumps, so it is worthwhile to inquire about a history of mumps after the age of 10 years.
Other viruses, including those that cause glandular fever (infectious mononucleosis) may also be associated with clinical or subclinical orchitis and damage. This association has also been reported with herpes, Coxsackie-, arbo-, Dengue and Marburg viruses. The testes can also be affected by nephritis, prostatitis and vesiculitis, especially when occurring with gonorrhea, chlamydia and other causes of nonspecific urethritis, all of which should be included in the routine history.
Trauma
Testicular trauma as a cause of andropause is not always obvious from a patient’s history. It can include hernial repair at any age, but particularly during infancy, when it may have been an aspect of the partial nondescent of the testes and impaired development of the inguinal canal. Direct blows to the testes, sufficient to cause bruising, may cause unilateral testicular atrophy, as can torsion, even when it is surgically corrected at an early stage.
Testicular trauma may be due to either a breach in the immunological defences of a testis or a prolonged sympathetic spasm that can affect both testes. Similar mechanisms may account for testicular atrophy or hypofunction, which may follow any operation on a testis, particularly when it involves trauma to the capsule, as in the removal of a varicocele, or damage to the vas, which may occur during a vasectomy [5]. Other operations on the prostate, particularly transurethral resection, may also damage the vas or their outflow, as shown by the retrograde ejaculation of semen into the bladder, and can possibly cause autoimmune orchitis.
Varicocele and hydroceles are also believed to impair the temperature regulation function of the scrotum, which, together with a refined counter-current heat exchange mechanism, normally keeps the testes 3°C to 4°C cooler than core body temperature. Impaired temperature regulation is thought to be crucial to the maintenance of both fertility and testosterone production, and may be one of the reasons why many andrologists interested in both infertility and andropause encourage scrotal cooling measures such as wearing loose fitting boxer shorts.
Aging
Although there is no abrupt cessation of function in an aging testis, there is a highly variable degree of atrophy, and both ‘spermatogenic efficiency’ and ‘testicular reserve for testosterone secretion’ tend to decrease with age [7]. The long established reduction in the number of Leydig’s cells with aging, with vascular changes underlying the measured decrease in testicular perfusion, as well as a decrease in the concentration of testosterone in the spermatic vein, make it probable that decreased androgen production with aging is mainly of testicular origin. This is confirmed by rising gonadotrophin levels and the reduced ability of an aging testis to produce more testosterone after human chorionic gonadotropin stimulation. The production of androgens is further reduced by the increasing influence of other diseases associated with aging and the drugs that are used to treat them.
Testosterone
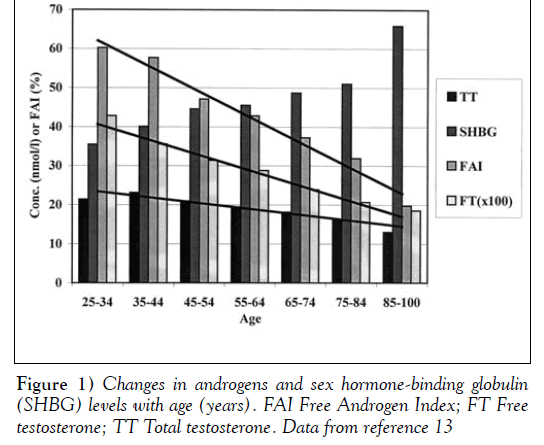
For all of the reasons outlined above, there is a highly variable decrease in both TT and, particularly, free testosterone (FT) levels with age. This variable decrease is shown in Figure 1, which is derived from the extensive work of Kaufman and Vermeulen [6,8].
The decrease in TT is usually gradual and highly dependent on an individual’s state of health and physical activity; however, as emphasized particularly by Tremblay [9], the reduction in the free and bioavailable testosterone fractions is far more marked, and these important endocrine variables are linked to the development of andropausal symptoms.
As a result of these changes in testosterone levels, the Free Androgen Index, though bearing a weaker theoretical relationship with FT compared with TT, is a useful clinical marker and decreases more rapidly with age. However, perhaps because of the apparent need of some men to have higher levels of androgens, there is a poor correlation in the intensity of andropausal symptoms when comparing Andropause Check List scores with either FT, bioavailable testosterone or the Free Androgen Index, other than some general cut-off levels above which symptoms seldom appear. Therefore, it is suggested that the patient should be given the benefit of the doubt when reporting symptoms; that characteristic andropausal symptomatology should be given precedence over laboratory measures; and that an adequate therapeutic trial of testosterone should be given for at least three months before andropause is excluded as a diagnosis.
