Advances in pharmacological therapy in reduced left ventricular heart failure patients with implantable cardiac defibrillator and cardiac resynchronization
2 Hospital Universitario de Elche Vinalopó, Universidad Católica de Murcia, Alicante, Spain
3 Grupo HM Hospitales, Universidad CEU San Pablo, Madrid, Spain, Email: Nunez@yahoo.com
Received: 28-Dec-2017 Accepted Date: Jan 10, 2018; Published: 14-Jan-2018
Citation: Carlos de D, Gonzalez-Torres L, Jose Maria N, et al. Advances in pharmacological therapy in reduced left ventricular heart failure patients with implantable cardiac defibrillator and cardiac resynchronization. Clin Cardiol J 2018;2(1):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
cardiac death (SCD) is a major cause of death in reduced left ventricular ejection fraction heart failure (rEFHF). Implantable cardiac defibrillator (ICD) significantly reduces SCD in rEFHF. Optimal heart failure medical therapy, including the blockage of renin-angiotensin system (ACEI or ARB), aldosterone system (MRA) or adrenergic system (BBK), has also proven to reduce SCD in these patients. Promisingly, a combined angiotensin receptor and neprilysin inhibition (ARNI) as compared to ACEI has demonstrated to further decrease SCD in rEFHF (by 22%). The decrease of myocardial wall tension induced by ARNI leads to a reduction in cardiac remodeling, fibrosis and repolarization dispersion, all major markers for ventricular arrhythmias. Therefore, ICD plus pharmacological therapy with ARNI, BBK and MRA are complementary tools to combat SCD.
Keywords
Cardiac resynchronization Therapy; Heart failure; Sudden cardiac death; Implantable cardiac defibrillator
Abbreviations
CRT: Cardiac Resynchronization Therapy; rEFHF: Reduced Left Ventricular Ejection Fraction Heart Failure; NYHA: New York Heart Association; LVEF= Left Ventricle Ejection Fraction; LVEF: Left Ventricle Ejection Fraction; ACEI: Angiotensin Coverting Enzyme inhibitors; ARB: Angiotensin Receptor Blocker; ARNI: Angiotensin Receptor and Neprilysin Inhibitor; MRA: Mineralocorticoid Receptor Antagonist; BBK: Betablockers.
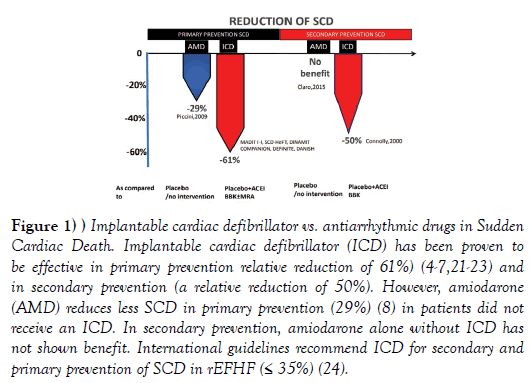
Decompensated congestive heart failure is frequently leading to a atrial and ventricular arrhythmias and vice versa. Cardiac arrhythmias, such as atrial fibrillation, frequent premature ventricular beats or a repetitive/ incessant ventricular tachycardia, can produce reduced left ventricular ejection fraction heart failure (rEFHF) [1]. Conversely, sudden cardiac death (SCD) due to ventricular arrhythmias is a major cause of death in rEFHF [2]. Implantable cardiac defibrillator (ICD) significantly reduces SCD in rEFHF (by~50% to 60%), [3-7] whereas antiarrhythmic drugs have failed to reduce the risk of SCD as effectively as ICDs (Figure 1) [6,8,9]. In addition, antiarrhythmic drugs are associated to adverse side effects. There is no doubt that ICD is an efficacious strategy for preventing SCD in patients with left ventricular ejection fraction (LVEF) ≤ 35%, however the following considerations should be done: 1) ICD is preventing 50-60% of SCD, not 100% of SCD in rEFHF, 2) Not all patients at high risk of SCD are ICD candidates, including those patients with New York Heart Association (NYHA) class IV heart failure, and 3) its worldwide use is limited by cost and access to the technology.
