A review of arsenic induced alternation in aquatic animals or mammals by its toxicity
Received: 27-Jan-2023, Manuscript No. PULJFDR-23-6105 (PQ); Editor assigned: 30-Jan-2023, Pre QC No. PULJFDR-23-6105 (PQ); Reviewed: 13-Feb-2023 QC No. PULJFDR-23-6105 (R); Revised: 27-Mar-2023, Manuscript No. PULJFDR-23-6105 (R); Published: 03-Apr-2023
Citation: Ebrahim M, Ayub H. A review of arsenic induced alternation in aquatic animals or mammals by its toxicity. J Food Drug Res 2023;7(1):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
This study evaluated the developmental and behavioral effects of long-term arsenite exposure in animals. The present investigation was therefore undertaken to study the effect of arsenic trioxide on the nephrotoxicity, hepatoxicity, nephrotoxicity etc. An attempt is made to observe effect of arsenic, based on an extensive literature research with special emphasis on the most recent works. Arsenic is a carcinogen to both humans and animals. Arsenicals have been associated with cancers of the skin, lung, and bladder. Arsenic is classified as a metalloid, and exhibits both metallic and nonmetallic properties. It is present in ores and crustal rocks at average concentrations. It exhibits a complex chemistry, occurring in four different valencies (-III, O, III and V) and in many different chemical forms, these being used for a wide variety of industrial and agricultural purposes.
Arsenous Oxide (As2O3) is the most important arsenic compound employed by industry, and is used to synthesize various other inorganic and organic’s arsenicals. Arsenic trioxide is now used to treat acute promyelocytic leukaemia. Absorption occurs predominantly from ingestion from the small intestine, though minimal absorption occurs from skin contact and inhalation. Acute arsenic poisoning is associated initially with nausea, vomiting, abdominal pain, and severe diarrhoea, and peripheral neuropathy are reported. Chronic arsenic toxicity results in multisystem disease. It is known that as can affect signaling pathways since it can activate proteins such as ERK2, p38 and JNK, as shown in mammals. A comparison between phosphorylation sites of these proteins is presented in order to determine whether the same effect triggered by as in mammals might be observed in aquatic animals.
Keywords
Arsenicals; Arsenous oxide; Chronic toxicity; Neuropathy; Valencies; Phosphorylation; Diseases
Introduction
Arsenic is a metalloid present in water, soil, and air from natural and man-made sources [1]. It exists in inorganic and organic forms and in different oxidation states (−3, 0, +3, +5). In the case of environmental exposure, toxicologists are primarily concerned with arsenic in the trivalent and pentavalent oxidation state. The more commonly known arsenic compounds, arsenate is anioic form of arsenic and arsenite is anionic form of arsenous acid. Monomethylarsonic acid (MMAV) and Dimethylarsinic acid (DMAV) are stable methylated mammalian metabolites of inorganic arsenic and are primarily excreted in the urine [2]. An item of interest is that DMAV and the sodium salts of MMAV have been used as herbicides.
Arsenic has been studied extensively because of its widespread occurrence in animal and vegetable tissues, its toxicity, and its therapeutic use. It is found in rocks and soil from concentrations of less than one part per million (ppm) to hundreds of ppm, the mean value in the earth's crust being 2 ppm [3]. Arsenic is found in high concentration in sulfide deposits where it is present mainly as sulfide with other metals such as lead, copper, silver, iron, etc.
Leaching of arsenic from soils or rocks containing high levels by hot-spring mineral waters has been reported in Japan, New Zealand and the United States [4]. Considerable concentrations of arsenic up to 1100 pg/l in drinking water have been reported in Chile, Argentian, Taiwan, the United States and the United Kingdom. Arsenic is also found in sea water at concentrations of 2-5 pg/l.
Arsenic has been used as a wood preservatives, desicants and herbicides in agriculture, a fining agent for glass industry and copper and lead base alloys. While the synthesis of arsenic compounds for agriculture and use of trioxide and metallic arsenic for industries has decreased, production and usage of high purity metallic arsenic for gallium arsenide semiconductor gradually increase [5]. Anthropogenic emission of arsenic in aquatic ecosystem including oceans during the past century have been estimated to be a high as 41000 metric tons per year, which is about five times the global emissions from natural sources estimated as 7800 metric tons per year. The greater input of arsenic in to the ocean promoted us to study its accumulation and toxic effects on marine organisms [6].
