Anatomical and surgical examination of the sural nerve and its clinical significance
2 University of Belgrade, Faculty of Medicine, Clinical and Hospital Center, Dragisa Misovic, Serbia
3 Department of Plastic and Reconstructive Surgery, Al Emadi Hospital, Doha, Qatar
4 Department of Neurosurgery, Clinical Center of Serbia, Faculty of Medicine,University of Belgrade, Serbia
Received: 01-Nov-2024, Manuscript No. ijav-24-7319; Editor assigned: 04-Nov-2024, Pre QC No. ijav-24-7319 (PQ); Reviewed: 20-Nov-2024 QC No. ijav-24-7319; Revised: 26-Nov-2024, Manuscript No. ijav-24-7319 (R); Published: 30-Nov-2024, DOI: 10.37532/1308-4038.17(11).454
Citation: Kapor S, et al. Anatomical and Surgical Examination of the Sural Nerve and Its Clinical Significance. Int J Anat Var. 2024;17(11): 675-678.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: The sural nerve (SN) is a sensory nerve of the lower extremities that arises from the junction between the medial sural cutaneous nerve and the lateral sural cutaneous nerve. The SN exhibits variability in its origin, leading to a distinction between three types of its origin. In type A, the SN arises from the connection of the medial cutaneous nerve and the lateral cutaneous nerve. Type B is an extension of the medial cutaneous nerve, while type C SN is a direct branch of the lateral sural cutaneous nerve.
Aim: Determine the variations in the origin of SN and their clinical significance.
Materials and Methods: The study was performed using cadaveric material from the Institute of Anatomy ‘Niko Miljanić’. A total of 27 cadavers of both sexes were used for the study. The measuring instruments used were a ruler and an electronic digital caliper (0-500 mm measuring range, 0.01 resolutions).
Results: According to the results obtained, most of the cadavers of both sexes had type A formation (56%). Type B was present in 26% of the cadavers examined, while the smallest percentage of the sample had a type C formation (18%).
Conclusion: The study showed that type A was formed in most cases. Due to the variability in formation, we suggest that the SN and surrounding structures in the posterior aspect of the lower leg should be protected, paying particular attention to the small saphenous vein. Lack of precautions may jeopardize these neurovascular structures
Keywords
Sural nerve; Anatomy; Surgery
INTRODUCTION
The sural nerve (SN) is a sensory nerve of the lower extremities that arises from the union of the medial sural nerve (MCSN) of the lower leg, a branch of the tibial nerve (TN), and the lateral sural nerve (LCSN) of the lower leg, a branch of the common fibular nerve (CFN). These two nerves usually join in the centre of the back of the lower leg at the level of the lower part of the gastrocnemius muscle. The SN runs downwards along the lateral part of the small saphenous vein (SSV) and gives cutaneous branches in the lower and lateral part of the back of the lower leg. It then bends laterally and forwards, making an arch between the heel and the lateral malleolus. At the level of the lateral malleolus, the SN gives off a branch for the portion of the lateral calcaneal branch and then continues across the lateral portion of the foot and gives off the terminal branch of the lateral dorsal cutaneous nerve of the foot [1]. The SN can continue on MSCN, which is also another way of its origin [2]. The SN also gives two branches that innervate the skin of the little and big toe, dorsal digital nerves as well as branches