Figure 1: Implantable cardiac defibrillator vs. antiarrhythmic drugs in Sudden Cardiac Death. Implantable cardiac defibrillator (ICD) has been proven to be effective in primary prevention relative reduction of 61%) (4-7,21-23) and in secondary prevention (a relative reduction of 50%). However, amiodarone (AMD) reduces less SCD in primary prevention (29%) (8) in patients did not receive an ICD. In secondary prevention, amiodarone alone without ICD has not shown benefit. International guidelines recommend ICD for secondary and primary prevention of SCD in rEFHF (≤ 35%) (24).
Therefore, physicians and researchers continue searching for approaches to reduce SCD in these patients.
Therefore, physicians and researchers continue searching for approaches to reduce SCD in these patients.
Literature Review
Optimization of heart failure medical therapy to reduce sudden cardiac death
Patients with rEFHF have benefited from blocking three pathways: renin-angiotensin system (with angiotensin coverting enzyme inhibitors; ACEI or angiotensin receptor blocker; ARB), aldosterone system (with mineralocorticoid receptor antagonist, MRA) and adrenergic system (betablockers, BBK) [1].
The blockage of these pathways has decreased global mortality and SCD in rEFHF patients.
Anatomical factors (such as fibrosis or cardiac remodeling) and/or electrophysiological factors (such as repolarization dispersion) play a major role in the development of sustained ventricular arrhythmias.
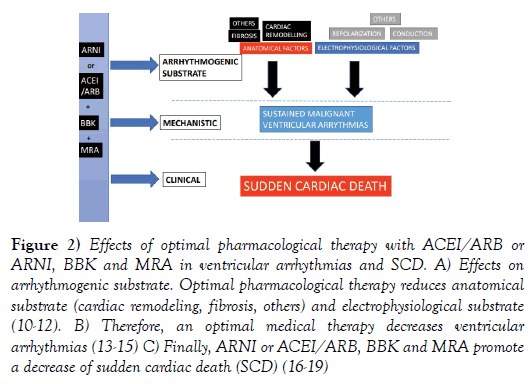
Numerous studies have proven that ACEi/ ARB, BBK and MRA reduced cardiac remodeling, fibrosis and dispersion of electrophysiological properties [10-12], all major markers for malignant ventricular arrhythmias. These mechanistic studies have been corroborated by large clinical studies. ACEi/ ARB, BBK and MRA have reduced ventricular arrhythmias [13-15] and SCD (Figure 2) [16-18].
Figure 2: Effects of optimal pharmacological therapy with ACEI/ARB or ARNI, BBK and MRA in ventricular arrhythmias and SCD. A) Effects on arrhythmogenic substrate. Optimal pharmacological therapy reduces anatomical substrate (cardiac remodeling, fibrosis, others) and electrophysiological substrate (10-12). B) Therefore, an optimal medical therapy decreases ventricular arrhythmias (13-15) C) Finally, ARNI or ACEI/ARB, BBK and MRA promote a decrease of sudden cardiac death (SCD) (16-19)
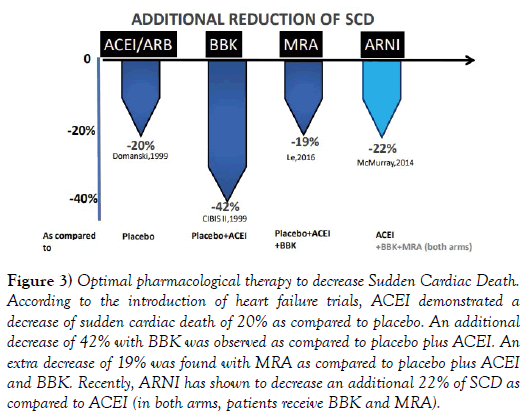
Considering the temporal sequence of rEFHF trials (Figure 3), ACEi/ARB decreased SCD by 20% [17]. Then, BBK reduced SCD by an additional 42% [16]. Subsequently, MRA demonstrated an extra reduction of 19% of SCD. [1] Recently, a combined angiotensin receptor and neprilysin inhibition (Sacubitril-Valsartan, ARNI) [19] has demonstrated greater benefit as compared to ACEI in decreasing SCD (by 22%). ARNI prevents the degradation of beneficial natriuretic peptides, decreasing myocardial wall stress, reducing cardiac remodeling and fibrosis, preventing ventricular arrhythmias as given with BBK and MRA [20].