When arsenic is mentioned, most people think of the lethal poison that has been used for centuries [7]. However, human exposure to arsenic more frequently occurs from other sources such as drinking water, food, dusts, and soils. Although it is plausible that a small amount of arsenic is a nutritional requirement, adverse health effects begin to occur at some elevated concentration of exposure [8]. This threshold concentration of exposure is the basis on which the current Maximum Containment Level (MCL) of 50~g/l for arsenic in drinking water was determined for the national interim primary drinking water regulations which were established under the safe drinking water act. However, this MCL is being reviewed as more information on the dose related health effects of arsenic exposure becomes available [9].
Arsenic (As) is a wide spread pollutant in various regions of the world. Arsenic and its derivatives are mobile in the environment [10]. Breakdown of rocks converts arsenic sulfides to arsenic trioxide, which enters the arsenic environment dust cycle or by dissolution in rain, rivers, or ground water. Chronic exposure to in organic arsenic can lead to cancer of the skin, lungs, bladder, and liver [11].
This metalloid is commonly found in several chemical forms with different toxicity; thus, inorganic forms of arsenic (arsenite and arsenate) are more toxic, while methylated forms (Methyl Arsonate, MMA and Dimethyl Arsinate, DMA) are consider edonly moderately toxic [12]. Other arsenic species, like Trimethylarsine Oxide (TMAO) and Tetramethyl Arsonium (TETRA) are also consider edmoderately toxic, whereas Arsenobetaine (AsB), Arsenocholine (AsC) and other Arsenosugars (AsS) show no toxicity.
Several epidemiological studies performed in different geographic areas have demonstrated that inorganic arsenic is a human carcinogen. However, the mechanism of its carcinogenicity is not yet known. The lack of cancer induction in in vivo animal models makes investigations carried out on in vitro cell systems very useful in elucidating the causes of arsenic carcinogenicity [13].
Some studies report that arsenic causes gene amplification, chromosome damage and inhibition of DNA repair as well as global DNA hypomethylation, decrease of DNA methyl transferase activity and protooncogene activation. Previously, we reported that V79-Cl3 Chinese hamster cells underwent either early genetic instability or apoptosis when exposed to Sodium Arsenite (SA).
In the observations performed both during and shortly after treatment, genetic instability was manifested in the presence of aneuploid and morphologically abnormal cells, but not by cells with chromosome aberrations. As dividing cells turned out to be the most sensitive to SA exposure, due to the arsenic's direct action on the mitotic spindle assembly we later ascertained the fate of genetically unstable cells escaping apoptosis, by harvesting mitotic rounded-up cells at the end of a 24 h treatment [14]. This cell population, examined after ~2 months of sub culturing (120 cell generations), was still genetically not stable.
In spite of tremendous development in the field of synthetic drugs during recent era, they have some or other side effects, whereas natural therapy with phytochemical substances still have been proposed to exert beneficial effects in many disease conditions [15].
Arsenic crosses the blood-brain barrier and accumulates in the brain where it can exert neurotoxic effects in several structures, such as the basal ganglia. Although information on the effects of arsenic in the central nervous system is limited, the basal ganglia appear to be particularly sensitive to the effects of toxins that affect energy metabolism, such as 3-nitropropionic acid, an inhibitor of succinic dehydrogenase, MPTP, and 6- hydroxydopamine as well as to metal exposure [16]. For example, manganese causes selective neuronal loss in the globus pallidus, and iron accumulates abnormally in one form of parkinsonism. Previous studies have focused on the effects of arsenic on monoamines in the basal ganglia and, although changes in content of monoamines have been observed, the results have been contradictory. Exposure to sodium arsenite in mice resulted in increased content of striatal dopamine, 3,4-Dihydroxyphenyl Acetic Acid (DOPAC), and 5-Hydroxyindole Acetic Acid (5-HIAA). Exposure to arsenic trioxide decreased DOPAC and Homovanillic Acid (HVA) in the striatum. In rats exposed to sodium arsenate, striatal serotonin content increased and dopamine content decreased in the nucleus accumbens. Decrements in the evoked release of dopamine and its metabolites (DOPAC and HVA) have been described in the striatum of rats exposed to a mining waste with high arsenic content. Dopamine is involved in a variety of functions, including movement control, learning and memory, cognition, and emotion. If arsenic exposure can modify monoamine content in the basal ganglia, it could also have a consequence on behavior. Studies on locomotor activity in mice reported deficits related to the dose of arsenic trioxide used. Deficits in an operant learning task have also been reported [17]. The paucity of data and the fact that previous studies did not examine the effects of dosage and time of exposure make it difficult to reach any conclusion regarding arsenic effects in the nervous system in an in vivo model. In order to study the possible neurotoxic effects of arsenic exposure, and given the relevance of the basal ganglia to functions such as learning, memory and movement, it would be important to evaluate if arsenic exposure produces alterations in these complex functions.
