Aberrant Anterior Portal Vein within the Extra Hepatic Portal Triad with Variation in the course of a Replaced Common Hepatic Artery
Received: 10-Aug-2021 Accepted Date: Jul 27, 2021; Published: 03-Sep-2021, DOI: 10.37532/1308-4038.21.14.129
Citation: Castellanos B, Ahmad S, Baez R et al. Aberrant Anterior Portal Vein within the Extra Hepatic Portal Triad with Variation in the course of a Replaced Common Hepatic Artery. Int J Anat Var. 2021;14(8):126-129.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The Hepatoduodenal ligament is a part of the lesser omentum that carries portal triad structures such as portal vein, common bile duct and proper hepatic artery to the liver. Following routine dissection of a 96-year-old female, a rare variation in location of the portal vein within the hepatoduodenal ligament was found. The portal vein is lying anterior to the common hepatic artery and common bile duct. The common hepatic artery was unusual in that it arises from the superior mesenteric artery bifurcating posterior to the portal vein on the right side into a gastroduodenal artery and an uncharacteristically short proper hepatic artery (PHA). The PHA quickly gave off the right and left hepatic arteries that coursed anterior to the portal vein before entering the liver. This is an aberrant type IX branching pattern from Michel’s classification of variant arterial hepatic anatomy, with significant difference in its anatomical position in relation to hepatoduodenal ligament and its arterial course in relation to the head and neck of the pancreas. This finding makes our case report very unique and clinically relevant in upper gastrointestinal surgeries and procedures.
Keywords
Anterior portal vein; Structures within the hepatoduodenal ligament; Replaced common hepatic artery; Celiac trunk variations, Hepatomesenteric trunk; Gastrosplenic trunk; Bifurcated celiac trunk
Introduction
The hepatic portal vein is contained within the hepatoduodenal ligament which is part of lesser omentum. The portal triad is formed by the portal vein (PV), the PHA and the common bile duct. The PV is formed by the unification of the superior mesenteric and the splenic vein. It is usually found posterior to the proper hepatic artery and the common bile duct (CBD) within the hepatoduodenal ligament as part of extra hepatic portal triad. About 75–80% of the blood that is received by the liver is from the portal vein [1].
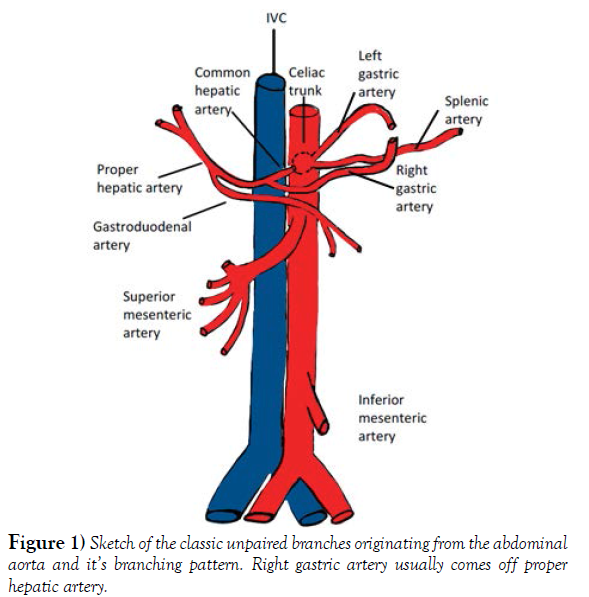
The abdominal aorta gives blood supply to the abdominal viscera via its three unpaired major branches: the celiac trunk (CT), the superior mesenteric artery (SMA) and inferior mesenteric artery (IMA). Typically, the celiac trunk gives off 3 main branches, the left gastric artery (LGA), the splenic artery (SA) and the common hepatic artery (CHA). The CHA is then divided into the gastro duodenal artery (GDA) and the proper hepatic artery (PHA). The PHA usually gives off the right gastric artery (RGA) before it further bifurcates into the right hepatic artery (RHA) and left hepatic artery (LHA) (Figure 1).