for the ankle, heel and tibiofibular joint – articular branches [3]. The SN is frequently used in surgery for autologous nerve transplantation [4]. Parts of the SN can be used in nerve grafting due to injury or damage, and SN surgeons find in relation to SSV. The SSV can serve as a good landmark for surgeons to find this nerve. Due to the different origin, surgeons usually make incisions on both legs and choose a better sample of the SN for transplantation [5]. The SN can be used to restore muscle tone in facial nerve palsies as well as for biopsy in neuropathies as this nerve is also superficial and easily accessible. The use of SN can also be included in the various electrophysiological studies comparing the speed of impulse conduction through the nerve in relation to the speed of distribution through the nerve fibre [6]. However, studies have shown variations in relation to samples from different cadavers as well as variability in samples from the left and right leg of the same cadaver [7, 8]. Based on these studies, the development of SN is categorised into three types [9]. In type A, SN arises from MCSN and LCSN, which has been demonstrated in 72% of cases. In type B, the SN is a continuation of the MCSN, while the CFN is absent. This variant is observed in 28% of cases. In type C, the SN was formed only from CFN. This type of SN formation was not found in this study [9]. In type A of SN formation, the site of fusion between MCSN and LCSN is quite variable. Their fusion can occur anywhere between the popliteal fossa (PF) and the lateral malleolus. The SN shows differences in length between the right and left leg and a difference in length between males and females. In men, the right leg was longer than the left, whereas this difference was in favour of the left leg in women [2-9]. In these studies, the symmetrical group had the same type of SN formation in both legs, although the percentage was slightly higher in men (62.5%) than in women (55.6%). In the asymmetrical group, SN formed in one leg according to type A, whereas in the second leg it formed according to type B or C. This variation in the origin of SN between the right and left leg was more common in women (44.4%) than in men (37.5%) [2]. SN has a wide clinical application. It can serve as a donor for many other nerves, such as the sciatic nerve (SN) [10], the femoral nerve (FN) [11], the oculomotor nerve (CN III) [12], the inferior alveolar nerve (IAN) [13] and the facial nerve (CN VII) [14]. During surgical procedures on the Achilles tendon, this nerve can be injured with a sensory deficit [15]. As the SN occasionally contains motor fibres responsible for abduction and adduction of the toes, both sensory and motor dysfunction may occur during surgery [16]. The aim of this study is to define variations in the origin of the SN and their clinical significance.
MATERIAL AND METHODS
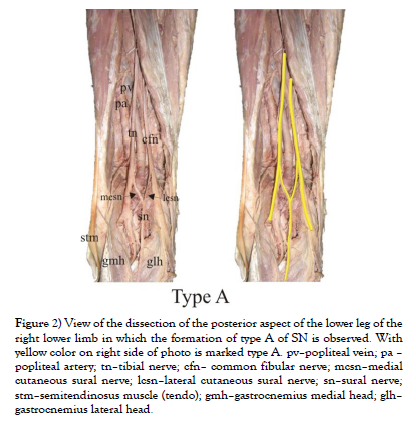
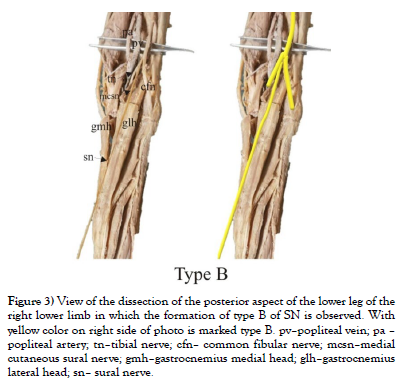
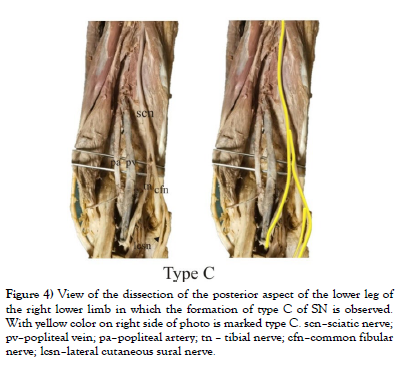
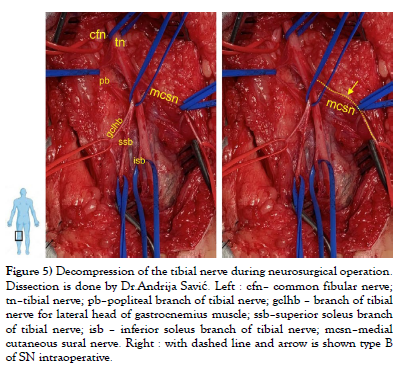
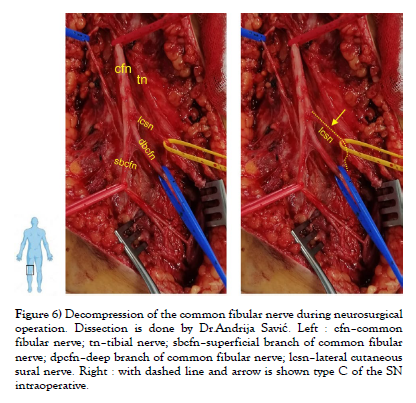