Arsenic can also induce male reproductive toxicity such as dose-dependent decrease in testes and accessory sex organ weights. It may also reduce epididymal sperm count viability, and motility. Normal morphology and the activity of antioxidant defense system. Alteration in the level of Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), testosterone and also massive degeneration of the germ cells in testis tissue is reported to be an account for arsenic toxicity. Oxidative stress and the generation of Reactive Oxygen Species (ROS) could also be a consequence of arsenic exposure [18]. ROS generation as well as the binding of arsenic to protein thiol groups can alter many protein functions. The integrity of sperm DNA is an important factor for the success of fertilization as well as normal development of the embryo, fetus and child. Several line of studies have shown the effect of environmental contaminates such as arsenic on DNA damages through inducing oxidative stress and the generation of ROS.
Literature Review
Inorganic arsenic is classified by the international agency for research on cancer and the US environmental protection agency as a known human carcinogen. This classification is based on several epidemiological studies which show an association of exposure to arsenic and the development of cancer. Cancer has developed in individuals exposed to arsenic through medical treatment with Fowler’s solution (potassium arsenite) occupational exposure via inhalation at copper or naturally contaminated drinking water [19]. Tumors that develop after inhalation of arsenic are observed primarily in the lung whereas they are initially observed in the skin after oral exposure to arsenic. However, additional studies indicate that cancer of internal organs occurs in individuals who chronically consume arseniccontaminated drinking water. Tumor sites include bladder, liver, and kidney. Although there is sufficient evidence to classify arsenic as a human carcinogen, whether it occurs in laboratory animals is not clear. In several studies, a carcinogenic effect was absent in animals after a life-time exposure to inorganic arsenic in either the drinking water or diet, or after oral intubation. This has been reported in rats dogs and monkeys. Mice exposed to arsenite for 26 weeks in their drinking water also did not develop tumors. However, there have been several positive reports on the carcinogenicity of arsenic in animal models. In the 1980, several studies reported on the carcinogenicity of inorganic arsenic in hamsters after intratracheal instillation. This route was used to simulate inhalation of arsenic. Chemicals used in these studies included Arsenic Trioxide (As2O3), Calcium Arsenate Ca(AsO4)2, and Arsenic Trisulfide (As2S3). The animals were dosed one time per week for 15 weeks and monitored for tumors throughout their life span. There was greater mortality with As2O3 compared to Ca(AsO4)2 and As2S3, and this effect was dose-dependent. Up to 50% of the animals in the As2O3 treated groups did not survive the dosing regimen, and this result must be considered in light of the tumorigenic response.
Methodology
A literature search was performed using pubmed and google scholar, sci-hub databases for identification of relevant studies from old one to recent one. Abstracts of studies that reported exposure to arsenic-contaminated drinking water.
Additional relevant studies were hand-searched and a cross-checked reference list from previous review articles was used for identification of studies not retrieved through the electronic database. We only considered articles published in peer-reviewed journals and in the English language. Each published article selected was critically evaluated and summarized in terms of location, design, pregnancy outcome, effect on organs, sample size, exposure assessment and magnitude of association between exposure to arsenic and its effect.
Historical therapeutic uses of arsenic
Arsenic was used as an attenuate agent after Greek physicians such as Hippocrates and Galen popularised its use. Arsenic compounds became obtainable as solutions, tablets, pastes, and in injectable forms. Fowler’s solution, a 1% arsenic trioxide preparation, was extensively used during the 19th century. In recent times 1958, the British pharmaceutical and therapeutic products handbook edited by Martindale, listed the indications for Fowler’s solution as: Leukaemia, skin conditions (psoriasis, dermatitis herpetiformis, and eczema), stomatitis and gingivitis in infants, and Vincent’s angina. Fowler’s solution was also advised as a health tonic. Chronic arsenic intoxication from the long term use of Fowler’s solution caused haemangiosarcoma, angiosarcoma of the liver, and nasopharyngeal carcinoma [20].
Current therapeutic uses of arsenic
Arsenic Trioxide (As2O3) is now broadly used to induce revocation in patients with acute promyelocytic leukaemia, based on its mechanism as an inducer of apoptosis (programmed cell death). Arsenic induces apoptosis by releasing an Apoptosis Inducing Factor (AIF) from the mitochondrial intermembrane space from where it translocates to the cell nucleus. AIF then effects apoptosis, resulting in altered nuclear biochemistry, chromatin condensation, DNA fragmentation, and cell death. AIF has been isolated and cloned and is a flavoprotein with a molecular weight of 57,000.20. Arsenic is more often a adulterate, sometimes with mercury and lead. The department of health services of California screened 251 products in retail herbal stores and detected arsenic in 36 products (14%) in concentrations from 20.4 to 114,000 parts per million (ppm) with a mean of 145.53 ppm and the median 180.5 ppm.
Coal contaning naturally high level of arsenic
Generally coal contains low arsenic concentration that poses no health risks to humans, as the arsenic content in most coals is less than 5 mg kg-1. However, some coals may contain up to 35,000 mg kg-1. Several studies have shown how burning of high arsenic coals can contaminate the indoor environment. For example the effect of coal burning on the residents of Guizhou province, PR China was investigated. Samples of coal, water, air, food, urine and hair were collected from endemic and controlled areas and analysed for arsenic. The mean arsenic concentrations in coal (mg kg-1), water (μgl-1), urine (μgl-1), and hair (mg kg-1) in the polluted areas were 2166.7, 5.2, 137, and 3.08 and for control areas were 2.5, 2.4, 23, and 0.97 respectively.
Choronic arsenic poisoning
The mechanisms of arsenic toxicity are inhibition of sulfhydryl groupcontaining cellular enzymes and replacement of phosphate molecules in ‘high-energy’ compounds (‘arsenolysis’). Trivalent arsenic compounds are more potent in inhibiting enzymes, whereas pentavalent compounds are more involved in arsenolysis. Trivalent arsenic compounds are human carcinogens causing tracheal and bronchogenic carcinomas, hepatic angiosarcomas and various skin cancers, such as intraepidermal carcinomas (bowen’s disease), squamous cell carcinomas, basal cell carcinomas, and ‘combined’ forms of skin cancer. Myelogenous leukemia may also occur. An increased risk of Hodgkin’s disease was found in arsenic-exposed French gold miners. Internal cancers of the lung, liver, bladder, and kidney have been associated with chronic ingestion of fowler’s solution or arsenic contaminated drinking water. About 60%-90% of soluble arsenic compounds are absorbed from the gastrointestinal tract following ingestion; inhalation exposure may be similar. Absorption through intact skin is usually negligible.
Acute arsenic poisoning
A single exposure to a high dose may lead to severe reactions such as diarrhea, vomiting, pain, dehydration and weakness. Nowadays, acute intoxication rarely occurs in Western European countries; if it occurs, it is usually the result of intentional (suicide or homicide) or accidental poisoning. Occupational exposure to as is rare and usually occurs in the form of arsine gas that causes symptoms different to those caused by as ingestion. Exposure often occurs, when arsine gas escapes during transport or when it is generated while arsenic containing ores or metals are treated with acid. Acute oral exposure to as is associated with gastrointestinal symptoms such as nausea, vomiting, abdominal pain and severe diarrhea. Cardiovascular and respiratory symptoms include hypotension, shock, pulmonary edema and heart failure.
Arsenic exposure
Arsenic exposure occurs from inhalation, absorption through the skin and, firstly, by ingestion of, for example, contaminated drinking water. Arsenic in food occurs as comparatively nontoxic organic compounds (arsenobentaine and arsenocholine). Seafood, fish, and algae are the richest organic sources. These organic compounds cause raised arsenic levels in blood but are rapidly excreted unchanged in urine. Arsenic intake is higher from solid foods than from liquids including drinking water. Organic and organic sources. These organic compounds cause raised arsenic levels in blood but are rapidly excreted unchanged in urine. Arsenic intake is higher from solid foods than from liquids including drinking water. Organic and inorganic arsenic compounds may enter the plant food chain from agricultural products or from soil irrigated with arsenic contaminated water.