Figure 3: Optimal pharmacological therapy to decrease Sudden Cardiac Death. According to the introduction of heart failure trials, ACEI demonstrated a decrease of sudden cardiac death of 20% as compared to placebo. An additional decrease of 42% with BBK was observed as compared to placebo plus ACEI. An extra decrease of 19% was found with MRA as compared to placebo plus ACEI and BBK. Recently, ARNI has shown to decrease an additional 22% of SCD as compared to ACEI (in both arms, patients receive BBK and MRA).
Pharmacological medical therapy in primary prevention ICD trials
Although clinical evidence is more solid in ischemic than in non-ischemic rEFHF, ICD reduces all-cause mortality by decreasing SCD as primary prevention in both ischemic and non-ischemic rEFHF. [4-7]
All-cause mortality benefit observed in these positive ICD trials is mainly due to the reduction in SCD.
In contrast, large studies such as the DINAMIT, [21] the DEFINITE [22] and the DANISH trials [23] failed to prove a decrease of global mortality from ICD in ischemic (within 40 days after myocardial infarction) or in non-ischemic rEFHF. In these negative trials, ICD also produce a relative reduction of SCD of ~50% to 60%. However, this decrease of SCD was not enough to generate a significant decrease in global mortality. The DANISH and DEFINITE trials studied non-ischemic rEFHF patients. In this population, the rate of SCD without ICD is ~6-8%, inferior to the rate in ischemic rEFHF (~10%). In addition to potentials explanations related to the type of rEFHF (non-ischemic in DEFINITE and DANISH trials) or the timing of ICD implantation (≤ 40 days post-myocardial infarction in DINAMIT trial), we analyzed others potential causes such as differences in pharmacological therapy.
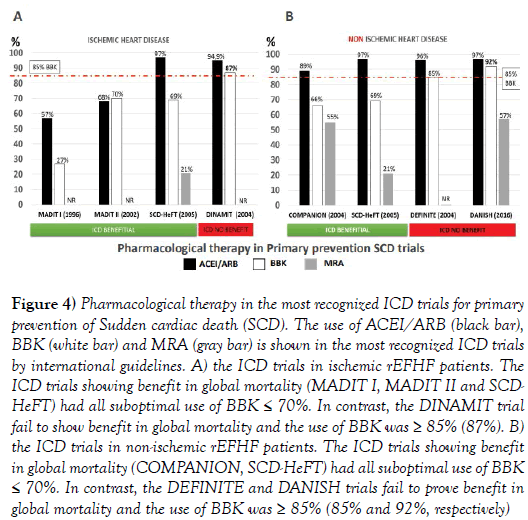
Figure 4 shows the use of ACEi/ ARB, BBK and MRA in the most relevant ICD trials for primary prevention of SCD.