Heredity and familial influences
Studies of monozygotic and dizygotic twins [10] have shown that familial, rather than genetic, factors accounted for twice as much of the concordance in TT and FT levels, and dihydrotestosterone, as well as virtually all SHBG and aromatase activity. In all of these factors, nurture appeared to be more important than nature. Only in estradiol and LH levels did heredity have a slightly greater influence. It is suggested that similar diet and physical activity levels in families may explain most of these factors in determining androgen levels and, hence, vulnerablity to andropause.
Diet, xenoestrogens and antiandrogens
Strict, low cholesterol diets have been shown to lower TT and FT levels by 14% (11). Vegetarian diets, especially if they are low in protein, can increase SHBG levels, thus further reducing FT. Weight-reducing regimes can also have a similar effect by lowering insulin resistance, reducing insulin levels and allowing SHBG levels to rise [7].
Many conditions, such as the nondescent of a testis or mumps that damage the testes and affect fertility, can also affect the production or action of testosterone. There is concern about environmental influences on fertility, particularly in relation to xenoestrogens and antiandrogens. Physicians need to consider the evidence in relation to the impact of such ‘hormonal havoc’ on endocrine balance in both the developing male fetus and men throughout their lives. Farmers are at particular risk for environmental influences, because they may often be exposed from an early age to a variety of growth-promoting hormones that are used in caponise chickens and turkeys, and that increase the yield of meat from cattle.
Drugs
As well as the psychotropic drugs mentioned above that interfere with GnRH and LH production, many substances can directly reduce the production of androgens at the testicular level or alter their metabolism. The most common of these substances is alcohol, which, in large amounts, is a well known cause of infertility and can irreversibly damage the Leydig’s cells, which lack the regenerative power of hepatocytes. Alcohol also promotes the conversion of testosterone to estrogen, which explains the beer belly and gynecomastia often seen in patients with a history of alcohol abuse.
Other drugs, such as aminoglutethamide and ketoconazole, can inhibit steroidogenic enzymes, causing rapid and dramatic reductions in testosterone levels. Some of these drugs, such as cimetidine, spironlactone and cyproterone acetate, act as androgen receptor antagonists. Both herbal preparations, including saw palmetto, and pharmaceutical drugs, such as finasteride, which is used to treat benign prostatic hypertrophy and hair loss, act as 5-alpha-reductases; they lower dihyrotestosterone (DHT) levels and, in some cases, contribute to reduced libido and erectile dysfunction.
Drugs that affect the level of SHBG also influence the levels of both TT and FT. These drugs include barbiturates, anticonvulsants and other hepatic enzyme inducers that have been shown to raise SHBG levels, which reduces both the clearance of testosterone and the level of FT, causing one of many examples of iatrogenic andropause.
A reverse effect, which is likely to have the therapeutic potential of restoring a more youthful androgen profile, is shown by the ethisterone derivative, danazol (Cyclomen; Sanofi-Smithelabo Canada Inc, Canada). This drug can be used to raise FT levels by lowering the high level of SHBG seen in many andropausal men because it reduces the hepatic synthesis of this carrier protein even when it is used in minimal doses and also displaces testosterone from its binding sites on SHBG [12]. This increase in FT appears to be associated with the relief of andropausal symptoms in patients with increased SHBG levels and means that they can be treated with lower doses of testosterone, especially oral preparations such as testosterone undecanoate (Andriol, Organon Canada Ltd, Canada).
Target Organs
As well as being affected by many of the above drugs, androgen receptors in many of the target organs, and the intracellular concentrations of both testosterone and DHT, decrease with age to a variable degree in different target organs.
Scrotal skin, for example, maintains the same levels of testosterone and DHT throughout life, whereas the pubic skin levels of both are decreased by one-half over the age of 60 years [7]. The same is thought to happen in the cavernosal androgen receptors that regulate nitric oxide synthase activity, which together could account for the loss in penile sensitivity and erectile dysfunction that occur as a part of the andropausal picture, and require high doses of testosterone treatment to reverse.