Our study was conducted on cadaveric material from the Institute of Anatomy “Niko Miljanić”, Faculty of Medicine, University of Belgrade, with the approval of the Ethics Committee for the Use of Human and Animal Material, Faculty of Medicine, University of Belgrade, Serbia (No. 323-07-00769/2021-05). The study included a total of 27 cadavers, 12 male and 15 female, aged between 64 and 79 years. First, a visual inspection of each limb was performed to exclude specimens with deformities and traces of trauma or surgery. After fixation in formalin solution, the extremities were carefully dissected, following standard procedures from dissection manuals. The limbs were placed in a prone position. A generous midline longitudinal incision was made from the middle of the popliteal fossa to the calcaneal tuberosity for each limb. The gastrocnemius muscles, gastrocnemius tendons, and the insertion of the Achilles tendons into the calcaneal tuberosity were visualized. The sural nerve was also identified, explored, and its course, visualized for all 54 legs. For our study, two groups of specimens were formed, comprising 54 lower limbs, of which 24 were in the male group and 30 in the female group. Based on previous studies [1-9], we hypothesized that SN could be formed in three ways, which we defined as type A, type B, and type C (Figure 1). We defined the origin of SN from the MCSN and LCSN as type A, while the origin of SN directly from the MCSN was defined as type B, i.e. type C in case of their origin from the LCSN. All parameters were measured on both sides. The measuring instruments used in the study are dissection instruments as well as a ruler and an electronic digital calliper (measuring range 0-500 mm, resolution 0.01 mm). The research methodology is shown schematically (Scheme 1), while the analysed parameters are shown in the figure (Figure 2, Figure 3 and Figure 4). Also, examples of our results are shown in (Figures 5 and Figure 6) which show type B and type C of SN. These figures are made by Dr. Andrija Savić who has done operations at Department of Neurosurgery, Clinical Center of Serbia. Operations are done during the decompression of CFN and TN.
Figure 2: View of the dissection of the posterior aspect of the lower leg of the right lower limb in which the formation of type A of SN is observed. With yellow color on right side of photo is marked type A. pv–popliteal vein; pa – popliteal artery; tn–tibial nerve; cfn– common fibular nerve; mcsn–medial cutaneous sural nerve; lcsn–lateral cutaneous sural nerve; sn–sural nerve; stm–semitendinosus muscle (tendo); gmh–gastrocnemius medial head; glh– gastrocnemius lateral head.
Figure 3: View of the dissection of the posterior aspect of the lower leg of the right lower limb in which the formation of type B of SN is observed. With yellow color on right side of photo is marked type B. pv–popliteal vein; pa – popliteal artery; tn–tibial nerve; cfn– common fibular nerve; mcsn–medial cutaneous sural nerve; gmh–gastrocnemius medial head; glh–gastrocnemius lateral head; sn– sural nerve.
Figure 4: View of the dissection of the posterior aspect of the lower leg of the right lower limb in which the formation of type C of SN is observed. With yellow color on right side of photo is marked type C. scn–sciatic nerve; pv–popliteal vein; pa–popliteal artery; tn – tibial nerve; cfn–common fibular nerve; lcsn–lateral cutaneous sural nerve.
Figure 5: Decompression of the tibial nerve during neurosurgical operation. Dissection is done by Dr.Andrija Savić. Left : cfn– common fibular nerve; tn–tibial nerve; pb–popliteal branch of tibial nerve; gclhb – branch of tibial nerve for lateral head of gastrocnemius muscle; ssb–superior soleus branch of tibial nerve; isb – inferior soleus branch of tibial nerve; mcsn–medial cutaneous sural nerve. Right : with dashed line and arrow is shown type B of SN intraoperative.
