Anatomic variation and introperative disposal of right hepatic artery in pancreaticoduodenectomy
Received: 01-Mar-2018 Accepted Date: May 24, 2018; Published: 05-Jun-2018, DOI: 10.37532/1308-4038.18.11.59
Citation: Cao X, Guan Q, Zhang F, et al. Anatomic variation and introperative disposal of right hepatic artery in pancreaticoduodenectomy. Int J Anat Var. 2018;11(2):59-62.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Anatomic variation of right hepatic artery (RHA) is not rare in clinical. we retrospective reviewed the clinical data of 179 patients underwent pancreaticoduodenectomy (PD) between January 2012 and June 2017 in Department of Hepatobiliary Surgery, Hospital of Binzhou Medical University, Shandong Province, China. 28 patients from them presented variant RHA including replaced RHA (rRHA) and accessory RHA (aRHA), and the incidence of aberrant RHA was about 15.64%. The variation was confirmed with preoperative imaging in 25 cases by contrast-enhanced computed tomographic (CT) and the preoperative diagnostic accuracy was up to 89.29% in our study. The duration of operation in variant group was prolonged than normal and there was a statistical difference between two groups, no difference was demonstrated in estimated amount of blood loss and postoperative complications including biliary fistula, pancreatic fistula, chylous fistula, intraperitoneal haemorrhage between two groups. It is essential for surgeons to be vigilant for rRHA or aRHA to avoid potentially disastrous complications and the best introperative disposal, only if transection and reconstruction is unavoidable, is to dissect and preserve despite it may cost much more time.
Keywords
Pancreaticoduodenectomy; Right hepatic artery; Variation
Abbreviations
aRHA: Accessory Right Hepatic Artery; CT: Computed Tomographic; CTA: Computed Tomography Angiography; GDA: Gastroduodenal Artery; IV: Inferior Vena Cava; LHA: Left Hepatic Artery; PD: Pancreaticoduodenectomy; PV: Portal Vein; rRHA: Replaced Right Hepatic Artery; RHA: Right Hepatic Artery; SMA: Superior Mesenteric Artery; SMV: Superior Mesenteric Vein.
Introduction
Anatomic variation of hepatic artery is not rare in abdominal surgery [1,2]. Early in 1966 Michels [3] put forward a point of view about newer anatomy of the liver and its variant blood supply and collateral circulation, and then drafted a classification about hepatic arterial anatomy. In which the incidence of variant hepatic artery was up to 45% and the anatomic variation of RHA was about 20%. The most common anomalies about variant RHA, according to the Michels classification, involved a replaced or accessory RHA originate from the superior mesenteric artery (SMA), were Michels type Ⅲ (11%) and type Ⅵ (7%) respectively. Both of which arise from the SMA, course behind or through the head of the pancreas, and run along the posterior aspect of the common bile duct. Recognition of the anatomic variations of RHA is more important than ever given recent advances in hepato biliary, pancreatic and liver transplantation surgery, especially in PD. Because major procedures lie in the head of pancreas and the right of hepatoduodenal ligament, once accidental ligation or cutoff of rRHA or aRHA happened, it may result in hepatic necrosis even hepatapostema, ischemic biliary injury or breakdown of biliary enteric anastomosis [4]. Besides, the presence of variant RHA may directly influence the local radicality of the resection especially in patients with pancreatic carcinoma or other malignant tumor [5]. In this report, we reviewed retrospectively the clinical data of 28 patients with variant RHA underwent PD between January 2012 and June 2017 in our institute, and analyzed preoperative diagnostic methods as well as intraoperative disposal of the variant RHA.
Patients and Methods
The study is a retrospective review and approved by the institutional ethics committee of Hospital of Binzhou Medical University, Shandong province, China.
In the study consecutive patients who underwent PD from January 2010 to June 2017 were identified, not only for benign but also for malignant pathology of the pancreas or of the periampullary region in the Department of Hepato Biliary Surgery, Hospital of Binzhou Medical University. All of the patients received CT examination preoperatively, a further examination of computed tomography angiography (CTA) was performed in patients may have variant RHA. All procedures of operation were performed by a single surgeon team with the same incison of upper middle abdominal incision, standard Whipple PD surgery including regional lymph nodes dissection and identical technique of digestive tract reconstruction.