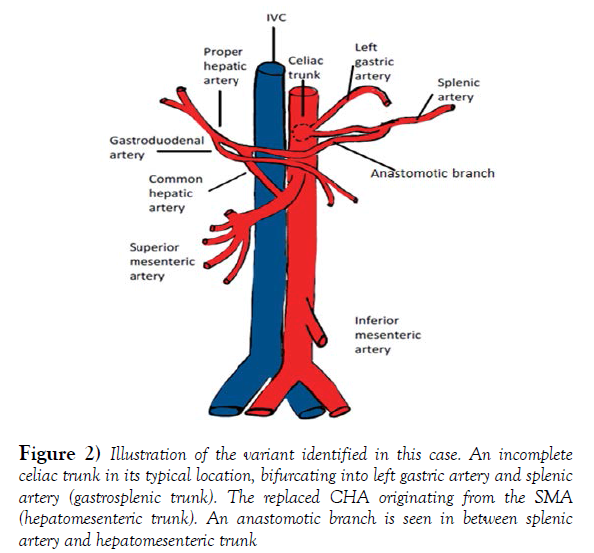
In the present case, we are describing an anterior portal vein within the extra hepatic portal triad and an incomplete celiac trunk bifurcating into left gastric and splenic artery, often referred as Gastro splenic trunk. The common hepatic artery is arising from the superior mesenteric artery (Hepato mesenteric trunk). The hepatic arterial branching pattern appears the same as Michel’s type IX classification yet the arterial course is significantly different, specifically with regards to the portal triad and the head and neck of the pancreas.
Out of the 3 unpaired branches arising from the abdominal aorta, celiac trunk variations have been described the most [2]. In fact, these variations have been classified by a few authors. Recent studies have shown that typical branching pattern of celiac trunk (trifurcation) was reported in about 84%- 90% of the cases and variations like bifurcation was reported in about 8% [3].
Knowledge of the normal anatomy of the portal vein, celiac trunk, and hepatic arteries as well as the variants, is the key to a successful diagnosis, appropriate preoperative planning, hepatobiliary surgeries, liver transplantation, portal embolization, trans jugular intrahepatic Porto systemic shunts, pancreatic oduodenectomy, chemotherapy and image guided interventions [4-8].
Case Report
During a routine dissection of the abdominal region for medical students in the Department of Anatomy at Touro College of Osteopathic Medicine. The anterior abdominal wall of a 96-year-old Caucasian female donor, was inspected and showed no evidence of surgical interventions. The wall was then dissected and reflected following the instructions from Grants dissector. The lesser omentum, the vessels and ducts within the Hepatoduodenal ligament were inspected. The celiac trunk, the SMA and the IMA along with their branches were traced to their destinations and dissected. Also following the same procedure, dissection of head and neck of pancreas was performed emphasizing on the formation of the Portal Vein. An infrequent branching pattern and course was observed. Further dissection of the blood supply to the abdominal viscera was performed, the vessels were colored and the specimen was photographed.
Discussion
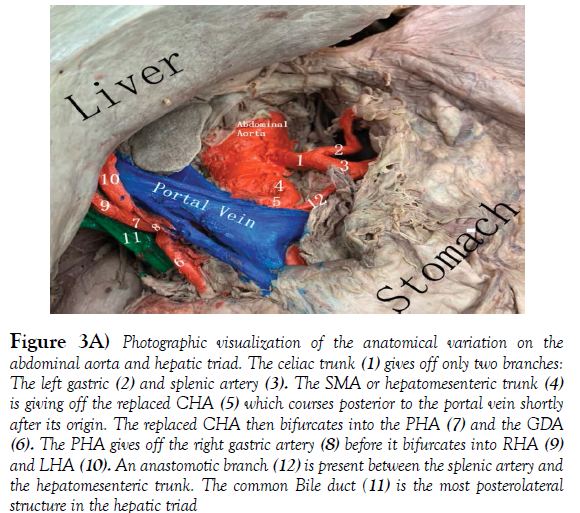
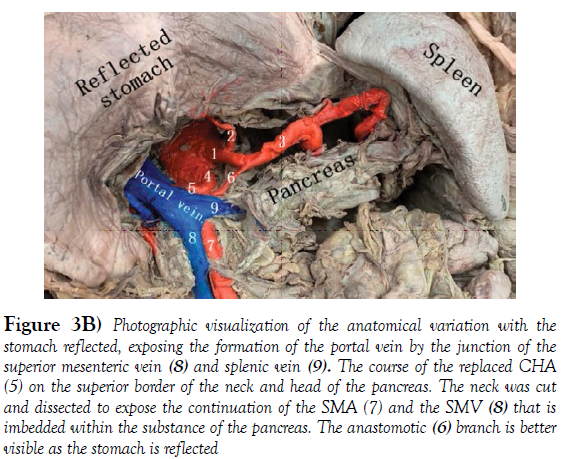
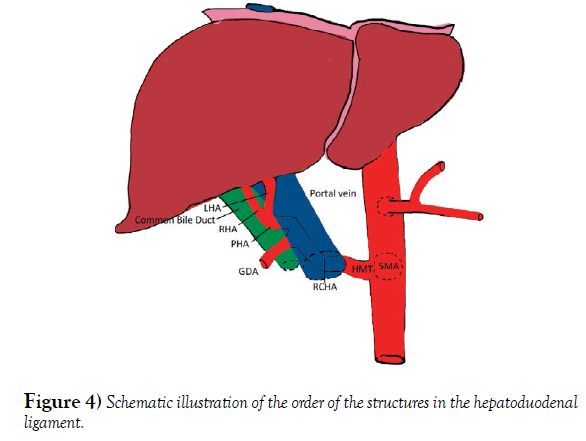
The hepatic portal vein is formed by the merger of the superior mesenteric and splenic veins, just posterior to the neck of the pancreas. In about 90% of the cases the portal vein shows a typical posterior location in the extra hepatic portal triad, variations are not frequent. The normal arrangement of the portal triad structures within the hepatoduodenal ligament is as follows: Portal vein is the most posterior structure with the PHA on the anteromedial wall of the PV, and the common bile duct (CBD) running alongside the lateral wall of the PHA [9]. The order of structures within the portal triad showed variations in the donor although the formation of the portal vein remained normal. The common bile duct was the most poster lateral structure, the PHA coursed anterior and medial to the CBD and the portal vein was the most anteromedial structure (Figure 3A, 3B).