Cardiac and skeletal muscle have both lower concentrations of androgen receptors than the accessory sex organs and lower levels of testosterone as age increases. Maintaining levels of cardiovascular and skeletomuscular fitness can reduce or even reverse this age-related decline, and together with testosterone supplementation, if needed, can help to maintain the condition and mass of both types of muscle, and to prevent osteoporosis.
The shift from aerobic to anaerobic cellular metabolism that is caused by changes that occur with aging, including reduced tissue oxygenation that results from processes such as impaired perfusion and impaired carbohydrate metabolism, particularly in patients with diabetes, leads to anoxia in both cardiac and skeletal muscle; in many cases, the anoxia or the tissular anoxia can be reversed by testosterone treatment [13].
Conclusion
Every level of the regulation, synthesis and action of androgens should be considered when assessing, diagnosing and treating andropause. Rather than just treating the problem with testosterone and hoping it resolves, depending on a patient’s history, a complete program of treatment may need to include, if possible, lifestyle changes such as stress management, relationship counselling, an exercise program, weight and alcohol reduction, and changes in drug regimens to those with less side effects.
This understanding of the causes of andropause leads to a broad approach to managing this increasingly common disorder and one that is well suited to the basic skills of the personal physician who is armed with the supplementary specialist knowledge contained in this paper.
References
- Tremblay RR, Morales AJ. Canadian practice recommendations for screening, monitoring and treating men affected by andropause or partial androgen deficiency. Aging Male 1998;1:213-8.
- Werner AA. The male climacteric: Report of two hundred and seventy-three cases. JAMA 1946;132:188-94.
- Heller CG, Myers GB. The male climacteric: Its symptomatology, diagnosis and treatment. JAMA 1944;126:472-7.
- Reiter T. Testosterone implantation: A clinical study of 240 implantations in ageing males. J Am Geriatr Soc 1963;11:540-50.
- Carruthers M. Male menopause: Restoring vitality and virility. London: HarperCollins, 1996.
- Kaufman JM, Vermeulen A. Androgens in male senescence. In: Nieschlag E, Behre HM, eds. Testosterone: Action, Deficiency, Substitution. Berlin: Springer Verlag, 1998:437-71.
- Vermeulen A. Androgens and male senescence. In: Nieschlag E, Behre HM, eds. Testosterone: Action, Deficiency, Substitution. Heidelberg: Springer, 1990:261-76.
- Vermeulen A. Declining androgens with age: An overview. In: Oddens B, Vermeulen A, eds. Androgens and the Aging Male. New York: The Parthenon Publishing Group, 1996:3-14.
- Tremblay RR. Practical consequences of the validation of a mathematical model in assessment of partial androgen deficiency in the aging male using bioavailable testosterone. Aging Male 2001;4:23-9.
- Meikle AW, Bishop DT, Stringham JD, West DW. Quantitating genetic and non genetic factors to determine plasma sex steroid variation in normal male twins. Metabolism 1987;35:1090-5.
- Hamalainen EK, Adlercreutz H, Puska P, Pietinen P. Decrease of serum total and free testosterone during a low-fat high-fibre diet. J Steroid Biochem 1983;18:369-70.
- Carruthers M. More effective testosterone treatment: Combination with sildenafil and danazol. Aging Male 2000;14:16.
- Moller J, Einfeldt H. Testosterone Treatment of Cardiovascular Diseases. Berlin: Springer-Verlag, 1984.
- Heinemann LJ, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘aging males’ symptoms’ rating scale. Aging Male 1999;2:105-14.
Gold Cross Medical Centre, London, England
- *Corresponding Author:
- Dr M Carruthers
Suite 20 Harmont House, 20 Harley Street, London W1N 1AL England.
Telephone: +44-0-20-8636-8223 Fax +44-0-20-8636-8292
E-mail: carruthers@goldcrossmedical.com
Citation: M Carruthers. A multifactorial approach to understanding andropause. J Sex Reprod Med 2001;1(2):69-74.