Absorption
The major site of absorption is the small intestine by an electro genic process involving a proton (H+) gradient. The optimal pH for arsenic absorption is 5.0, though in the milieu of the small bowel the pH is approximately 7.0 due to pancreatic bicarbonate secretion.
Metabolism
Arsenic toxicity varies widely with its oxidation states. Inorganic species generally are more toxic than organic ones, and Arsenite (AsIII) is about 60 times more toxic than Arsenate (AsV). This last species, in turn, is about 70 times more toxic than methylated species as Monomethylarsonic Acid (MMA), and Dimethylarsinic Acid (DMA), with the last two forms being considered only moderately toxic. In mammals, after reduction, arsenite is methylated by arsenite methyltransferase. Results from in vitro assay systems containing rat liver cytosol, arsenite and methylarsonous diiodide (CH3AsI2) showed that arsenite was the preferred substrate for the methylation reaction, with the conversion of arsenite to methylated metabolites being faster than for arsenate.
The absorbed arsenic undertake hepatic biomethylation to form monomethylarsonic acid and dimethylarsinic acid that are less toxic but not completely innocuous. About 50% of the ingested dose may be eliminated in the urine in three to five days. Dimethylarsinic acid is the presiding urinary metabolite (60%-70%) contrast with monomethylarsonic acid. A small amount of inorganic arsenic is also excreted unchanged. After acute poisoning electrothermal atomic absorption spectrometry studies show that the excessive concentration of arsenic is in the kidneys and liver. In chronic arsenic ingestion, arsenic accumulates in the liver, kidneys, heart, and lungs and smaller amounts in the muscles, nervous system, gastrointestinal tract, and spleen. Though most arsenic is cleared from these sites, residual amounts remain in the keratin-rich tissues, nails, hair, and skin. After about two weeks of take in, arsenic is deposited in the hair and nails.
Biological cycle of arsenic
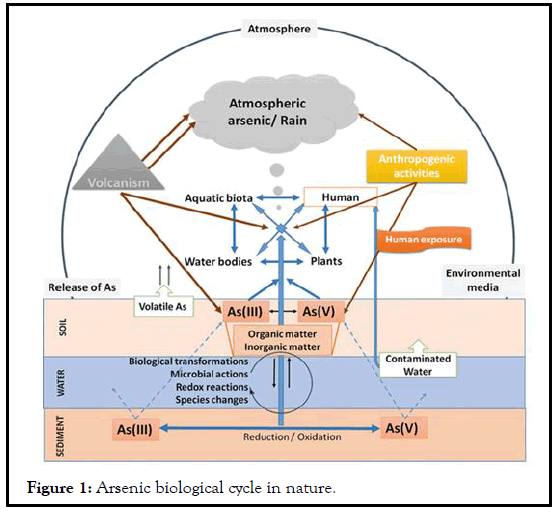
In water, arsenic can be found as arsenate or arsenite. There also exist methylated arsenic compounds that occur naturally in the environment as the result of biological activity. The major sources of contamination and occupational exposure with arsenic are the burning of coal and industrial metal smelting Tamaki, et al. and more recently the semiconductor industry. Arsenic toxicity is highly dependent on its oxidation state: Trivalent arsenicals are at least 100 times more toxic than the pentavalent derivatives. Toxicity of Arsenite (As(Ill)) is due to its binding to protein sulfhydryl groups. Arsenate (As(V)), on the other hand, is a toxic 357 analog for inorganic phosphorylating activities. The insoluble arsenides can be transformed to the soluble forms of arsenate and arsenite by industrial processes and rectal smelting. The presence of arsenate, arsenite and methyl-arsenic can be detected in exposed individuals. Arsenite and arsenate are interconverted to an extent by biological redox reactions and arsenite can be methylated by bacteria, fungi and algae. Some living organisms such as algae and crustaceans concentrate arsenic well over the level in the environment. The arsenic biological cycle in nature is summarized in Figure 1.