Figure 6: Decompression of the common fibular nerve during neurosurgical operation. Dissection is done by Dr.Andrija Savić. Left : cfn–common fibular nerve; tn–tibial nerve; sbcfn–superficial branch of common fibular nerve; dpcfn–deep branch of common fibular nerve; lcsn–lateral cutaneous sural nerve. Right : with dashed line and arrow is shown type C of the SN intraoperative.
RESULTS
The results of our study are presented in tabular form (Table 1 and Table 2). Analysing the obtained results according to the table in which they are presented, we concluded that the largest percentage of the examined extremities of both sexes has the occurrence of the SN origin according to type A, this percentage is 56% of the examined sample. Type C has the lowest percentage of SN formation and was observed in 10/54 cadavers, with this type accounting for 18% of the total sample. Type B is represented by 26% of the total sample, where the SN is formed as MCSN directly from the TN. From (Table 1), we can see that all three types occur equally in both extremities, and all three types are more common in males than females (type A – 53% in males, 47% in females; type B – 57% in males, 43% in females; type C – 60% in males, 40% in females).
| SN origin | Type A | Type B | Type C |
|---|---|---|---|
| Total number (%) | 30/54 (56%) | 14/54 (26%) | 10/54 (18%) |
| Males | 16/30 (57%) | 8/14 (57%) | 6/10 (60%) |
| Females | 14/30 (47%) | 6/14 (43%) | 4/10 (40%) |
Table 1) Tabular review of results conducted in our study. In the table are presented three different types of SN with % in males and females. All three types are presented on both extremities equally (results are shown in Table 2).
| Country | USA(150) | China (143) | Japan(90) | Russia (250) | India (25) | Ourstudy (27) |
|---|---|---|---|---|---|---|
| Symmetrical type of origin of the SN | 82.7 % | 83.9% | 82.2% | 78.8% | 60% | 100% |
| Asymmetrical type of origin of the SN | 17.3% | 16.1% | 17.8% | 21.2% | 40% | 0% |
Table 2) Tabular presentation of the distribution of the origin of SN in different studies. The number of cadavers is given in brackets.
DISCUSSION
The SN can originate in three ways. Origin type A means that the SN arises from the MCSN, and the LCSN. The SN can arise directly from MCSN, which corresponds to type B of its origin, while type C implies the formation of the SN directly from LCSN. Based on the measurements in this study, we concluded that type A is the most common with a frequency of 56%. Type B is represented with 26%, while type C has the lowest frequency of only 18%. In this study, we also investigated the symmetry of the origin of SN, and thus its mode of origin on different extremities of the same cadaver. The symmetry of the origin of SN means that it occurs in the same type on both the left and the right leg. Asymmetry is the origin of the sural nerve according to one type on one limb, and according to another type on the limb on the opposite side. The asymmetry of the origin of the SN was not found between both sexes in our study. SN is widely used in diagnostics as well as for therapeutic purposes [17-20]. Diagnostic studies, i.e. SN conduction studies, are performed in focal neuropathies to investigate conduction velocity, amplitude, and action potential. In addition, it is important for the diagnosis of focal neuropathies, compressive lesions, and diffuse polyneuropathy diseases [21, 22]. A good anatomical knowledge of the SN is crucial for interventions on the SSV. As the SN is located close to the vein, surgical procedures can lead to complications due to inflammation of the SN [23]. The SN is safest in the proximal third of the leaflet, where it is protected by the deep fascia [24]. Endogenous thermal ablation (ETA) has become a widely used, more effective and safer alternative for the treatment of incompetent SSV previously treated with the conventional method of open surgery [25]. There is a study showing that in 80% of cases, SN results from a combination of MCSN, and LCSN, while in 20% of cases, SN is the continuation of MCSN. In this study, there was not a single example of the origin of SN from LCSN [26]. There is also a study that looked at the symmetry of the origin of SN in the left and right leg. According to this study, the asymmetric group had an anastomosing type A in one leg and a non-anastomosing type B, i.e. type C in the other leg. Huelke et al [22] conducted a study on 150 cadavers in which 124 cadavers showed symmetry with respect to the origin of the SN, while 26 cadavers were asymmetrical according to the type of origin. The American, Chinese, Japanese and Russian groups showed almost the same percentage of symmetrical, and asymmetrical distributions [22]. A study conducted on South Indian cadavers showed that the symmetry of the origin of SN was lower than in other studies (60%). This can be explained by the fact that the study was performed on a small sample. Similar results were found in the foetal study on the anatomical variation of SN [27]. The discrepancy between the results of the current study and the results of some previous studies is due to several factors. Firstly, this difference can be explained by the small size of our sample compared to other studies conducted on several hundred lower limbs. The ethnic differences of the populations studied should also be taken into account, which certainly contributes to the diversity of the data obtained. Considering that the previous studies were conducted mainly on the Asian continent (China, Japan, India and Russia - as a Eurasian country), and the American continent, and this study dealt with the European area, such a large variation in the results obtained can be explained (Table 2).