Abstract
Every level of the regulation, synthesis and action of androgens should be considered when assessing, diagnosing and treating andropause. It is important to distinguish the symptoms of andropause from those of male mid-life crisis. While the former symptoms most commonly present in the 45- to 55-year age group and are brought on by androgen deficiency, the latter typically occur between ages 35 and 45 years and constitute a psychological, existential crisis. Although andropause and mid-life crisis are often confused by lay people and professionals, to the detriment of the diagnosis and treatment of both conditions, they have widely different clinical pictures, which can and should be distinguished. The present paper defines the syndrome of andropause and considers the many factors that may contribute to its causation.
-Keywords
Diet profile; Familial history; Lifestyle; Medical problems; Sedentary status; Stress factors
The best current definition of the term ‘andropause’ probably is that proposed by Tremblay and Morales [1]: “when men exhibit several of the symptoms and/or clinical features of reduced testosterone availability to various systems or organ functions”. Tremblay and Morales’ key article [1] provides a detailed list of symptoms that are strikingly similar both in content and frequency to those originally listed by Werner [2] and other authors over the past 60 years, as shown in the comparison of andropausal symptoms in Table 1 [1,3,4,5].
The characteristic ‘identikit’ pattern of andropausal symptoms presented in Table 1 is the same as that generally seen in androgen-deficient adult males, regardless of whether it is caused by testicular damage, suppression of testosterone by a prolactinoma, antiandrogens or increases in sex hormone-binding globulin (SHBG).
| Author (reference) | |||||||
|---|---|---|---|---|---|---|---|
| Werner (2) | Heller andMeyer (3) | Reiter (4) | Carruthers* | Carruthers (5) | Tremblay and Morales (1) | Heineman et al (14) |
|
| Year | 1946 | 1944 | 1963 | 1989 | 1996 | 1998 | 1999 |
| Number of patients in study Symptoms | 273 | 23 | 100 | 990 | 1533 | about 300 | 116 |
| Erectile dysfunction | 95 | † | † | 84 | 83 | † | 88 |
| Libido, sex drive or desire | 90 | † | † | 82 | 87 | † | 84 |
| Reduced fatigue or energy | 76 | † | ‡ | 76 | 94 | ‡ | 80 |
| Depression | 89 | ‡ | † | 60 | 88 | ‡ | 75 |
| Anxiety or nervousness | 100 | † | † | † | 85 | ‡ | 69 |
| Memory or concentration | 87 | ‡ | ‡ | 37 | 90 | ‡ | N/A |
| Irritability or anger | 59 | ‡ | ‡ | 54 | 85 | ‡ | 72 |
| Aches or pains in joints | 75 | ‡ | N/A | 55 | 83 | N/A | 77 |
| Sweating, especially at night | 35 | ‡ | N/A | 49 | 63 | ‡ | 66 |
| Vasomotor or flushes | 46 | ‡ | N/A | 27 | N/A | ‡ | N/A |
| Age older than 50 years | N/A | N/A | N/A | N/A | 40 | 55 | 59 |
| Dry or thinning skin | 30 | ‡ | N/A | 39 | 63 | N/A | N/A |
Table 1: Comparison of andropausal symptoms reported by various authors
It is important to distinguish the symptoms of andropause from those of a male mid-life crisis. While the former most commonly present in the 45- to 55-year age group and are brought on by androgen deficiency, the latter typically occur from ages 35 to 45 years and constitute a psychological, existential crisis. Although andropause and midlife crisis are often confused by lay people and professionals, to the detriment of the diagnosis and treatment of both conditions, they have widely different clinical pictures, which can and should be distinguished [5].
Having defined the syndrome of andropause, the present paper considers the many factors that may contribute to its causation. These factors can conveniently be considered by looking at every level of the sequence of events that regulates the production and action of androgens (Table 2).
| Cerebral cortex |
| Stress (overload and underload) |
| Hypothalamus and pituitary disorders |
| Gonadotrophin-releasing hormone (decreased release) |
| Luteinizing hormone and follicle-stimulating hormone |
| (increased but insufficient) |
| Testes |
| Damage and aging (infections, warming, trauma) |
| Testosterone |
| Decreased synthesis and action of sex hormone-binding globulin, |
| estrogens and antiandrogens |
| Target organs |
| Decrease in androgen and receptor content; decrease in 17-beta-estradiol |
Table 2: Factors contributing to andropause
The High Testosterone Male
Testosterone has been called ‘the hormone of kings – the king of hormones’ [5], and the level to which a man becomes accustomed during puberty and youth may determine the level of androgens at which a man develops andropausal symptoms later in life. An age-related decline in total testosterone (TT) levels and, to an even greater extent, in free or biologically active testosterone levels, has been documented in even the healthiest men as they age [6]; however, the threshold at which the onset of symptoms occurs shows great variability.