Figure 4: Pharmacological therapy in the most recognized ICD trials for primary prevention of Sudden cardiac death (SCD). The use of ACEI/ARB (black bar), BBK (white bar) and MRA (gray bar) is shown in the most recognized ICD trials by international guidelines. A) the ICD trials in ischemic rEFHF patients. The ICD trials showing benefit in global mortality (MADIT I, MADIT II and SCDHeFT) had all suboptimal use of BBK ≤ 70%. In contrast, the DINAMIT trial fail to show benefit in global mortality and the use of BBK was ≥ 85% (87%). B) the ICD trials in non-ischemic rEFHF patients. The ICD trials showing benefit in global mortality (COMPANION, SCD-HeFT) had all suboptimal use of BBK ≤ 70%. In contrast, the DEFINITE and DANISH trials fail to prove benefit in global mortality and the use of BBK was ≥ 85% (85% and 92%, respectively)
Please note that the BBK use is different in trials in which ICD was beneficial or not beneficial in global mortality. In trials in which ICD was beneficial, the BBK use was suboptimal (≤70%). In trials in which ICD was not beneficial (DEFINITE, DANISH and DINAMIT trials), the BBK use was always ≥ 85%.
Figure 5 shows global mortality and SCD (primary prevention) in ICD trials with favorable results in global mortality (MADIT I, MADIT II, SCDHeFT, COMPANION trials, all with BBK use ≤ 70%) and in ICD trials with negative results (DINAMIT, DEFINITE and DANISH trials, all with BBK use ≥ 85%).
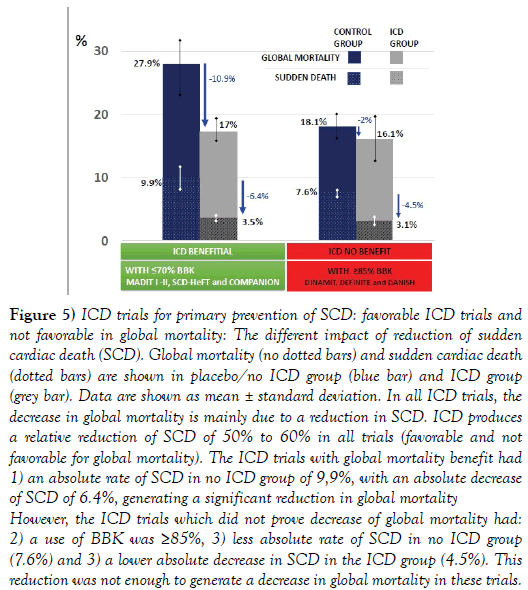
Figure 5: ICD trials for primary prevention of SCD: favorable ICD trials and not favorable in global mortality: The different impact of reduction of sudden cardiac death (SCD). Global mortality (no dotted bars) and sudden cardiac death (dotted bars) are shown in placebo/no ICD group (blue bar) and ICD group (grey bar). Data are shown as mean ± standard deviation. In all ICD trials, the decrease in global mortality is mainly due to a reduction in SCD. ICD produces a relative reduction of SCD of 50% to 60% in all trials (favorable and not favorable for global mortality). The ICD trials with global mortality benefit had 1) an absolute rate of SCD in no ICD group of 9,9%, with an absolute decrease of SCD of 6.4%, generating a significant reduction in global mortality However, the ICD trials which did not prove decrease of global mortality had: 2) a use of BBK was ≥85%, 3) less absolute rate of SCD in no ICD group (7.6%) and 3) a lower absolute decrease in SCD in the ICD group (4.5%). This reduction was not enough to generate a decrease in global mortality in these trials.
In the favorable ICD trials, the rate of SCD without ICD was higher (~10%) and ICD generated a higher absolute reduction of SCD (~6.4%).
In trials in which ICD was not beneficial, the rate of SCD without ICD was lower (~7.6%) and ICD produced a lower absolute reduction of SCD (~4.5%).
Therefore, the higher use of BBK in ICD trials, the lower rate of SCD without ICD and lower reduction rate of SCD in the ICD group, making more complicated to demonstrate differences impacting in global mortality.
Optimal medical therapy and ICD indications
The DANISH trial proved the importance of an optimal medical therapy in non- ischemic rEFHF patients. In this trial, ICD did not reduce global mortality despite a significant reduction of SCD, as patients were well treated with heart failure pharmacological therapy. In the DANISH trial, patients received 97% of ACEI/ARB, 92% of BBK and 57% of MRA. However, tolerance to ACEI/ARB, BBK and MRA is not always possible. Moreover, the results of DANISH may be attributed to a prolonged follow-up (>5 years) and the time course nature of rEFHF, which progresses along time, increasing gradually heart failure mortality. The recently published AHA/ACC/ HRS guidelines for ventricular arrhythmias prevention, still recommend implanting an ICD in non-ischemic rEFHF [24]. However, the DANISH, the DINAMIT and the DEFINITE trials highlight the relevance of an optimal medical therapy to reduce SCD. It is important to take in consideration that patients who are not receiving ACEI/ARB, BBK and/or MRA, due to lack of optimization or intolerance, are more vulnerable for SCD. Optimization of medical therapy (≥ 3 months) is not only needed before implanting an ICD. After implanting an ICD, optimization of medical therapy is also crucial, with ARNI, BBK and MRA (Figure 3) [13-15,20].
Although ICD significantly reduces SCD, does not prevent all the events of SCD such as cardiogenic shock, incessant ventricular tachycardia or electromechanical dissociation. [4-7,21-23].
It is well recognized that, the indications of ICD have been more debated in non-ischemic rEFHF. The introduction of a new therapy, sacubitrilvalsartan (ARNI), which reduces SCD by an extra ~22% as compared to ACEI, will necessarily have an impact in SCD endpoints and global mortality in future ICD trials. The rEFHF therapies have dramatically advanced in the last decade. ARNI improves NYHA functional class [19] and increases left ventricular ejection fraction (LVEF) by ̴ 5% [20]. There are the major findings that we use to indicate an ICD. New ICD trials will be carried out to elucidate the value of ICD in both rEFHF, midllerange EFHF and preserved EFHF.
Pharmacological strategies to decrease ventricular arrhythmias and ICD shocks
The prevention of SCD does not end after ICD implantation and continuous optimization of medical therapy improves clinical outcomes.
There is a robust correlation between ICD shocks and increased mortality in ICD recipients [24,25]. It is uncertain if shocks are purely a marker of a more severe cardiovascular disease or directly contribute to the increase in mortality.
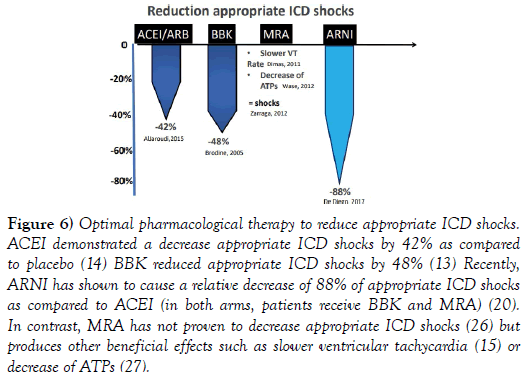
A variety of strategies have been implemented to diminish ICD shocks such as ICD programming, remote monitor, antiarrhythmic drugs or catheter ablation. Optimization of pharmacological therapy is also an effective method to diminish ICD shocks (Figure 6). The use of ACEi and/or BBK have associated to a reduction of appropriate ICD shocks [13,14].
Figure 6: Optimal pharmacological therapy to reduce appropriate ICD shocks. ACEI demonstrated a decrease appropriate ICD shocks by 42% as compared to placebo (14) BBK reduced appropriate ICD shocks by 48% (13) Recently, ARNI has shown to cause a relative decrease of 88% of appropriate ICD shocks as compared to ACEI (in both arms, patients receive BBK and MRA) (20). In contrast, MRA has not proven to decrease appropriate ICD shocks (26) but produces other beneficial effects such as slower ventricular tachycardia (15) or decrease of ATPs (27).
