A severe case of hyperostosis frontalis interna and multiple comorbidities
Tiffany Champion and Janet M.Cope*
Department of Physical Therapy Education, Elon University, Elon, North Carolina, USA.
- *Corresponding Author:
- Janet M. Cope
Department of Physical Therapy Education, School of Health Sciences Campus Box 2085, Elon University Elon, NC 27244, USA.
Tel: +1 (336) 278-6355
E-mail: jcope2@elon.edu
Date of Received: February 4th, 2012
Date of Accepted: September 9th, 2012
Published Online: November 9th, 2012
© Int J Anat Var (IJAV). 2012; 5: 76–78.
[ft_below_content] =>Keywords
hyperostosis frontalis interna,bony overgrowth
Introduction
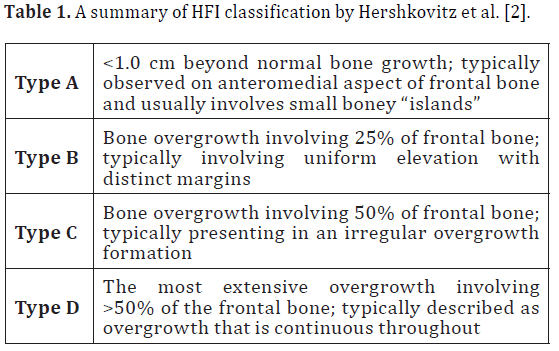
overgrowth of the frontal region of the endocranial surface appearing in the scientific literature as early as 1719 [1]. Formerly HFI was associated with Morgagni’s syndrome (HFI, obesity, virilism), Stewart-Morel syndrome (HFI, obesity, neuropsychiatric disorders) and Troell-Junet syndrome (HFI, acromegaly, goiter, diabetes) but is now considered to be an entity entirely of its own. HFI is observed independently as well as in conjunction with systemic pathologies, psychological impairments, and there may even be a genetic component [2]. In any case, HFI is most often reported in modern day postmenopausal women [3,4] and potentially related to prolonged estrogen stimulus in females and males with hormonal imbalance [2]. Hershkovitz and colleagues classified HFI by the extent of bony overgrowth in the frontal bone region of the endocranium. A summary of this classification can be seen in Table 1 [2].
Case Report
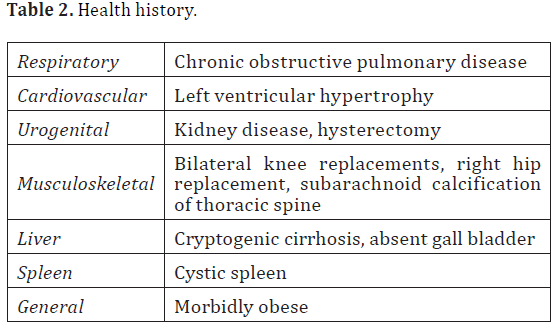
The donor was a 77-year-old female at her time of death from “cryptogenic liver disease”. She was of European dissent and had multiple co-morbidities. Some were listed in her health history while others were observed during dissection and included: cardiopulmonary, urogenital, and multiple musculoskeletal conditions. Please see Table 2 for a complete list of the donor’s health history.
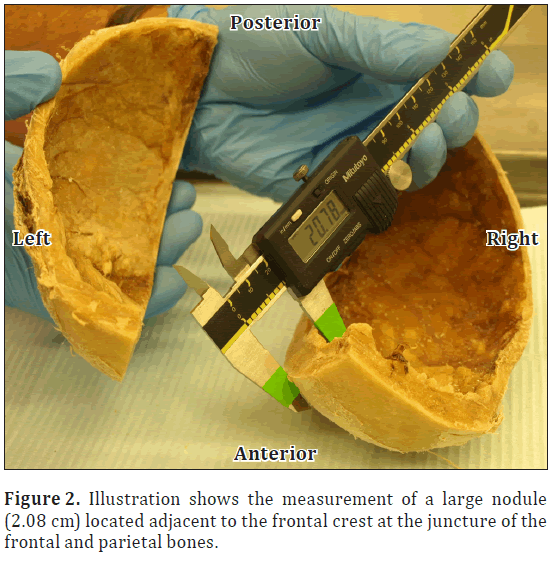
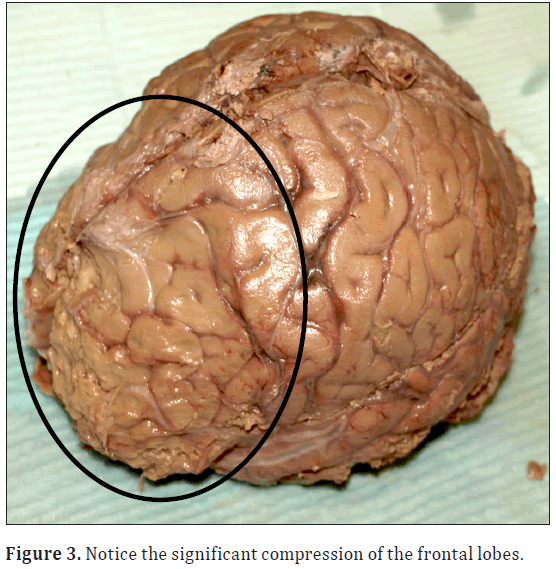
During a routine dissection of the donor’s calvaria in the human anatomy laboratory at Elon University, Elon, North Carolina; it was noted that she had significant bony overgrowth of the endocranium. Bony overgrowth was diffuse throughout the frontal bone extending slightly into the parietal region with midline sparing (Figure 1). Cranial thickness was noted at multiple points on the calvaria using digital calipers. Bony overgrowth ranged from 1.0 cm thickness in the temporal region, to 1.3 cm adjacent to midline in the frontal region. Large individual nodules were located along either side of the frontal crest at the intersection of the frontal and parietal bones. The largest nodules measured 2.08 cm on the right and 1.81 cm in thickness on the left (Figure 2). As a result, the pathway of the middle meningeal artery was tortuous. In addition there was significant depression bilaterally of the frontal lobes and surrounding neural tissues (Figure 3).