Dominance among men has throughout recorded history been decided by a combination of predominantly testosterone- related physical and mental attributes. The anabolic action of testosterone, particularly when combined with the physical activity of sports, hunting or fighting, builds the muscular physique; however, in large amounts, it may limit height by causing early closure of the epiphyses. Sexual dominance was highly prized among leaders, and its loss often signalled the end of power for a potentate.
The above considerations led to the idea that each man may have his own ‘reference range’ for several endocrine variables, including androgens. As well as postural, diurnal and possibly seasonal variations, clinical experience suggests that genetic and behavioural patterns can play a major role in deciding at what level an individual feels he is functioning best in terms of general and sexual drive, energy, enthusiasm and physical activity. Certainly, the author’s 10 years of clinical experience with a range of captains of industry, tycoons, politicians and those who are sometimes called ‘movers and shakers’ shows that they need higher doses of testosterone than less ambitious males to restore their competitive edge.
Testosterone and Stress – The Cortex
A wealth of evidence from both primate and human research shows that a man’s testosterone level changes when his status changes, rising when he achieves or defends a dominant position and falling when he is dominated. Results of studies of men in various competitive and stressful situations suggest that when a man achieves a rise in status through his own efforts and experiences an elated mood over the achievement, he is most likely to have a rise in testosterone levels.
Both excessive and unpleasant physical and mental stress can activate the hypothalamo-pituitary-adrenal axis and reduce either the amount or activity of androgens. For example, extreme endurance training in military cadets that involved psychological stress, and the deprivation of food and sleep resulted in a marked drop in testosterone levels, as have intensive on-duty periods in resident physicians.
Less acute psychological stress, such as financial problems, serious quarrels and the loss of close friends or relatives, also have been shown to lower androgen levels. Physical illnesses, ranging from life-threatening trauma to a variety of chronic diseases, have also been related to reduced testosterone levels, although it is difficult to establish which came first.
Hypothalamus and Pituitary Function
Detailed studies of the gonadal axis in older men have shown the disappearance of nyctohemeral variation in T levels, increased sensitivity of the gonadostat to sex hormone feedback and decreased opioid tonus, which suggests altered regulation at the hypothalamo-pituitary level [6]. The loss of circadian rythmicity of luteinizing hormone (LH) and hence testosterone with age, has been attributed to a reduced number and size of LH pulses.
Although there tends to be higher gonadotrophin levels in older men, this increase is insufficient to maintain declining androgen levels. The rise in follicle-stimulating hormone tends to be greater than that in LH and falls with testosterone treatment. It is known that both of these gonadotrophins are required for the development and maintenance of testicular function, and while LH is the most important hormone for the control of Leydig’s cell function, other hormones and locally produced factors also play a role in testicular function.
Reductions in testosterone production may also occur with increased production of prolactin. This increase may result from stress, but prolactin production rarely goes over 500 pmol/L due to reductions in testosterone. Prolactin may be regarded as ‘nature’s contraceptive’ in both sexes; it suppresses the libido and fertility in both sexes during times when procreation may detract from the immediate survival of the human species, as well as during lactation in women.
Larger increases in prolactin, usually to over 1000 pmol/L, are seen with prolactinomas and can cause marked suppression of testosterone levels. Although prolactinoma tumours are rare, occurring in approximately 0.4% of andropausal patients, they are eminently treatable and should be considered to be diagnostic possibilities in younger patients with very low testosterone levels and no other obvious cause of dysfunction of the hypothalamopituitary axis.
A wide variety of drugs (including dopamine antagonists, such as phenothiazines and imipramine, and those antagonists that interfere with dopamine synthesis [eg, alpha-methyldopa], the depletion of dopamine stores [eg, reserpine] or the direct stimulation of prolactin production [eg, H2-blockers and estrogens]) can also raise prolactin levels. As well as inhibiting gonadotrophin-releasing hormone (GnRH) secretion, chronic renal failure and hypothyroidism can also cause hyperprolactinemia, and, as with other causes, may be associated with gynecomastia. Opiates, such as heroin, morphine and methadone, all markedly suppress LH and testosterone secretion by suppressing GnRH release.
Testes
Impaired development
Men with nondescent or late descent of one or both testes are often hypogonadal throughout their lives, and when testosterone treatment is stopped, they develop typical andropausal symptoms. Even when the defect has been anatomically corrected by orchidopexy, testicular function may still be impaired in terms of both sperm and testosterone production. Sometimes, there is no overt history of testicular problems, but when the patient presents in middle age or later, there may be a life long history of low sex drive and activity, unexplained infertility and poor secondary sexual characteristics. Physical examination may show small, easily retractile testes in a poorly developed scrotum.
Impaired development of the testes is a reminder that testosterone is active in promoting the development of the male urogenital tract from the ninth week of intrauterine life onwards and reaches a peak during the first few weeks after birth, which is not reached again until puberty. If the levels of this hormone are insufficient in utero or if they are opposed by estrogens, then not only may penile development be impaired, with resulting micropenis or hypospadias, but the active part played by the gubernaculums in steering the testes toward the developing scrotum is also retarded, which can impair their function for life. Minor degrees of these developmental disorders can be missed surprisingly often and predispose a patient to hypogonadism in later life.
Infections
Mumps is the classic example of an infection that causes an endocrine disorder. The resultant orchitis, first described by Hippocrates, occurs in 25% of postpubertal cases and, as with many testicular disorders, may affect endocrine function and sperm production. As in women, there is a tendency for puberty to occur earlier in men who have had mumps, so it is worthwhile to inquire about a history of mumps after the age of 10 years.
Other viruses, including those that cause glandular fever (infectious mononucleosis) may also be associated with clinical or subclinical orchitis and damage. This association has also been reported with herpes, Coxsackie-, arbo-, Dengue and Marburg viruses. The testes can also be affected by nephritis, prostatitis and vesiculitis, especially when occurring with gonorrhea, chlamydia and other causes of nonspecific urethritis, all of which should be included in the routine history.
Trauma
Testicular trauma as a cause of andropause is not always obvious from a patient’s history. It can include hernial repair at any age, but particularly during infancy, when it may have been an aspect of the partial nondescent of the testes and impaired development of the inguinal canal. Direct blows to the testes, sufficient to cause bruising, may cause unilateral testicular atrophy, as can torsion, even when it is surgically corrected at an early stage.
Testicular trauma may be due to either a breach in the immunological defences of a testis or a prolonged sympathetic spasm that can affect both testes. Similar mechanisms may account for testicular atrophy or hypofunction, which may follow any operation on a testis, particularly when it involves trauma to the capsule, as in the removal of a varicocele, or damage to the vas, which may occur during a vasectomy [5]. Other operations on the prostate, particularly transurethral resection, may also damage the vas or their outflow, as shown by the retrograde ejaculation of semen into the bladder, and can possibly cause autoimmune orchitis.
Varicocele and hydroceles are also believed to impair the temperature regulation function of the scrotum, which, together with a refined counter-current heat exchange mechanism, normally keeps the testes 3°C to 4°C cooler than core body temperature. Impaired temperature regulation is thought to be crucial to the maintenance of both fertility and testosterone production, and may be one of the reasons why many andrologists interested in both infertility and andropause encourage scrotal cooling measures such as wearing loose fitting boxer shorts.
Aging
Although there is no abrupt cessation of function in an aging testis, there is a highly variable degree of atrophy, and both ‘spermatogenic efficiency’ and ‘testicular reserve for testosterone secretion’ tend to decrease with age [7]. The long established reduction in the number of Leydig’s cells with aging, with vascular changes underlying the measured decrease in testicular perfusion, as well as a decrease in the concentration of testosterone in the spermatic vein, make it probable that decreased androgen production with aging is mainly of testicular origin. This is confirmed by rising gonadotrophin levels and the reduced ability of an aging testis to produce more testosterone after human chorionic gonadotropin stimulation. The production of androgens is further reduced by the increasing influence of other diseases associated with aging and the drugs that are used to treat them.
Testosterone
For all of the reasons outlined above, there is a highly variable decrease in both TT and, particularly, free testosterone (FT) levels with age. This variable decrease is shown in Figure 1, which is derived from the extensive work of Kaufman and Vermeulen [6,8].