CONCLUSION
Our study shows that the highest prevalence of the origin of SN is type A. These results are consistent with previous studies that we have presented in the discussion. Accordingly, we believe that a detailed assessment of the SN and its surrounding elements, especially the SSV, should be performed before any invasive procedure in the posterior aspect of the lower leg in order to avoid complications in reconstructive and transplantation procedures on these neurovascular structures. In conclusion, we can say that there is a need for further research in this area to improve the existing knowledge. One of the directions that will advance research in the future is the examination of the factors that influence the extraordinary variability in the occurrence of SN. In recent years many new, and innovative computational approaches have been developed to analyze, and evaluate tissue structure [28-29]. The rapid advancement in machine learning and artificial intelligence techniques has led to significant advancement in contemporary anatomy, histology and pathology research [28-29]. It is conceivable that in the future, our results, in conjunction with the results from other similar future studies, could be used for training and testing of various artificial intelligence models that could further enhance their applicability in anatomical and pathohistological evaluation [28-29].
FUNDING/ ACKNOWLEDGEMENTS
The authors acknowledge the support of the Ministry of Science, Technological Development and Innovation, subgrant entitled „Development of artificial intelligence models based on the random forest algorithm for the detection of discrete structural changes in the cell nucleus“ (Head of the subgrant Prof. Igor Pantić).
REFERENCES
- Marinković S, Filipović B, Puškaš L. Anatomija čoveka. Beograd, Sebija. 2016;233.
- Kavyashree AN, Subhash LP, Asha KR, Bindu Rani MK. Anatomical Variations in Formation of Sural Nerve in Adult Indian Cadavers. Journal of Clinical and Diagnostic Research. 2013;7(9):1838-1841.
- Mrvaljević D. Anatomija donjeg ekstremiteta. (Anatomija čoveka 1) Beograd, Srbija: Savremena administracija. 2010;100.
- Coert HJ, Dellon AL. Clinical implication of the surgical anatomy of the sural nerve. Plast Reconstr Surg. 1994;94(6):850-855.
- Moore KL, Agur AMR. Essential clinical anatomy. 3 Edition. Baltimore: Williams and Wilkins. 2007;429.
- Pyun SB, Kwon HK. The effect of sural nerve anatomical variations on nerve conduction studies. Am J Phys Med Rehabil. 2008;87(6):438-442.
- Huene DB, Bunnell WP. Operative anatomy of nerves encountered in the lateral approach to the distal portion of the fibula. The Journal of Bone and Joint Surgery. 1995;77(7):1021-1024.
- Webb J, Moorjani N, and Radford M. Anatomy of the sural nerve and its relationship to the Achilles tendon. Foot and Ankle International. 2000;21(6):475-477.
- Huelke DF. The origin of the communicating peroneal nerve in adult man. Anat Rec. 1958;132:81-92.