Results
HFI presents in predominantly postmenopausal women, normally ranging in thickness from 0.5–1cm, and has been associated with diabetes, obesity, and chronic headaches. In the literature, there are multiple independent cases of HFI and subarachnoid calcification as was observed in this donor’s thoracic spine. Subarachnoid calcification is commonly observed during the dissection of human donors.
Etiology for this pathology remains unclear, but the plaques are often discovered in the lower thoracic and lumbar spinal regions [5–7]. In addition, there is no literature suggesting a relationship between HFI, subarachnoid calcification and other orthopedic conditions as were observed in this donor (Table 1).
As previously discussed, HFI can be categorized based on the extent of bony overgrowth. In this case the donor exhibited Type D, which involves the most extensive overgrowth (more than 50%) of the frontal bone and is continuous throughout. As a result, there was significant compression of the donor’s meninges, meningeal blood supply and specific regions of neural tissues in the frontal lobe including: Broca’s area, the premotor cortex, and the prefrontal association cortex. Compression in these areas of the brain could result in headaches and personality disturbances (ranging from passive to disinhibited), as well as impairments with cognition, motor planning, speech, eye movement, nonverbal communication, and memory [8].
Conclusion
There is currently no documented underlying pathology that connects HFI with all of the comorbidities observed in this donor. As a result of HFI, patients may have significant cognitive impairments in addition to other medical conditions. There is a need for continued efforts in reporting cases of HFI along with a complete assessment for co-morbidities to better understand this pathology and the clinical implications for patients.
Acknowledgements
Special thanks to Dr. Stewart Holt for assisting with obtaining images for this case report and Angela Susan Ramer for assisting with the literature search.
References
- Moore S. Hyperostosis Cranii. Springfield, Illinois: Charles C. Thomas. 1955; 3–195.
- Hershkovitz I, Greenwald C, Rothschild BM, Latimer B, Dutour O, Jellema LM, Wish-Baratz S. Hyperostosis frontalis interna: an anthropological perspective. Am J Phys Anthropol. 1999; 109: 303–325.
- Talarico EF Jr, Prather AD, Hardt KD. A case of extensive hyperostosis frontalis interna in an 87-year-old female human cadaver. Clin Anat. 2008; 21: 259–268.
- Glab H, Szostek K, and Kaczanowski K. Hyperostosis frontalis interna, a genetic disease?: Two medieval cases from Southern Poland. Homo. 2006; 57: 19–27.
- Knoblich R and Olsen B. Calcified and ossified plaques of the spinal arachnoid membranes. J Neurosurg. 1966; 25: 275–279.
- Wijdicks CA, Williams JM. Spinal arachnoid calcifications. Clin Anat. 2007; 20: 521–523.
- Lovering RM, Anderson LD. Leptomeningeal plaques, a “common” finding. Clin Anat. 2006; 19: 696–697.
- Blumenfeld H. Neuroanatomy through clinical cases. Sunderland, MA, Sinauer Associates. 2002; 821–854.
Tiffany Champion and Janet M.Cope*
Department of Physical Therapy Education, Elon University, Elon, North Carolina, USA.
- *Corresponding Author:
- Janet M. Cope
Department of Physical Therapy Education, School of Health Sciences Campus Box 2085, Elon University Elon, NC 27244, USA.
Tel: +1 (336) 278-6355
E-mail: jcope2@elon.edu
Date of Received: February 4th, 2012
Date of Accepted: September 9th, 2012
Published Online: November 9th, 2012
© Int J Anat Var (IJAV). 2012; 5: 76–78.
Abstract
Hyperostosis frontalis interna (HFI) is a condition that involves thickening of the frontal region of the endocranial surface with midline sparing. It is observed in radiographic images of the cranium in living subjects and upon dissection in deceased subjects. There is little information related to the impact that hyperostosis frontalis interna has on living patients. In this case of severe hyperostosis frontalis interna, the subject was a 77-year-old female human donor who had an overwhelming number of co-morbidities. The purpose of this case study is to report an unusual case of hyperostosis frontalis interna.