During exploration of arterial branching pattern of the upper abdominal vasculature, we found the celiac trunk from the anterior surface of the abdominal aorta at the typical vertebral level, between the 12th thoracic vertebrae (T12) and the 1st lumbar vertebrae(L1). It bifurcated into the left gastric and splenic arteries (Figure 2). The Gastro splenic trunk, according to the Uflacker’s system is classified as a type V variation of the celiac trunk (Table 1) [10].
| Type | Variation |
|---|---|
| I | Classic celiac trunk |
| II | Hepatosplenic trunk |
| III | Hepatogastric trunk |
| IV | Hepatosplenomesenteric trunk |
| V | Gastrosplenic trunk |
| VI | Celiac-mesenteric trunk |
| VII | Celiac-Colic trunk |
| VIII | No Celiac trunk |
TABLE 1 Celiac trunk variations. Uflacker’s classification.
Figure 2) Illustration of the variant identified in this case. An incomplete celiac trunk in its typical location, bifurcating into left gastric artery and splenic artery (gastrosplenic trunk). The replaced CHA originating from the SMA (hepatomesenteric trunk). An anastomotic branch is seen in between splenic artery and hepatomesenteric trunk
Figure 3A) Photographic visualization of the anatomical variation on the abdominal aorta and hepatic triad. The celiac trunk (1) gives off only two branches: The left gastric (2) and splenic artery (3). The SMA or hepatomesenteric trunk (4) is giving off the replaced CHA (5) which courses posterior to the portal vein shortly after its origin. The replaced CHA then bifurcates into the PHA (7) and the GDA (6). The PHA gives off the right gastric artery (8) before it bifurcates into RHA (9) and LHA (10). An anastomotic branch (12) is present between the splenic artery and the hepatomesenteric trunk. The common Bile duct (11) is the most posterolateral structure in the hepatic triad
Figure 3B) Photographic visualization of the anatomical variation with the stomach reflected, exposing the formation of the portal vein by the junction of the superior mesenteric vein (8) and splenic vein (9). The course of the replaced CHA (5) on the superior border of the neck and head of the pancreas. The neck was cut and dissected to expose the continuation of the SMA (7) and the SMV (8) that is imbedded within the substance of the pancreas. The anastomotic (6) branch is better visible as the stomach is reflected
The third branch that typically arises from the celiac trunk, the common hepatic artery branched off of the superior mesenteric artery commonly referred as Replaced Common Hepatic artery (RCHA) [11]. Anatomical variations of the hepatic arterial system were classified by Michel’s; Hiatt later on modified Michel’s classification. We categorized our arterial findings based on both classifications.