- Shin DS, Seo JS, Shon OJ, Han JH, Park SK, et al. Sural nerve graft after resection of a schwannoma in the sciatic nerve: a case report. J Korean Orthop Assoc. 2006;41:926-931.
- Tsuchihara T, Nemoto K, Arino H, Amako M, Murakami H, et al. Sural nerve grafting for long defects of the femoral nerve after resection of a retroperitoneal tumour. J Bone Joint Surg Br. 2008;90:1097-1100.
- Mariniello G, Horvat A, Dolenc VV. En bloc resection of an intracavernous schwannoma of the oculomotor nerve and grafting of the oculomotor nerve with the sural nerve. Case report and review of the literature. J Neurosurg. 1999;91:1045-1049.
- Chang YM, Rodriguez ED, Chu YM, Tsai CY, Wei FC. Reconstruction of the inferior alveolar nerve with an interpositional sural nerve graft: a useful adjunct to single-stage mandibular reconstruction. J Plast Reconstr Aesthet Surg. 2012;65(6):757-762.
- Lee MC, Kim DH, Jeon YR, Rah DK, Lew DH, et al. Functional outcomes of multiple sural nerve grafts for facial nerve defects after tumour-ablative Surgery. Arch Plast Surg. 2015;42:461-468.
- Porter KJ, Robati S, Karia P, Portet M, Szarko M, et al. An anatomical and cadaveric study to investigate the risk of sural nerve injury during percutaneous Achilles tendon repair with the Achillon device. Foot Ankle Surg. 2014;20:90-93.
- Amoiridis G, Schoels L, Ameridis N, Przuntek H. Motor fibres in the sural nerve of man. Neurology. 1997;49:1725-1728.
- Williams DD. A study of the human fibular communication nerve. Anat Rec. 1954;120:533-543.
- George BM, Nayak S. Entrapment of the sural nerve in the gastronemius muscle - a case report. Neuroanatomy. 2007;6:41-42.
- Sankar DK, Bhanu SP, Susan PJ. Aberrant formation of the sural nerve and its distribution on the dorsum of the foot. International Journal of Anatomical Variations. 2008;1:33-34.
- Kim YT, Moon JS, Kim JK. Anatomical study of the sural nerve and its associated nerves. J Korean Acad Rehabil Med. 2003;27(5):723-726.
- Ikiz AZA, Ucerler H, Bilge O. The anatomic features of the sural nerve with an emphasis on its clinical importance. Foot Ankle Int. 2005;26(7):560-567.
- Huelke DF. A study of the formation of the sural nerve in adult man. Am. J. Phys. Anthropol. 1957;15:137-145.
- Park JY, Galimzahn A, Park HS, et al. Midterm results of radiofrequency ablation for incompetent small saphenous vein in terms of recanalisation and sural neuritis. Dermatol Surg. 2014;40:383-389.
- Ricci S, Moro L, and Antonelli IR. Ultrasonography of the sural nerve: ultrasound anatomy and rationale for examination. Eur J Vasc Endovasc Surg. 2010;39:636-641.
- Samuel N, Carradice D, Wallace T, et al. Randomised clinical trial of endovenous laser ablation versus conventional surgery for small saphenous veins. Ann Surg. 2013;257:419-426.
- Ortiguela ME, Wood MB, Cahill DR. Anatomy of the sural nerve complex. J Hand Surg. 1987;12(6):1119-1123.
- Shankar N, Selvam RP, Dhanpal N, Reddy R, Alapati A. Anatomical variations of the sural nerve in the leg: a foetal study. Neurol India. 2010;58:24-28.
- Pantic I, Paunovic J, Cumic J, Valjarevic S, Petroianu GA, et al. Artificial neural networks in contemporary toxicology research. Chem Biol Interact. 2023;369.
- Pantic IV, Cumic J, Valjarevic S, Shakeel A, Wang X, et al. Computational approaches for evaluating morphological changes in the corneal stroma associated with decellularization. Front Bioeng Biotechnol. 2023;11.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref