A rare case of scapular winging due to a novel trpv4 mutation
Received: 12-Aug-2017 Accepted Date: Sep 04, 2017; Published: 11-Sep-2017
Citation: Brown KA, Nguyen TP. A rare case of scapular winging due to a novel trpv4 mutation. J of Genet Disord and Genet Med. 2017;1(1): 2-3.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Hereditary scapular winging is an important clue for neuromuscular differential diagnosis. We report a case of a family with a novel phenotype of isolated scapular winging due to a mutation in Transient Receptor Potential Vallinoid 4 (TRPV4). We believe this report may broaden the differential diagnosis of patients with scapular winging. Phenotypes associated with mutations in this gene have been previously described and are known to vary markedly. It has been previously reported that mutations in this gene are associated with Charcot Marie Tooth disease 2C (CMT 2C), scapuloperoneal spinal muscular atrophy and congenital distal spinal atrophy as well as various skeletal dysplasias. Associated features include hearing loss, vocal cord paresis and pes cavus. To our knowledge, this is the first reported case of isolated scapular winging associated with TRPV4 mutation.
Keywords
Charcot marie tooth disease; Scapuloperoneal muscular atrophy; Neurogenetics; Neuromuscular disease; Motor neuron disease
Case Study
Our patient is a 36-year-old woman with a past medical history of Sjogren’s disease who presented to the neuromuscular clinic complaining of muscle fasciculations and cramping. Of note, the patient did not complain of weakness and was a gymnast as a child. The patient reported that she was the product of a normal full term pregnancy and had no developmental delays or complications. She has had muscle spasms and cramps since childhood but denied any history of myoglobinuria. When acquiring a family history it was discovered that she has a fourteen-year-old son, father and sister with similar symptoms of spasms and cramping.
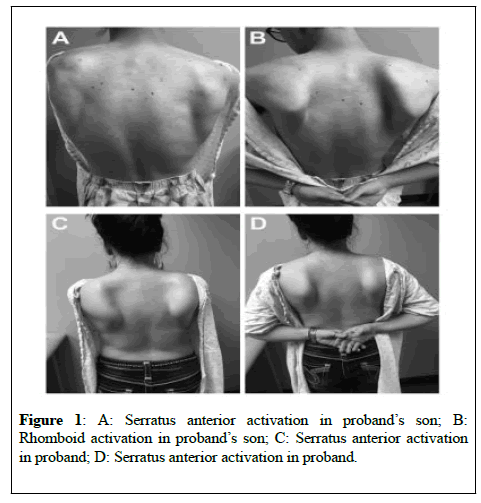
On neurological exam, her mental status and cranial nerve examination were normal. Voice was normal. She reported right-sided hearing loss but could hear finger rub on examination. She was noted to have normal motor strength except for weakness of the bilateral rhomboids and serratus anterior muscles. Motor examination revealed asymmetric, bilateral scapular winging with rounded shoulders (Figure 1). Sensation was normal. She was noted to have brisk reflexes throughout without clonus and her plantar response was flexor. Gait and coordination were also normal. There was no pes cavus.
An extensive work-up was then initiated including an electromyography and nerve conduction study which showed an incidental, chronic, right cervical radiculopathy at C7, as well as chronic neurogenic changes in the right serratus anterior muscle. MRI cervical spine did reveal right, severe, neuroforaminal stenosis at C7. A muscle biopsy of the right deltoid was also performed which showed chronic denervation and reinnervation process. Fascioscapulohumeral dystrophy testing was negative.
The patient elected to undergo whole exome sequencing. Genomic DNA from the patient, affected relatives (father, sister and son) and unaffected relatives (mother) were obtained from total blood samples. The Agilent Clinical Research Exome kit was used to target exonic regions and flanking splice junctions of the genome. These targeted regions were sequenced simultaneously by massively parallel (NextGen) sequencing on an Illumina HiSeq 2000 sequencing system. Capillary seuqncing or another appropriate method was used to confirm all potentially pathogenic variants identified in the patient and relative samples.
Whole exome testing revealed a heterozygous TRPV4 mutation, resulting in a change in cytosine to thymine at position 956, which resulted in an amino acid change from serine to Leucine (c.956C>T, p.Ser319 Leu). This S319L variant in the TRPV4 gene has not been published as mutation. To our knowledge, it has not been reported as a benign polymorphism. Additionally, the NHLBI Exome Sequencing project did not observe that variant at any significant frequency in 6500 individuals. Targeted genetic testing of other family members showed the same mutations in the patient’s sister, father and son, who were all reportedly symptomatic. The unaffected mother did not have this variant. This was the only variant found in our patient.
Due to distance, we were only able to examine the patient’s son. He was 17 at age of evaluation. He reported muscle soreness and fasciculatons. He also reported right sided hearing loss, but neither he nor his mother underwent formal assessment. On neurologic examination, he had normal strength testing in all muscle groups except for significant weakness of rhomboids (Figure 1A and 1B). Sensory and reflex examinations were normal.