The Superior mesenteric artery emerged one-centimeter inferior to the celiac trunk at the level of L1 vertebra. The common hepatic artery arises from the SMA or also called Hepatomensenteric trunk, which traversed on the superior border of the pancreas without perforating the pancreatic substance and then crossed the portal vein posteriorly shortly after its origin [12]. The branching pattern of the RCHA was unremarkable, it bifurcated posterior to the portal vein on the right side into a Gastro duodenal artery and an uncharacteristically short proper hepatic artery. The PHA quickly gave off the right gastric artery and then bifurcated into the right and left hepatic arteries that coursed anterior to the portal vein right before entering the liver. There was an additional anastomotic branch connecting the hepatomesenteric trunk and the splenic artery, providing communication between branches of the first two unpaired branches of the abdominal aorta (Figure 4). The branching pattern observed in the Donor’s abdominal aorta has been classified as a type IX Michel’s variation and type V according to Hiatt’s (Table 2). These replaced arteries are usually found running lateral to the portal vein, posterior to the head of the pancreas and entering the free edge of the hepatoduodenal ligament poster lateral to the common bile duct [13].
| Hepatic arterial system | Michel’s Classification | Hiatt Classification |
|---|---|---|
| Normal anatomy | Type I | Type I |
| Replaced LHA originating from LGA | Type II | Type II |
| Replaced RHA originating from SMA | Type III | Type III |
| Type I and II association | Type IV | Type IV |
| Accessory LHA originating from LGA | Type V | Type II |
| Accessory RHA originating from SMA | Type VI | Type III |
| Accessory LHA originating from LGA + accessory RHA originating form SMA | Type VII | Type IV |
| Accessory LHA originating from LGA + replaced RHA originating from SMA | Type VIII | Type IV |
| CHA originating from SMA | Type IX | Type V |
| RHA and LHA originating from LGA | Type X | - |
| CHA originating from the Aorta | - | Type VI |
TABLE 2 Hepatic arterial systems variations. Michel’s and Hiatt’s classifications.
The abdominal aorta and the rest of its branches were inspected and dissected. The aorta was found to be tortuous in the thoracic cavity and followed the normal branching pattern.
The results reported here depart from the typical Michel’s Type IX classification in two areas: firstly, the replaced CHA coursed on the superior border of the head and neck of the pancreas, rather than through its substance or posterior to it and secondly, the PHA runs anteromedial instead of the typical poster lateral route in relation to the CBD and posterior to the portal vein within the hepatoduodenal ligament. These variations have not been reported before and make our case report unique.
Portal vein variations were reported earlier [14]. Portal vein is formed by selective involution of vitelline veins and dorsal anastomotic network surrounding the duodenum [15]. The embryological variations related to celiac trunk and the superior mesenteric artery were studied [16,17}. The ventral splanchnic (vitelline) arteries arising from the abdominal aorta gradually fuse and form the arteries in the dorsal mesentery of the gut. Initially, the roots are connected by ventral longitudinal anastomosis which later disappear to form the arteries of the primordial gut. The development of celiac trunk is through the union of the first three roots that represent the left gastric artery, the splenic artery and the common hepatic artery. In our case, longitudinal anastomosis associated with the first two roots regressed in a normal manner, while the one associated with the third root did not form and further arose from the fourth root. The fourth root gives rise to superior mesenteric artery that supplies the midgut. It has been reported that celiac mesenteric variations occur due to persistent ventral longitudinal anastomoses and uncommon regression of roots of vitelline arteries [18]. The current donor has a hepatomesenteric trunk that most likely arose from the fourth root [19].
In the past, variations in portal vein and arterial branching and patterns were clinically irrelevant, nowadays this tendency has changed. Recent studies have demonstrated that patients with variants in the celiac trunk had longer surgical time and more blood loss, compared to patients who displayed normal anatomy. In this way, angiographic studies are encouraged in patients with whom variant anatomies are found in, not to simply end at the branching patterns and arterial origins themselves, but to pay attention to the vessels course as well. Our reported type IX displayed an unusual position in the hepatic triad, resulting in serious consideration of surgical planning before any major intervention such as liver transplant.
Ideally, detection of these variations should take place before surgery. Knowledge and establishment of variant arterial anatomy provides a quick and efficient method to avoid complications during and after surgery. The decision about surgical approach and intraoperative procedures by the surgical team, should be made taking these variations into consideration. In surgeries like pancreatoduodenectomy the main difficulties that could result in RCHA variation are partial liver ischemia and necrosis [20, 21]. Surgeons can benefit from early detection and so avoid iatrogenic complications and malpractice.
During surgery, palpation of the portal triad also serves as a heuristic approach to quickly recognize the vascular anatomy and determine significant arterial anomalies. An absent pulse in the free edge of the hepatoduodenal ligament would suggest a replaced CHA. Although 61% of patients display normal hepatic arterial anatomy, palpation of the arterial pulse is encouraged in routine operations in which the portal triad is involved [22].