The accumulation of arsenic by aquatic biota
Plankton: Relatively little has been published on the concentrations of total arsenic in plankton, although phytoplankton have received considerable attention because of their role in defining the speciation of the element in marine water. Leatherland, et al. reported total arsenic concentrations of 14 to 42 μg g-1 dry weight in three species of zooplankton from offshore waters of the North-East Atlantic Ocean. Similar or slightly lower concentrations have been reported for zooplankton from other waters. More recently, it has been shown that while arsenate is directly toxic to zooplankton only at grossly elevated levels in solution, its impacts on phytoplankton species composition (favouring tolerant species) may nevertheless be an important factor affecting the abundance of both phytoplankton and zooplankton in coastal waters.
Echinoderms: Data for arsenic in echinoderms are sparse. Vaskovsky, et al. found high concentrations of total arsenic (which varied with location of sampling) in the lipids of several species. Leatherland and Burton reported concentrations of 5.8 and 10 μg g-l dry weight respectively for the asteroids Marthasterias glacialis and Asterias rubens from Portland, Dorset.
Polychaetes: Total arsenic levels in most species of polychaetes appear unremarkable. Thus, for example, Nereis diversicolor has been reported to contain between 4 and 87 μg g-1 dry weight of the element, depending on location of sampling.
Other species: Isolated data exist concerning arsenic in species other than those discussed above. For example, Leatherland, et al. found 11 μg g-1 dry weight of total arsenic in a scyphozoan (Pelagia sp.) from the North Atlantic. Leatherland, et al. reported 72 μg g-1 dry weight of arsenic in the coelenterate tealiafelina from Southampton water, but much lower concentrations (2.8-6.6 gg g-1 dry weight) in a sponge and two tunicate species.
Mechanism of arsenic toxicity for causing adverse pregnancy outcomes
The mechanisms by which arsenic exposure causes adverse pregnancy outcomes are not completely understood, however, a number of possible mechanisms based on experimental evidence have been suggested. Arsenic occurs either in the elemental form, as part of organic compounds or as a part of inorganic compounds. In waters, inorganic species are dominant (international agency for research on cancer, 2012). Inorganic arsenic is eliminated from the body via rapid urinary excretion or a sequential methylation process which predominantly occurs in the liver. Various epidemiological studies were identified that have investigated the association between arsenic exposure via drinking water and adverse pregnancy outcomes.
A number of epidemiological studies from Bangladesh, India, Sweden, Hungary, Mongolia, and the United States of America (USA) have reported an increased risk of spontaneous abortion (loss of a clinically recognised pregnancy prior to 20 weeks completed gestation) and stillbirth (loss of a clinically recognised pregnancy following 20 weeks completed gestation) in association with chronic arsenic exposure. Only a few studies have been conducted exploring the association between chronic arsenic exposure and neonatal death (mortality within the first 28 days following delivery) and post neonatal death (mortality between 28 days and 364 days following delivery). A prospective cohort study conducted in two Chilean cities with contrasting drinking water arsenic levels, Antofagasta (40 ppb) and Valparaiso (<1 ppb) found that moderate arsenic exposure from drinking water (<50 ppb) during pregnancy was associated with low birth weight.
Effect of arsenic on skin
Numerous skin changes occur with long term exposure. Dermatological changes are a common feature and the initial clinical diagnosis is often based on hyperpigmentation, palmar and solar keratosis. The keratosis may appear as a uniform thickening or as discrete nodules. It is emphasized that both palmar and solar keratosis are a significant diagnostic criterion. Arsenic may cause a basal cell carcinoma in a non-melanin pigmented skin.
Despite the high responsiveness of human skin to both arsenic’s toxicity and carcinogenicity, wild-type rodent skin models of carcinogenicity have not been very responsive to arsenic. Mouse skin is well known to be sensitive to many chemical carcinogens; rat skin is extremely unresponsive to chemical carcinogenesis.
Effect of arsenic on neurological system
The neurological system is the major target for the toxic effects of a number of metals, especially the heavy metals such as mercury, lead, and arsenic. The neurological effects are many and varied. The most frequent finding is a peripheral neuropathy mimicking Guillain-Barre syndrome with similar electromyographic findings. The neuropathy is initially sensory with a glove and stocking anesthesia.