Recently, an additional decline in ICD shocks was found after ARNI as compared to ACEI [20]. In contrast, MRA did not reduce ICD shocks, [26] but other beneficial effects were demonstrated such as decrease of ATPs or slower rate of ventricular tachycardia [15,27] (Figure 7).
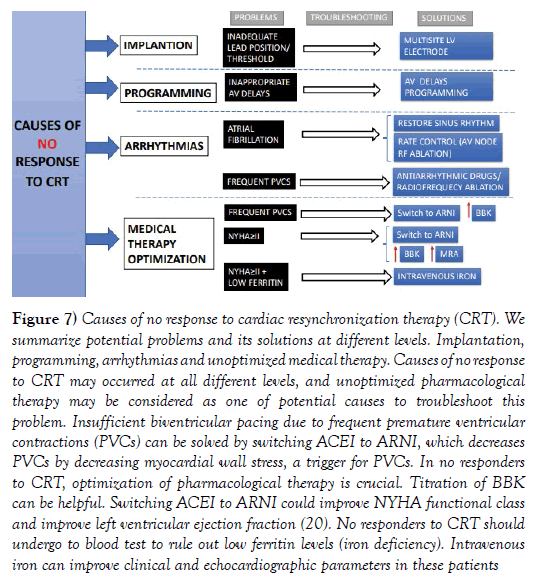
Figure 7: Causes of no response to cardiac resynchronization therapy (CRT). We summarize potential problems and its solutions at different levels. Implantation, programming, arrhythmias and unoptimized medical therapy. Causes of no response to CRT may occurred at all different levels, and unoptimized pharmacological therapy may be considered as one of potential causes to troubleshoot this problem. Insufficient biventricular pacing due to frequent premature ventricular contractions (PVCs) can be solved by switching ACEI to ARNI, which decreases PVCs by decreasing myocardial wall stress, a trigger for PVCs. In no responders to CRT, optimization of pharmacological therapy is crucial. Titration of BBK can be helpful. Switching ACEI to ARNI could improve NYHA functional class and improve left ventricular ejection fraction (20). No responders to CRT should undergo to blood test to rule out low ferritin levels (iron deficiency). Intravenous iron can improve clinical and echocardiographic parameters in these patients
Medical therapy approaches to improve response to CRT
In patients with cardiac resynchronization therapy (CRT), the percentage of biventricular pacing has prognostic implications. An insufficient biventricular pacing is correlated to poor clinical outcomes and no response to CRT [28]. Different causes may impair the percentage of biventricular pacing such as inadequate AV delays, atrial fibrillation, intrinsic conducted sinus rhythm or frequent premature ventricular contractions (PVCs). Inappropriate AV delays can be fixed by device programming. Atrial fibrillation rate control can be achieved by atrioventricular node radiofrequency ablation. Pharmacological therapies can be also valuable to increase biventricular pacing percentage. Intrinsic conducting sinus rhythm can also decrease biventricular pacing, which can be solved optimizing BBK dosage.
Recently, sacubitril-valsartan has shown to significantly reduce PVC burden increasing biventricular pacing percentage [20].
About one third of patients does not respond to CRT. First, optimization of pharmacological therapy should be ensured. Patients with rEFHF and NYHA ≥II benefit from switching ACEI to ARNI. BBK and MRA titration should be optimized. Ferritin levels should be taken every 6-12 months. Iron deficiency is a powerful predictor of adverse outcomes in rEFHF patients [29] and also in CRT patients [30]. Iron deficiency is highly prevalent in CRT patients, up to 56% and correlates to worse NYHA functional class, poor reverse remodeling and heart failure hospitalization. Therefore, intravenous iron is highly recommended in CRT patients if NYHA ≥ II and low ferritin according to international guidelines [1].