-Keywords
hyperostosis frontalis interna,bony overgrowth
Introduction
overgrowth of the frontal region of the endocranial surface appearing in the scientific literature as early as 1719 [1]. Formerly HFI was associated with Morgagni’s syndrome (HFI, obesity, virilism), Stewart-Morel syndrome (HFI, obesity, neuropsychiatric disorders) and Troell-Junet syndrome (HFI, acromegaly, goiter, diabetes) but is now considered to be an entity entirely of its own. HFI is observed independently as well as in conjunction with systemic pathologies, psychological impairments, and there may even be a genetic component [2]. In any case, HFI is most often reported in modern day postmenopausal women [3,4] and potentially related to prolonged estrogen stimulus in females and males with hormonal imbalance [2]. Hershkovitz and colleagues classified HFI by the extent of bony overgrowth in the frontal bone region of the endocranium. A summary of this classification can be seen in Table 1 [2].
Case Report
The donor was a 77-year-old female at her time of death from “cryptogenic liver disease”. She was of European dissent and had multiple co-morbidities. Some were listed in her health history while others were observed during dissection and included: cardiopulmonary, urogenital, and multiple musculoskeletal conditions. Please see Table 2 for a complete list of the donor’s health history.
table 2: Health history.
During a routine dissection of the donor’s calvaria in the human anatomy laboratory at Elon University, Elon, North Carolina; it was noted that she had significant bony overgrowth of the endocranium. Bony overgrowth was diffuse throughout the frontal bone extending slightly into the parietal region with midline sparing (Figure 1). Cranial thickness was noted at multiple points on the calvaria using digital calipers. Bony overgrowth ranged from 1.0 cm thickness in the temporal region, to 1.3 cm adjacent to midline in the frontal region. Large individual nodules were located along either side of the frontal crest at the intersection of the frontal and parietal bones. The largest nodules measured 2.08 cm on the right and 1.81 cm in thickness on the left (Figure 2). As a result, the pathway of the middle meningeal artery was tortuous. In addition there was significant depression bilaterally of the frontal lobes and surrounding neural tissues (Figure 3).
Results
HFI presents in predominantly postmenopausal women, normally ranging in thickness from 0.5–1cm, and has been associated with diabetes, obesity, and chronic headaches. In the literature, there are multiple independent cases of HFI and subarachnoid calcification as was observed in this donor’s thoracic spine. Subarachnoid calcification is commonly observed during the dissection of human donors.
Etiology for this pathology remains unclear, but the plaques are often discovered in the lower thoracic and lumbar spinal regions [5–7]. In addition, there is no literature suggesting a relationship between HFI, subarachnoid calcification and other orthopedic conditions as were observed in this donor (Table 1).
As previously discussed, HFI can be categorized based on the extent of bony overgrowth. In this case the donor exhibited Type D, which involves the most extensive overgrowth (more than 50%) of the frontal bone and is continuous throughout. As a result, there was significant compression of the donor’s meninges, meningeal blood supply and specific regions of neural tissues in the frontal lobe including: Broca’s area, the premotor cortex, and the prefrontal association cortex. Compression in these areas of the brain could result in headaches and personality disturbances (ranging from passive to disinhibited), as well as impairments with cognition, motor planning, speech, eye movement, nonverbal communication, and memory [8].
Conclusion
There is currently no documented underlying pathology that connects HFI with all of the comorbidities observed in this donor. As a result of HFI, patients may have significant cognitive impairments in addition to other medical conditions. There is a need for continued efforts in reporting cases of HFI along with a complete assessment for co-morbidities to better understand this pathology and the clinical implications for patients.
Acknowledgements
Special thanks to Dr. Stewart Holt for assisting with obtaining images for this case report and Angela Susan Ramer for assisting with the literature search.
References
- Moore S. Hyperostosis Cranii. Springfield, Illinois: Charles C. Thomas. 1955; 3–195.
- Hershkovitz I, Greenwald C, Rothschild BM, Latimer B, Dutour O, Jellema LM, Wish-Baratz S. Hyperostosis frontalis interna: an anthropological perspective. Am J Phys Anthropol. 1999; 109: 303–325.
- Talarico EF Jr, Prather AD, Hardt KD. A case of extensive hyperostosis frontalis interna in an 87-year-old female human cadaver. Clin Anat. 2008; 21: 259–268.
- Glab H, Szostek K, and Kaczanowski K. Hyperostosis frontalis interna, a genetic disease?: Two medieval cases from Southern Poland. Homo. 2006; 57: 19–27.
- Knoblich R and Olsen B. Calcified and ossified plaques of the spinal arachnoid membranes. J Neurosurg. 1966; 25: 275–279.
- Wijdicks CA, Williams JM. Spinal arachnoid calcifications. Clin Anat. 2007; 20: 521–523.
- Lovering RM, Anderson LD. Leptomeningeal plaques, a “common” finding. Clin Anat. 2006; 19: 696–697.
- Blumenfeld H. Neuroanatomy through clinical cases. Sunderland, MA, Sinauer Associates. 2002; 821–854.