Discussion
TRPV4 encodes for a calcium-permeable channel expressed in various tissues and cells. The TRPV4 protein consists of six transmembrane domains. At the N-terminus, individual ankyrin repeats are comprised of antiparallel helices followed by a finger loop. Neuropathy causing mutations as well as our family’s mutation were identified in ankyrin repeats 1 through 4. All previously identified neuropathy causing mutations in the TRPV4 ankyrin repeat domains have involved arginine residues [1-6]. The exact role of this calcium-permeable channel still remains unclear [1,3,5-10].
In laboratory studies, TRPV4 knockout mice and mice expressing mutant TRPV4 have not been able to fully recreate the human phenotypes associated with mutations in this gene [11,12]. However, some key features seen in humans (hearing impairment, skeletal dysplasia) were also found in the TRPV4 mice models [11,12]. At this time, the precise mechanism in which TRPV4 mutations affect the calcium channel is unclear. One proposed hypothesis is that mutations in this protein lead to increased probability of the calcium channel being open, as well as increased sensitivity of the channel to agonists.
Mutations in this gene presents with a wide variety of phenotypes [2,4,13]. The most widely reported conditions associated with this mutation are CMT2C and scapuloperoneal spinal muscular atrophy [2,4,6,13,14]. Skeletal dysplasias including brachyolmia, brachydactly and arthrogryposis are also known manifestations of TRPV4 mutations [1,2]. There appears to be some overlap in the phenotypes of the patients with neuropathy and those with skeletal dysplasias. Interestingly, some patients with CMT2C are also found to have changes in the pelvis and upper leg on radiographs [2,4,6].
Less well known features of TRPV4 mutations include vocal cord paralysis, respiratory failure and sensorineural hearing loss [1,2]. Hearing loss was also seen in the mouse TRPV knockout model, however, the exact etiology has not been elucidated [12].
There is a wide variation of penetrance associated with these mutations [1,2,6,13-18]. Individuals can be phenotypically normal. Others may die early due to respiratory failure. [1,13-18]. These phenotypically normal individuals have not been reported in the families with TRPV4 mutations associated with skeletal dysplasias. The age of onset of symptoms also vary widely with some patients presenting antenatally with arthrogryposis, while others do not present until adulthood [1,14]. Given the incomplete penetrance and phenotypic variability, we do believe that our case illustrates a rare cause of isolated scapular winging that may broaden its phenotypic spectrum.
Given the young age of both the proband and her son, we cannot exclude the possibility that her symptoms may progress beyond isolated scapular winging to involve other muscle groups. However, a 30 year plateau phase of lack of clinically perceptible progression to other muscle groups has never been reported, to our knowledge.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
REFERENCES
- Schindler A, Sumner C, Hoover-Fong JE. TRPV4-Associated Disorders. GeneReviews(R) 1993.
- McEntagart M. TRPV4 axonal neuropathy spectrum disorder. J Clin Neurosci 2012;19:927-33.
- Sullivan JM, Zimanyi CM, Aisenberg W, et al. Novel mutations highlight the key role of the ankyrin repeat domain in TRPV4-mediated neuropathy. Neurol Genet 2015;1:e29.
- Fleming J, Quan D. A case of congenital spinal muscular atrophy with pain due to a mutation in TRPV4. Neuromuscul Disord 2016; 26:841-43.
- Klein CJ, Shi Y, Fecto F, et al. TRPV4 mutations and cytotoxic hypercalcemia in axonal Charcot-Marie-Tooth neuropathies. Neurology 2011;76:887-94.
- Deng HX, Klein CJ, Yan J, et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nature genetics 2010;42:165-69.
- Aharoni S, Harlalka G, Offiah A, et al. Striking phenotypic variability in familial TRPV4-axonal neuropathy spectrum disorder. Am J Med Genet A 2011;155A:3153-156.
- Auer Grumbach M, Olschewski A, Papic L, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nature genetics 2010;42:160-64.
- Fecto F, Shi Y, Huda R, et al. Mutant TRPV4-mediated toxicity is linked to increased constitutive function in axonal neuropathies. J Biol Chem 2011;286:17281-291.
- Goswami C, Kuhn J, Heppenstall PA, et al. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PloS one 2010;5:e11654.
- Weinstein MM, Tompson SW, Chen Y, et al. Mice expressing mutant Trpv4 recapitulate the human TRPV4 disorders. J Bone Miner Res 2014;29:1815-22.
- Tabuchi K, Suzuki M, Mizuno A, et al. Hearing impairment in TRPV4 knockout mice. Neurosci Lett 2005;382:304-8.
- Evangelista T, Bansagi B, Pyle A, et al. Phenotypic variability of TRPV4 related neuropathies. Neuromuscul. Disord 2015;25:516-21.
- DeLong R, Siddique T. A large New England kindred with autosomal dominant neurogenic scapuloperoneal amyotrophy with unique features. Arch Neurol 1992;49:905-8.
- Berciano J, Baets J, Gallardo E, et al. Reduced penetrance in hereditary motor neuropathy caused by TRPV4 Arg269Cys mutation. J Neurol 2011;258:1413-21.
- Chen DH, Sul Y, Weiss M, et al. CMT2C with vocal cord paresis associated with short stature and mutations in the TRPV4 gene. Neurology 2010;75:1968-75.
- Zimon M, Baets J, Auer Grumbach M, et al. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 2010;133:1798-809.
- Landoure G, Zdebik AA, Martinez TL, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat genet 2010;42:170-4.