Prognostic ICD/ICD-CRT events related to mortality and heart failure hospitalizations
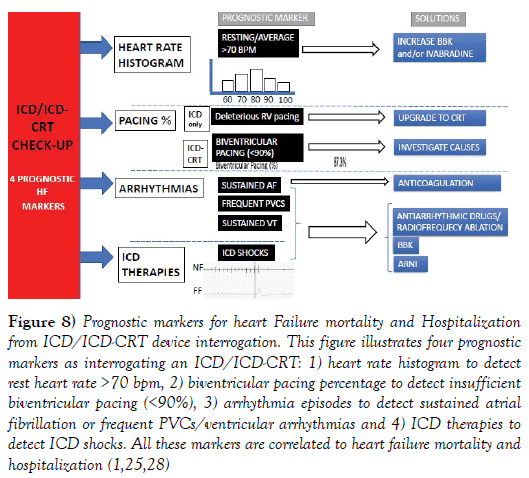
The ICD/ICD-CRT interrogation provides relevant prognostic markers for heart failure hospitalization and mortality in rEFHF. Figure 8 illustrates the four key points as interrogating an ICD/ICD-CRT: heart rate histogram to detect rest heart rate >70 bpm, biventricular pacing percentage to detect insufficient biventricular pacing (<90%), arrhythmia episodes to detect sustained atrial fibrillation or frequent PVCs/ventricular arrhythmias and ICD therapies to detect ICD shocks. All these markers correlate to poorer clinical outcomes and heart failure hospitalization. In ICD patients (without CRT), the percentage of right ventricular pacing is also a marker for worse prognosis, indicating the need for upgrading to ICD-CRT. Remote monitor is particularly beneficial transmitting these important markers in a monthly fashion [25].
Figure 8: Prognostic markers for heart Failure mortality and Hospitalization from ICD/ICD-CRT device interrogation. This figure illustrates four prognostic markers as interrogating an ICD/ICD-CRT: 1) heart rate histogram to detect rest heart rate >70 bpm, 2) biventricular pacing percentage to detect insufficient biventricular pacing (<90%), 3) arrhythmia episodes to detect sustained atrial fibrillation or frequent VCs/ventricular arrhythmias and 4) ICD therapies to detect ICD shocks. All these markers are correlated to heart failure mortality and hospitalization (1,25,28)
Future perspective: Heart failure-arrhythmia unit with simultaneous heart failure optimization and ICD interrogation
Heart failure units have been created worldwide to decrease mortality and heart failure hospitalizations. Heart failure units generally optimize medical therapy and are led by a general cardiologist with training in heart failure.
Patients with rEFHF also visit arrhythmia units, where ICD/ICD-CRT device is interrogated. These arrhythmia units are led by a electrophysiologist. Physical visits to arrhythmia unit are complemented with remote monitor of ICD devices, which is also reviewed by arrhythmia units.
Discussion
In most centers, heart failure unit visit (optimization medical treatment) and arrhythmia unit visit (ICD device interrogation) are not simultaneous, occurring in different days or visits. Therefore, important prognostic markers for heart failure hospitalization provided by ICD device interrogation are not immediately available in the heart failure unit visit. In our center, we created an integral unit with a heart failure cardiologist and an electrophysiologist reviewing patients simultaneously the same day in the same consultation. The components of our heart failure-arrhythmia unit, our proposed model, include: a nurse with heart failure training (for patient education and intravenous medications), a cardiologist with heart failure training (for optimizing medical treatment, providing an assistance telephone, performing echocardiogram, blood test with natriuretic peptide and ferritin levels) and a cardiologist with electrophysiology training to perform: Physical device interrogation and remote monitor device reviewing to detect heart failure markers, and to evaluate SCD risk. A single cardiologist with heart failure training and electrophysiology/device interrogation training can also perform point 2 and 3, optimizing human resources.
Conclusion
We anticipate that the future directions of heart failure treatment will include strengthening the collaboration between heart failure units and arrhythmia units with fruitful results in combating heart failure as recently published [20].