The decrease in TT is usually gradual and highly dependent on an individual’s state of health and physical activity; however, as emphasized particularly by Tremblay [9], the reduction in the free and bioavailable testosterone fractions is far more marked, and these important endocrine variables are linked to the development of andropausal symptoms.
As a result of these changes in testosterone levels, the Free Androgen Index, though bearing a weaker theoretical relationship with FT compared with TT, is a useful clinical marker and decreases more rapidly with age. However, perhaps because of the apparent need of some men to have higher levels of androgens, there is a poor correlation in the intensity of andropausal symptoms when comparing Andropause Check List scores with either FT, bioavailable testosterone or the Free Androgen Index, other than some general cut-off levels above which symptoms seldom appear. Therefore, it is suggested that the patient should be given the benefit of the doubt when reporting symptoms; that characteristic andropausal symptomatology should be given precedence over laboratory measures; and that an adequate therapeutic trial of testosterone should be given for at least three months before andropause is excluded as a diagnosis.
Heredity and familial influences
Studies of monozygotic and dizygotic twins [10] have shown that familial, rather than genetic, factors accounted for twice as much of the concordance in TT and FT levels, and dihydrotestosterone, as well as virtually all SHBG and aromatase activity. In all of these factors, nurture appeared to be more important than nature. Only in estradiol and LH levels did heredity have a slightly greater influence. It is suggested that similar diet and physical activity levels in families may explain most of these factors in determining androgen levels and, hence, vulnerablity to andropause.
Diet, xenoestrogens and antiandrogens
Strict, low cholesterol diets have been shown to lower TT and FT levels by 14% (11). Vegetarian diets, especially if they are low in protein, can increase SHBG levels, thus further reducing FT. Weight-reducing regimes can also have a similar effect by lowering insulin resistance, reducing insulin levels and allowing SHBG levels to rise [7].
Many conditions, such as the nondescent of a testis or mumps that damage the testes and affect fertility, can also affect the production or action of testosterone. There is concern about environmental influences on fertility, particularly in relation to xenoestrogens and antiandrogens. Physicians need to consider the evidence in relation to the impact of such ‘hormonal havoc’ on endocrine balance in both the developing male fetus and men throughout their lives. Farmers are at particular risk for environmental influences, because they may often be exposed from an early age to a variety of growth-promoting hormones that are used in caponise chickens and turkeys, and that increase the yield of meat from cattle.
Drugs
As well as the psychotropic drugs mentioned above that interfere with GnRH and LH production, many substances can directly reduce the production of androgens at the testicular level or alter their metabolism. The most common of these substances is alcohol, which, in large amounts, is a well known cause of infertility and can irreversibly damage the Leydig’s cells, which lack the regenerative power of hepatocytes. Alcohol also promotes the conversion of testosterone to estrogen, which explains the beer belly and gynecomastia often seen in patients with a history of alcohol abuse.
Other drugs, such as aminoglutethamide and ketoconazole, can inhibit steroidogenic enzymes, causing rapid and dramatic reductions in testosterone levels. Some of these drugs, such as cimetidine, spironlactone and cyproterone acetate, act as androgen receptor antagonists. Both herbal preparations, including saw palmetto, and pharmaceutical drugs, such as finasteride, which is used to treat benign prostatic hypertrophy and hair loss, act as 5-alpha-reductases; they lower dihyrotestosterone (DHT) levels and, in some cases, contribute to reduced libido and erectile dysfunction.
Drugs that affect the level of SHBG also influence the levels of both TT and FT. These drugs include barbiturates, anticonvulsants and other hepatic enzyme inducers that have been shown to raise SHBG levels, which reduces both the clearance of testosterone and the level of FT, causing one of many examples of iatrogenic andropause.
A reverse effect, which is likely to have the therapeutic potential of restoring a more youthful androgen profile, is shown by the ethisterone derivative, danazol (Cyclomen; Sanofi-Smithelabo Canada Inc, Canada). This drug can be used to raise FT levels by lowering the high level of SHBG seen in many andropausal men because it reduces the hepatic synthesis of this carrier protein even when it is used in minimal doses and also displaces testosterone from its binding sites on SHBG [12]. This increase in FT appears to be associated with the relief of andropausal symptoms in patients with increased SHBG levels and means that they can be treated with lower doses of testosterone, especially oral preparations such as testosterone undecanoate (Andriol, Organon Canada Ltd, Canada).