In order to compare the influence of variant RHA on PD, we classified patients enrolled in our study into two groups: group of normal RHA and group of variant RHA. Most data of both groups were gathered from our clinical database, including the information about age, gender, duration of operation, estimated amount of perioperative blood loss, pathological diagnosis and incidence of postoperative complications such as biliary fistula, pancreatic fistula, chylous fistula, intraperitoneal hemorrhage and so on and so forth. The type of anatomic variation of RHA, according to the description in the surgical record, was classified with Michels classification.
The patient characteristics and outcomes of operation were described using descriptive statistics and expressed as the mean and standard deviation (SD). Data comparisons between the groups were performed with SPSS version 16.0 (IBM Co, Armonk, NY, USA). P value less than 0.05 was considered indicative of statistically significant differences.
Results
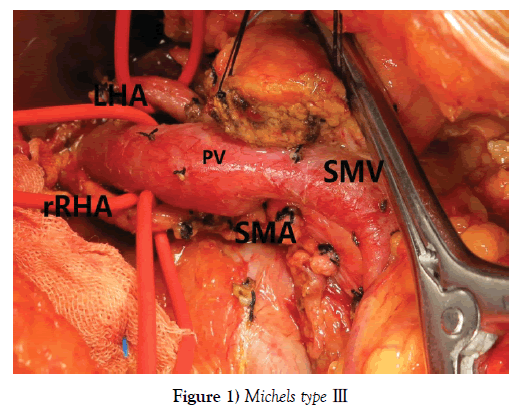
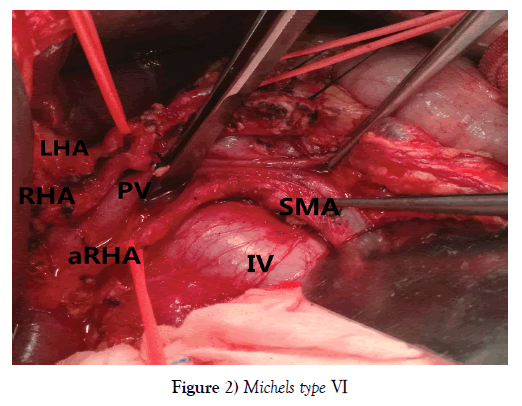
One hundred and seventy-nine PDs were performed during the period, among whom 151 patients (84.36%) with normal RHA and 28 (15.64%) with aberrant RHA, including 23 rRHA Michels type Ⅲ, 12.85% (Figure 1) and 5 aRHA Michels type Ⅵ, 2.79% (Figure 2), no other type of variant RHA was encountered in our study. The demographic characteristics of patients in each group are listed in Table 1.