As Dr. professor Jerónimo Farré, a pioneer electrophysiologist and a visionary, said “if an electrophysiologist does not treat heart failure with medical therapy, she/he is not a real electrophysiologist, because the main cause of arrhythmias is due to heart failure.
REFERENCES
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-200.
- Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794-801.
- Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. antiarrhythmics vs implantable defibrillator study. Cardiac arrest study Hamburg. Canadian implantable defibrillator study. Eur Heart J. 2000;21:2071-8.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-40.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-83.
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-37.
- Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-50.
- Piccini JP, Berger JS, O'Connor CM. Amiodarone for the prevention of sudden cardiac death: A meta-analysis of randomized controlled trials. Eur Heart J. 2009;30:1245-53.
- Claro JC, Candia R, Rada G, et al. Amiodarone versus other pharmacological interventions for prevention of sudden cardiac death. Cochrane Database Syst Rev. 2015:CD008093.
- Lijnen P, Petrov V. Antagonism of the renin-angiotensin system, hypertrophy and gene expression in cardiac myocytes. Methods Find Exp Clin Pharmacol. 1999;21:363-74.
- Wei S, Chow LT, Sanderson JE. Effect of carvedilol in comparison with metoprolol on myocardial collagen postinfarction. J Am Coll Cardiol. 2000;36:276-81.
- Kassotis J, Mongwa M, Reddy CV. Effects of angiotensin-converting enzyme inhibitor therapy on QT dispersion post-acute myocardial infarction. Pacing Clin Electrophysiol. 2003;26:843-8.
- Brodine WN, Tung RT, Lee JK, et al. Effects of beta-blockers on implantable cardioverter defibrillator therapy and survival in the patients with ischemic cardiomyopathy (from the Multicenter Automatic Defibrillator Implantation Trial-II). Am J Cardiol. 2005;96:691-5.
- AlJaroudi WA, Refaat MM, Habib RH, et al. Effect of angiotensin-converting enzyme inhibitors and receptor blockers on appropriate implantable cardiac defibrillator shock in patients with severe systolic heart failure (from the GRADE Multicenter Study). Am J Cardiol. 2015;115:924-31.
- Dimas V, Ayers C, Daniels J, et al. Spironolactone therapy is associated with reduced ventricular tachycardia rate in patients with cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:309-14.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9-13.
- Domanski MJ, Exner DV, Borkowf CB, et al. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta-analysis of randomized clinical trials. J Am Coll Cardiol. 1999;33:598-604.
- Willenheimer R, van Veldhuisen DJ, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426-35.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
- De Diego C, Gonzalez-Torres L, Nunez JM, et al. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm. 2017.
- Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481-8.
- Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-8.
- Kober L, Thune JJ, Nielsen JC, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221-30.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017.
- Li A, Kaura A, Sunderland N, et al. The significance of shocks in implantable cardioverter defibrillator recipients. Arrhythm Electrophysiol Rev. 2016;5:110-6.
- Zarraga IG, Dougherty CM, MacMurdy KS, et al. The effect of spironolactone on ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2012;5:739-47.
- Wase A, Garikipati N, Mufti O, et al. Effect of spironolactone on ventricular arrhythmias in patients with left ventricular systolic dysfunction and implantable cardioverter defibrillators. Indian Heart J. 2012;64:123-7.
- Gasparini M, Galimberti P, Ceriotti C. The importance of increased percentage of biventricular pacing to improve clinical outcomes in patients receiving cardiac resynchronization therapy. Curr Opin Cardiol. 2013;28:50-4.
- Drozd M, Jankowska EA, Banasiak W, et al. Iron therapy in patients with heart failure and iron deficiency: Review of iron preparations for practitioners. Am J Cardiovasc Drugs. 2017;17:183-201.
- Martens P, Verbrugge F, Nijst P, et al. Impact of iron deficiency on response to and remodeling after cardiac resynchronization therapy. Am J Cardiol. 2017;119:65-70.