Target Organs
As well as being affected by many of the above drugs, androgen receptors in many of the target organs, and the intracellular concentrations of both testosterone and DHT, decrease with age to a variable degree in different target organs.
Scrotal skin, for example, maintains the same levels of testosterone and DHT throughout life, whereas the pubic skin levels of both are decreased by one-half over the age of 60 years [7]. The same is thought to happen in the cavernosal androgen receptors that regulate nitric oxide synthase activity, which together could account for the loss in penile sensitivity and erectile dysfunction that occur as a part of the andropausal picture, and require high doses of testosterone treatment to reverse.
Cardiac and skeletal muscle have both lower concentrations of androgen receptors than the accessory sex organs and lower levels of testosterone as age increases. Maintaining levels of cardiovascular and skeletomuscular fitness can reduce or even reverse this age-related decline, and together with testosterone supplementation, if needed, can help to maintain the condition and mass of both types of muscle, and to prevent osteoporosis.
The shift from aerobic to anaerobic cellular metabolism that is caused by changes that occur with aging, including reduced tissue oxygenation that results from processes such as impaired perfusion and impaired carbohydrate metabolism, particularly in patients with diabetes, leads to anoxia in both cardiac and skeletal muscle; in many cases, the anoxia or the tissular anoxia can be reversed by testosterone treatment [13].
Conclusion
Every level of the regulation, synthesis and action of androgens should be considered when assessing, diagnosing and treating andropause. Rather than just treating the problem with testosterone and hoping it resolves, depending on a patient’s history, a complete program of treatment may need to include, if possible, lifestyle changes such as stress management, relationship counselling, an exercise program, weight and alcohol reduction, and changes in drug regimens to those with less side effects.
This understanding of the causes of andropause leads to a broad approach to managing this increasingly common disorder and one that is well suited to the basic skills of the personal physician who is armed with the supplementary specialist knowledge contained in this paper.
References
- Tremblay RR, Morales AJ. Canadian practice recommendations for screening, monitoring and treating men affected by andropause or partial androgen deficiency. Aging Male 1998;1:213-8.
- Werner AA. The male climacteric: Report of two hundred and seventy-three cases. JAMA 1946;132:188-94.
- Heller CG, Myers GB. The male climacteric: Its symptomatology, diagnosis and treatment. JAMA 1944;126:472-7.
- Reiter T. Testosterone implantation: A clinical study of 240 implantations in ageing males. J Am Geriatr Soc 1963;11:540-50.
- Carruthers M. Male menopause: Restoring vitality and virility. London: HarperCollins, 1996.
- Kaufman JM, Vermeulen A. Androgens in male senescence. In: Nieschlag E, Behre HM, eds. Testosterone: Action, Deficiency, Substitution. Berlin: Springer Verlag, 1998:437-71.
- Vermeulen A. Androgens and male senescence. In: Nieschlag E, Behre HM, eds. Testosterone: Action, Deficiency, Substitution. Heidelberg: Springer, 1990:261-76.
- Vermeulen A. Declining androgens with age: An overview. In: Oddens B, Vermeulen A, eds. Androgens and the Aging Male. New York: The Parthenon Publishing Group, 1996:3-14.
- Tremblay RR. Practical consequences of the validation of a mathematical model in assessment of partial androgen deficiency in the aging male using bioavailable testosterone. Aging Male 2001;4:23-9.
- Meikle AW, Bishop DT, Stringham JD, West DW. Quantitating genetic and non genetic factors to determine plasma sex steroid variation in normal male twins. Metabolism 1987;35:1090-5.
- Hamalainen EK, Adlercreutz H, Puska P, Pietinen P. Decrease of serum total and free testosterone during a low-fat high-fibre diet. J Steroid Biochem 1983;18:369-70.
- Carruthers M. More effective testosterone treatment: Combination with sildenafil and danazol. Aging Male 2000;14:16.
- Moller J, Einfeldt H. Testosterone Treatment of Cardiovascular Diseases. Berlin: Springer-Verlag, 1984.
- Heinemann LJ, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘aging males’ symptoms’ rating scale. Aging Male 1999;2:105-14.