Therefore, it is crucial to detect variations in celiac trunk and superior mesenteric artery, which will help in avoiding complications associated with invasive upper abdominal procedures such as liver transplants, hepatobiliary interventions, laparoscopic surgeries, chemoembolization of liver malignancies including minimally invasive radiological procedures [23].
Conclusion
The coexistence of variations like this could make the clinical diagnosis of disease of the aortic arch branches and vascular thoracic outlet syndrome difficult, particularly, when the subclavian artery is involved. It should also be emphasized that the existence of the scalenus minimus and the compartmentalization of the interscalene triangle could be a cause of brachial plexopathy or isolated ventral nerve root and/or arterial compression syndromes in any of these narrow triangular spaces. This would also complicate preprocedural diagnosis, intraprocedural approach and postprocedural outcomes when dealing with the roots of the brachial plexus and the subclavian artery in the triangle. Therefore, surgeons, radiologists, anesthesiologists and others involved in carrying out various procedures in these areas should be cognizant of such variations in order to make the correct diagnosis, apply the suitable approach and institute the proper treatment for a better clinical outcome.
Conflict of Interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research did not utilize grant funds.
Acknowledgement
The authors thank Nestor D. Rodriguez and Nicholas Vanterpool for their contribution to this project.
REFERENCES
- Moore KL, Dalley AF, Agur A. Clinically oriented anatomy (8th ed.). Lippincott Williams and Wilkins. (2017).
- Hemamalini. Variations in the branching pattern of the celiac trunk and its clinical significance. Anat Cell Biol. 2018;51(3):143-149.
- Selvaraj L, Sundaramurthi I. Study of Normal Branching Pattern of the Coeliac Trunk and its Variations Using CT Angiography. JCDR. 2015;9(9):1–4.
- Bhardwaj, Nikha. Anomalous Origins of Hepatic Artery and Its Significance for Hepatobiliary Surgery. J Anat Soc India. (2010);59:173-176.
- Ugurel MS, Battal B, Bozlar U, et al. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. BJR. (2010);83(992): 661–667.
- Araujo Neto SA, Franca HA, de Mello Júnior. Anatomical variations of the celiac trunk and hepatic arterial system: an analysis using multidetector computed tomography angiography. Radiologia brasileira. (2015);48(6): 358–362.
- Sureka B, Patidar Y, Bansal K et al. Portal vein variations in 1000 patients: surgical and radiological importance. BJR. (2015); 88:10-55
- Iqbal S, Iqbal R, Iqbal F. Surgical Implications of Portal Vein Variations and Liver Segmentations: A Recent Update. JCDR. (2017);11(2):1-5.
- Bianchi H, Algieri, Rubén S. Multiple Anatomical Variations of the Hepatic Pedicle. Int J Morphol 2014;32. 782-785.
- Achantani K, Raju P, Kumar R. Variants of Coeliac Trunk, Hepatic Artery and Renal Arteries in Puducherry Population. (2018).
- Kahn CI, MacNeil M, Fanola CL. Complex arterial patterning in an anatomical donor. Transl Res Anat 12;11-19.
- Furukawa H, Shimada K, Iwata R et al. A replaced common hepatic artery running through the pancreatic parenchyma. Surgery. 2000;127(6): 711–712.
- Michels NA. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Philadelphia: J.B. Lippincott Co. 1955
- Hu GH, Shen LG, Yang J et al. Insight into congenital absence of the portal vein: is it rare? World J Gastroenterol 2008;14:5969–79.
- [15] Walsh G, Williams MP. Congenital anomalies of the portal venous system: CT appearance with embryological considerations. Clin Radiol 1995; 50: 174–6.
- Tandler J. Uberdie Varietaten der arteriacoelia caunderen Entwiklung. Anat Hefte. 1904; 25:473-500.
- Morita M. Reports and conception of three anomalous cases of the superior mesenteric arteries. Igaku Kenkyu. 1935; 9:
- Ramesh CS, Joshi S, Gupta KK, et al. Celiacomesenteric trunk and its variants a multidetector row computed tomographic study. J Anat Soc India. 2015;64(1):32-41.
- Poorwa B. The Hepatomesentric Trunk. JCDR. 2013;7(2): 386-388.
- Sitarz R, Berbecka M, Mielko J et al. Awareness of hepatic arterial variants is required in surgical oncology decision making strategy: Case report and review of literature. Oncology letters, 2018;15(5), 6251–6256.
- Shukla PJ, Barreto SG, Kulkarni A et al. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17(1):186–193.
- Kardile PB, Ughade JM, Ughade MN et al. Anomalous origin of the hepatic artery from the hepatomesenteric trunk. JCDR. 2013;7(2), 386–388.