Enhanced oxidative stress has been suggested to be an important mechanism in arsenic induced neurotoxicity. Arsenic exposure has been found to cause oxidative damage to the biological system by enhancing generation of free radical species which in turn may be responsible for increased lipid peroxidation, protein carbonyl and decreased GSH levels. Glutathione is an important biomolecule involved in the defense against toxicants. The decrease in GSH level following arsenic exposure has been associated with high affinity of arsenic with GSH.
As neurotransmitters play an important role in modulating an array of behavior and signaling cascade, a number of experimental studies have been carried out to investigate the effect of arsenic on their levels and metabolites. Alterations in dopaminergic, cholinergic, serotonergic and glutamatergic systems have been reported in rats and mice exposed to arsenic. Consistent changes in the levels of brain neurotransmitters have not been observed due to variations in the dose of arsenic used, duration and route of exposure. However, a decrease in dopamine levels in striatum and whole brain has been reported largely following prolonged exposure to arsenic.
Arsenic effect on liver
The observed histological alterations in hepatic tissues on arsenic treatment include cytoplasmic vacuolation, degeneration of the cells and focal necrosis. Degeneration of liver tissue and necrosis of central vein could be due to the accumulation of neutrophils and lymphocytes. Our results are corroborated by the similar findings of liver pathology in mice. In the present study the observed decrease in blood Hb and increase in bilirubin levels may be due to the alterations in hematopoietic function encountered due to arsenic intoxication which is in agreement with that of Patric Lyn. Arsenic compound is a protoplasmic poison that reacts with sulfhydrylcontaining molecules including Hb with high affinity and forms complexes thereby gets accumulated in the blood and leads to hematological abnormalities including anemia and leucopenia. This property of arsenic is considered to be the mechanism to exert its toxic effect.
The initiation of oxidative stress following exposure to hepatotoxic agents is an important factor underlying chronic liver diseases associated with fibrosis. Disturbed membrane molecular properties through damage to such as membrane lipids, building blocks of proteins (amino acids), sugars (carbohydrates) and nucleic acids, is likely the primary factor of chemical induced hepatocellular injury. The liver is the center of metabolism of various chemicals including inorganic arsenic in a number of animals including mammals and reduced GSH in hepatocytes acts as a vital antioxidant.
Biochemical and morphological evidence of arsenite induced hepatotoxicity was observed in developing rat livers. The evidence was reduced levels of GSH (leading to increased oxidative stress), increased size of hepatocyte nuclei, sporadic vacuolation of hepatocyes and sinusoidal dilatation in the animals administered arsenic. To understand the exact mechanisms underlying arsenic induced toxicity at cellular and molecular levels, broader studies using various dosages of sodium arsenite and varied durations of exposure at different developmental periods should be considered.
Arsenic effect on kidney
Kidney is vulnerable to arsenic-induced damage because of large perfusion and the increased concentration of excreted compounds that occur in renal tubular cells. According to our results the increased serum urea, uric acid and creatinine levels confirm the high degree of involvement of the renal tubular cells in the excretion of arsenic metabolites which makes them more susceptible to damage and renal dysfunction.
Arsenic trioxide may have an effect on bone marrow, kidney and hemoglobin metabolism. Young, et al. showed clearly that any substance which significantly affects the values of red blood cells and associated parameters would have effects on the bone marrow, kidney and haemoglobin metabolism.
In the present study a remarkable hepatic and renal oxidative stress was induced on arsenic intoxication as reflected by decrease in the activities of cellular antioxidant enzymes which are in agreement with the earlier reports by Wang, et al.
Discussion
Arsenic compounds are toxic substances with very profound effects on animal’s health. It has been established that arsenic toxicity is related to its chemical forms. In fact, some authors have suggested that as is incorporated by cells through AQPs, in mammals and other organisms, after which it can be bio transformed and its metabolites can also exert toxic effects. Although it is known that as can affect signaling pathways mostly in mammals, in this review we compared phosphorylation sites in different mammalian and non-mammalian species.
Conclusion
As a result, we can infer that in aquatic animals it is possible that the same effects observed in mammalian models can in fact occur. Furthermore, it has been shown that arsenic can induce oxidative stress in mammals and also some aquatic animals. The present work demonstrates that animals intoxicated with arsenic trioxide display a pronounced impairment in hepatic and renal functions. There are few reports of as exposure effects on development of sensory systems. Also, it capable of induced marked alterations in some biochemical parameters, liver and kidney also. There is consistent and convincing evidence present to acknowledge a positive association between exposure to high concentration of inorganic arsenic exposure (>50 ppb) in drinking water and spontaneous abortion, stillbirth and low birth weight.