| Normal RHA | Variant RHA | Total | |||
|---|---|---|---|---|---|
| Type Ⅲ | Type Ⅵ | ||||
| Age (mean ± SD years) | 58.52 ± 10.14 | 58.04 ± 10.73 | 59.20 ± 5.17 | 58.59 ± 9.94 | |
| Gender | |||||
| Male | 88 | 13 | 3 | 104 (58.10%) | |
| Female | 63 | 10 | 2 | 75 (41.90%) | |
| Postoperative pathology | |||||
| Cholangiocarcinoma | 38 | 7 | 1 | 46 (25.70%) | |
| Pancreatic adenocarcinoma | 31 | 7 | 2 | 40 (22.35%) | |
| Ampullary adenocarcinoma | 32 | 4 | 1 | 37 (20.67%) | |
| Duodenal adenocarcinoma | 28 | 3 | 1 | 32 (17.88%) | |
| Ampullary adenoma | 7 | 1 | 8 (4.47%) | ||
| Heterotopic pancreas | 4 | 4 (2.23%) | |||
| Chronic pancreatitis | 3 | 3 (1.68%) | |||
| Pancreatic neuroendocrine tumor | 2 | 1 | 3 (1.68%) | ||
| Gallbladder carcinoma | 1 | 1 (0.56%) | |||
| Others | 5 | 5 (2.79%) | |||
Table 1: Demographic characteristic of patients in each group
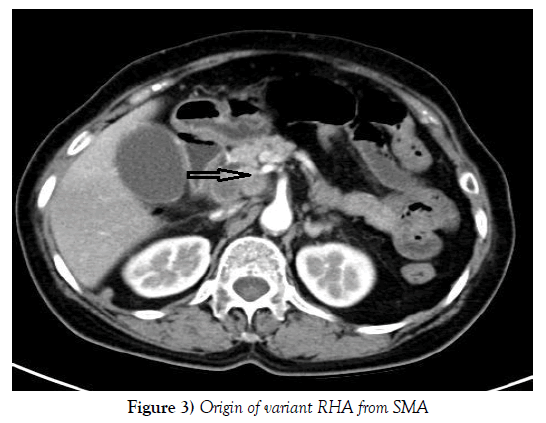
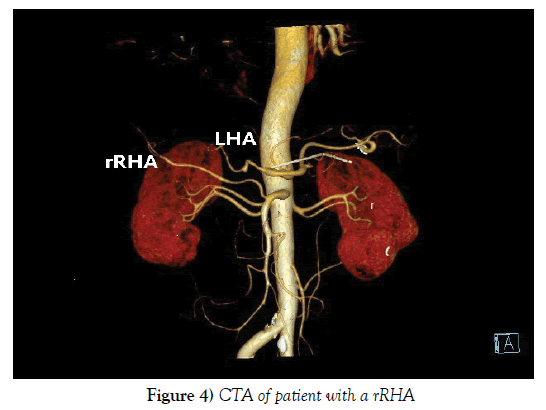
Compared the preoperative CT examination with intraoperative findings, 25 out of 28 patients with variant RHA were demonstrated before operation and the diagnostic accuracy of CT examination was up to 89.29% (Figures 3 and 4). All of those 3 failures were patients with variant RHA of Michels type Ⅵ whose diameter of aRHA was too thin to confirm preoperatively.
Although not all aberrant RHA were confirmed preoperatively, none of them suffered an accidental ligation or cutoff. Still, the introperative disposal of variant RHA cost much more time causing a prolonged duration of surgery (P<0.05), but there is no statistical difference in postoperative complications including estimated amout of perioperative blood loss, incidence of postoperative biliary fistula, pancreatic fistula, intraperitoneal hemorrhage (P>0.05). Comparisons of surgical parameter between two groups are listed in Table 2.
| Normal RHA (n=151) | Variant RHA (n=28) | P value | |
|---|---|---|---|
| Duration of operation (h) | 6.15 ± 1.93 | 8.33 ± 1.31 | 0.00* |
| Estimated amount of blood loss (ml) | 313.58 ± 238.25 | 367.14 ± 112.31 | 0.065 |
| Biliary fistula (%) | 8 (5.30%) | 2 (7.14%) | 1.000 |
| Pancreatic fistula (%) | 14 (9.27%) | 3 (10.71%) | 1.000 |
| Chylous fistula (%) | 3 (1.99%) | 1 (3.57%) | 1.000 |
| Intraperitoneal hemorrhage (%) | 7 (4.64%) | 2 (7.14%) | 1.000 |
| Hepatapostema (%) | 0 | 0 | -- |
| Mortality in hospital (%) | 8 (5.30%) | 2 (7.14%) | 1.000 |
Table 2: Comparison of parameters between two groups
Discussion
The first description of aberrant hepatic arteries was published in 1756 by Haller [6]. Variant patterns occurred in 24.3-49% of all individuals [7]. These arteries could be defined as accessory, occurring in addition to the normal arterial supply, or replaced, representing the primary arterial supply to the lobe. Biehl [8] analyzed preoperative visceral angiography of the hepatic arteries in 64 cases undergoing PD and reported that variant patterns of the hepatic arteries were found in 18.8% of the patients. Rong [9] reported that the incidence of aberrant hepatic artery was 34% for angiographic study of patients with pancreatic malignant neoplasms and 31% for cases undergoing PD. While in study [3] of Michels’ the incidence of aberrant hepatic artery was up to 45%. Although the incidence of variant hepatic artery may be controversial in these studies, all reports indicate that the anatomic variation of hepatic artery is not rare during the procedure of PD.
Among so many anatomic variations of hepatic artery, the most common and important variation in PD is variant RHA, especially type Ⅲ and Ⅵ in Michels classification. Because complications of injury of rRHA or aRHA include hepatic ischaemia and bilioenteric anastomosis breakdown [10]. Although hepatic parenchymal ischaemia may be minimized by compensatory portal venous blood flow and intrahepatic collateral flow, biliary fistula is likely to occur if arterial blood flow is not maintained to the remaining bile duct at the bilioenteric anastomosis. Most of the arterial supply to the common bile duct is derived from the retroduodenal artery, which is a branch of the gastroduodenal artery (GDA). The proximal biliary tree is the susceptible watershed area; it is supplied by the RHA, which, in combination with the retroduodenal artery, produces an arterial plexus at the 3-o’clock and 9-o’clock positions of the duct. Because the GDA is routinely sacrificed during PD, the remaining bile duct becomes completely reliant on the RHA for its blood supply [11], thus accidental ligation or cutoff of rRHA or aRHA will be bound to cause a series of disastrous complications.
Although high-quality images of the liver and pancreas are performed in nearly every patient before the operation, imaging methods are merely limited to MRI or CT [4], especially contrast-enhanced CT and subsequently CTA. The sensitivity, specificity, and accuracy of axial spiral CT in detection of variant hepatic artery were up to 96%, 87%, and 88% respectively according to the report of Chambers in 1995 [12]. Unfortunately, some small variant RHA may be easily obscured because of the thin diameter of artery especially of aRHA, just as shown in our research, 3 out 5 patients with aRHA were failed to confirm preoperative. Visceral angiography may be another effective mean to detect the rRHA or aRHA preoperatively and make up for the deficiency of CT, but it is invasive and seldom be used in clinical at present. But, undeniable is, with the development of digital medical technology, accuracy in detection of variant hepatic artery may be greatly improved in the future [13,14].
So far, the disposal of variant RHA includes four possible scenarios [15]: (i) sacrifice; (ii) preoperative embolization; (iii) dissection and preservation, (iv) transection and reconstruction. Among which sacrifice or preoperative embolization is ideal for aRHA with a small diameter and is unlikely to have clinically significant consequences. Dissection and preservation are ideal for all types of variant hepatic artery, but may not always be possible. Transection and reconstruction, includes active and passive manipulation, among which passive manipulation is commonly seen in accidental injury of variant RHA while active manipulation commonly seen in circumstances that aRHA or rRHA run through the parenchyma of head of pancreas and difficult to dissect from it, may be accomplished by primary anastomosis or through implantation at a suitable arterial site [16] such as GDA.
Our experiences about how to dissect and preserve the rRHA or aRHA include points as following: (i) Start from the dissection of hepatoduodenal ligament and maintain sharp vigilance with right of the ligament; (ii) Dissect retrogradely till to truncus coeliacus; (iii) Protect the origin of rRHA or aRHA from SMA when disconnect the uncinate process of pancreas and (iv) Choose other surgical approaches such as SMA first approach, uncinate process of pancreas first approach or posterior pancreatic approach [17-20].
But in cases of accidental cutoff or a small resection of the artery, a primary anastomosis can generally be achieved. If a large segment of artery is resected, reconstruction with prosthetic graft is a best choice [15,21].
However, there are still several limitations in our study. First, it is a single centre study and only 28 patients with variant RHA of Michels type Ⅲ and type Ⅵ were enrolled in which may limit the external validity of our findings. Second, although our experiences about how to dissect and preserve the rRHA or aRHA include choice of SMA first approach or uncinate process of pancreas first approach, we cannot compare merits and drawbacks between each approach because of the limitation by sample size.