References
- Abernathy CO, Liu YP, Longfellow D, et al. Arsenic: Health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999;107(7):593-97.
[Crossref] [Google Scholar] [PubMed]
- Akter KF, Owens G, Davey DE, et al. Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol. 2005;184:97-49.
[Crossref] [Google Scholar] [PubMed]
- An D, He YG, Hu QX, et al. Poisoning by coal smoke containing arsenic and fluoride. Fluoride. 1997;30(1):29-32.
- Aposhian HV. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol. 1997;37(1):397-419.
[Crossref] [Google Scholar] [PubMed]
- Aposhian HV, Aposhian MM. Newer developments in arsenic toxicity. J American Colle Toxicol. 1989;8(7):1297-305.
- Bates MN, Smith AH, Hopenhayn-Rich C. Arsenic ingestion and internal cancers: A review. Am J Epidemiol. 1992;135(5):462-76.
[Crossref] [Google Scholar] [PubMed]
- Benramdane L, Accominotti M, Fanton L, et al. Arsenic speciation in human organs following fatal arsenic trioxide poisoning-a case report Clin Chem. 1999;45(2):301-06.
[Google Scholar] [PubMed]
- Bohn A, McElroy RO. Trace metals (As, Cd, Cu, Fe, and Zn) in arctic cod, Boreogadus saida, and selected zooplankton from Strathcona Sound, northern Baffin Island. J Fish Board Can. 1976;33(12):2836-40.
- Bohn A. Arsenic in marine organisms from West Greenland. Mar Pollut Bull. 1975;6(6):87-9.
- Buchet JP, Lison D, Ruggeri M, et al. Assessment of exposure to inorganic arsenic, a human carcinogen, due to the consumption of seafood. Arch Toxicol. 1996;70:773-78.
[Crossref] [Google Scholar] [PubMed]
- Chattopadhyay S, Bhaumik S, Chaudhury AN, et al. Arsenic induced changes in growth development and apoptosis in neonatal and adult brain cells in vivo and in tissue culture. Toxicol Lett. 2002;128(1-3):73-84.
[Crossref] [Google Scholar] [PubMed]
- Chiou HY, Hsueh YM, Liaw KF, et al. Incidence of internal cancers and ingested inorganic arsenic: A seven-year follow-up study in Taiwan. Cancer Res. 1995;55(6):1296-300.
[Google Scholar] [PubMed]
- Cuzick J, Sasieni P, Evans S. Ingested arsenic, keratoses, and bladder cancer. Am J Epidemiol. 1992;136(4):417-21.
[Crossref] [Google Scholar] [PubMed]
- Ding Z, Zheng B, Long J, et al. Geological and geochemical characteristics of high arsenic coals from endemic arsenosis areas in southwestern Guizhou Province, China. Appl Geochem. 2001;16(11-12):1353-60.
- Edmonds JS, Francesconi KA. Transformations of arsenic in the marine environment. Experientia. 1987;43(5):553-7.
[Crossref] [Google Scholar] [PubMed]
- Farmer JG, Johnson LR. Assessment of occupational exposure to inorganic arsenic based on urinary concentrations and speciation of arsenic. Br J Ind Med. 1990;47(5):342-48.
[Crossref] [Google Scholar] [PubMed]
- Flora SJ, Bhadauria S, Pant SC, et al. Arsenic induced blood and brain oxidative stress and its response to some thiol chelators in rats. Life Sci. 2005;77(18):2324-37.
[Crossref] [Google Scholar] [PubMed]
- Goddard MJ, Tanhehco JL, Dau PC. Chronic arsenic poisoning masquerading as Landry-Guillain-Barre syndrome. Electromyogr Clin Neurophysiol. 1992;32(9):419-23.
[Google Scholar] [PubMed]
- Gonzalez MJ, Aguilar MV, Martinez MC. Mechanisms of absorption of As2O5 from rat small intestine: The effect of different parameters. J Trace Elem Med Biol. 1997;11(4):239-47.
[Crossref] [Google Scholar] [PubMed]
- Hopenhaynrich CL, Smith AH, Goeden HM. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993;60(2):161-77.
[Crossref] [Google Scholar] [PubMed]