Conclusion
In conclusion, anatomic variation of RHA is not rare in clinical and it is essential for surgeons to be vigilant for rRHA or aRHA to avoid potentially disastrous complications. Contrast-enhanced CT and subsequently CTA play a critical part in the preoperative evaluation for the procedures whenever and wherever possible. The best introperative disposal, only if transection and reconstruction is unavoidable, is to dissect and preserve despite it may cost much more time.
REFERENCES
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1,000 cases. Ann Surg. 1994;220:50-2.
- Covey AM, Brody LA, Maluccio MA, et al. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542-7.
- Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337-47.
- Yang F, Long J, Fu DL, et al. Aberrant hepatic artery in patients undergoing pancreaticoduodenectomy. Pancreatology. 2008;8:50-4.
- Eshuis WJ, Olde Loohuis KM, Busch OR, et al. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB (Oxford). 2011;13:161-7.
- Koops A, Wojciechowski B, Broering DC, et al. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239-44.
- Murakami Y, Uemura K, Yokoyama Y, et al. Celiac axis occlusion with replaced common hepatic artery and pancreatoduodenectomy. J Gastrointest Surg. 2004;8:520-2.
- Biehl TR, Traverso LW, Hauptmann E, et al. Preoperative visceral angiography alters intraoperative strategy during the Whipple procedure. Am J Surg. 1993;165:607-12.
- Rong GH, Sindelar WF. Aberrant peripancreatic arterial anatomy: considerations in performing pancreatectomy for malignant neoplasms. Am Surg. 1987;53:726-9.
- Traverso LW, Freeny PC. Pancreaticoduodenectomy: the importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg. 1989;55:421-6.
- Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379-84.
- Chambers TP, Fishman EK, Bluemke DA, et al. Identification of the aberrant hepatic artery with axial spiral CT. J Vasc Interv Radiol. 1995;6:959-64.
- Zeh H, Choyke PL, Alexander HR, et al. Gad-olinium-enhanced 3D MRA prior to isolated hepatic perfusion for metastases. J Comput Assist Tomogr. 1999;23:664-9.
- Lee MW, Lee JM, Lee JY, et al. Preoperative evaluation of the hepatic vascular anatomy in living liver donors: comparison of CT angiography and MR angiography. J Magn Reson Imaging. 2006;24:1081-7.
- Stauffer JA, Bridges MD, Turan N, et al. Aberrant right hepatic arterial anatomy and pancreaticoduodenectomy: recognition, prevalence and management. HPB 2009;11:161-5.
- Nakano H, Kikuchi K, Seta S, et al. A patient undergoing pancreaticoduodenectomy in whom involved common hepatic artery anomalously arising from the superior mesenteric artery was removed and reconstructed. Hepato gastroenterology. 2005;52:1883-5.
- Satoshi O, Claudius C, Kenichiro A, et al. Posterior approach for laparoscopic pancreaticoduodenectomy to prevent replaced hepatic artery injury. Ann Surg Oncol. 2013;20:3120.
- Rose JB, Rocha F, Alseidi A, et al. Posterior Superior mesenteric artery first approach for resection of locally advanced pancreatic cancer. Ann Surg Oncol. 2014;21:1927-8.
- Hackert T, Werner J, Weitz J, et al. Uncinate processes first-a novel approach for pancreatic head resection. Langenbecks Arch Surg. 2010;395:1161-4.
- Shrikhande SV, Barreto SG, Bodhankar YD, et al. Superior mesenteric artery first combined with uncinate process approach versus uncinate process first approach in pancreatoduodenctomy:a comparative study evaluating peripperative outcomes. Langenbecks Arch Surg. 2011;396:1205-12.
- Allendorf JD, Bellemare S. Reconstruction of the replaced right hepatic artery at the time of pancreaticoduodenctomy. J Gastrointest Surg 2009;13:555-7.