Antibacterial activity in a collection of amps from a Hirudo medicinalis
Received: 28-Jun-2022, Manuscript No. puljmbr-22-5116; Editor assigned: 01-Jul-2022, Pre QC No. puljmbr-22-5116 (PQ); Accepted Date: Jul 13, 2022; Reviewed: 04-Jul-2022 QC No. puljmbr-22-5116 (Q); Revised: 05-Jul-2022, Manuscript No. puljmbr-22-5116 (R); Published: 22-Jul-2022, DOI: : 10.37532/puljmbr.2022.5(4).35-38
Citation: Sanchez A, Victoria M, Portillo A, et al. Antibacterial activity in a collection of amps from a Hirudo medicinalis. J Mic Bio Rep. 2022; 5(4):35-38.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
In 2020, the Centers for Disease Control and Prevention (CDC) estimated that over 2.8 million people in the United States had been diagnosed with drug-resistant infections resulting in an estimated 35,000 deaths. According to the World Health Organization, mortality is about 700,000 people a year, and this is expected to increase to 10 million a year by 2050, at the cost of 100 billion dollars annually. Bacteria have developed strategies to resist most current antibiotics, making it difficult to find safe, effective antibiotic alternatives. Many animals have evolved their own antimicrobial factors, especially invertebrates. Antimicrobial activity has been shown to exist in the central nervous system of the medicinal leech. By working with the genomic library of Hirudo medicinalis, we found a gene family associated with earthworms, initially identified in Lumbricus rebellus. Molecular and microbiological techniques were employed to confirm the existence of antibacterial activity, specific for Gram-positive and Gram-negative bacteria, from different tissues of Hirudo medicinalis. We show a protein extract with antimicrobial properties, where a family of 4 lumbricin-like peptides are the leading player in this family of antibiotics. However, each peptide presents less antibacterial activity individually against marine bacteria than the complete protein extract.
Keywords
Antibiotics; Antimicrobials; New medicines; Hirudo medicinalis
Introduction
One of the species most used as a model in medicine is Hirudo Omedicinalis due to the production of substances in its saliva resultiing from its innate immune defense system, which is derived from its feeding process and surviving strategies in its habitat. This organism secretes more than 100 substances, although only a few of them have an active action as an anti-inflammatory, antimicrobial, analgesic, and anticoagulant activity, among other properties [1]
There have been found Antimicrobial Peptides (AMPˈs) in leeches between them destabilase, chloromycetin, theromacin, theromyzin and p--eptide B; however, studies on proteomics and transcriptome of saliva have demonstrated more peptides with antibacterial effects [2-4].
AMPˈs are produced for all living creatures in nature; they are oligopeptides with a higher basic lysine and arginine amino acids and cationic and amphipathic molecules. They are natural antibiotics since they can inhibit gram-positive and negative bacteria growth, virus, fungi, and parasites [5]. AMPˈs from leeches have had attention in the pharmaceutical industry for their host’s low blood cell lysis and toxicity. However, their low concentration has difficulties their discovery.
AMPs are produced by different organisms [6,7]. Since their discovery in the 1980s, AMPs promise to be novel antibiotics and have been reported in various animals, plants, and bacteria. In animals, there are 2298 (76.9%) AMPs described; in plants, 349 (11.7%) and bacteria 342 (11.4%). Only 20 AMPs have been discovered in worms [7,8].
Recently it has been found AMP´s in the microbiome of leech using metagenomic analysis and they showed strong antimicrobial activity against Gram negative and Gram positive bacteria [4,9]. After being challenged with pathogenic strains, this study sought to detect AMPs from H. medicinalis using computer algorithms and measuring gene expression based on qPCR. A family of 4 lumbricin-like peptides is the main players in this family of antibiotics.
We synthesized them and challenge them in different concentrations, against E. coli, B. subtilis, V. alginoliticus and E. faecalis, and compared them against the complete salivary gland protein extract and commercial antibiotics. Resulted that the individual synthetic peptides presented lower antibacterial activity than the complete protein extract or commercial well know antibiotics.
Research Methodology
Computational algorithms
To identify new AMPs in the leech, the unpublished draft genome for H. medicinalis and lumbricin I from L. rebellus annelid as a sequence anchor using a search matrix of similar sequences to lumbricin. Found four sequences with great homology that promised to have antimicrobial activity [10]. Then with the Beltran and Brizuela [11], DNA algorithm tool, which shows an efficient automatic AMP/non-AMP classifier, we corroborate and confirm the peptide sequences as potential antimicrobials.
Bacterial inoculum
The gram-negative Vibrio alginolyticus was used and Enterococcus faecalis, as a representative of Gram-positive bacteria. V. alginolyticus was grown in TSA broth with 2% NaCl and E. faecalis in L.B. broth. They were used individually. Cultures were incubated for 24 hours at 32°C. After incubation was complete, the liquid culture was centrifuged at 3000 g. Then the pellet was suspended in one ml of sterile distilled water. The tubes were heated at 100°C for ten minutes to inactive the cells. The inoculation was adjusted to 1.5 × 108 cfu. mL-1.
Treatment and bacterial inoculation
Eight adult leeches (4 g-5 g) were injected with 100 µL of E. faecalis, V. alginolyticus, or Ringer's solution as a negative control. The leeches were left to rest on a bed of ice with Wenning's solution with 8% ethanol for 20 minutes to prevent movements before administering the injection. Then inoculum or control was injected into each gland (1.5 cm below the head parallel to the midline) and was left to rest for 24 hours, 48 hours, or 72 hours (eight per time). After that, the leeches were placed in alcohol to help numb them and later on ice with Wenning's solution with 8% ethanol for 10 minutes.
Leeches after the inoculation were left for periods of 24 hours, 48 hours, and 72 hours, continuously observed before tissue extraction. A longitudinal section was made at the center of the body before the end, where there are glands.
Protein extraction
Only the salivary gland tissue from the bacterial-challenged leeches was used. It was gently triturated and homogenized on ice with a pestle. The protein was extracted using 500 ml of TRIzol®. Each sample was homogenized and then cold-centrifuged for 10 minutes at 6000 g. Then the supernatant was used for RNA purification as the protocol of the brand said. The organic phase with the protein was recuperated in a new tube.
cDNA and qPCR
Different tissues from the central nervous system, salivary glands, nephridia (kidneys), and (dorsal) body wall were dissected and used to extract RNA. RNA was purified using Trizol®, following the instructions of the commercial brand. 0.5 µg of RNA was used for cDNA synthesis using SuperScript III Reverse Transcriptase (Thermo-Scientific®) and lumbricin-like gene primers.
The primers were selected based on the lumbricin-like gene resulting from the genome analysis of H. medicinalis. The amino acid sequences from the putative lumbricin-like were aligned to the lumbricin I from L. rebellus (Genbank, AAC64517.1), and the homologous sequence from the annelid polychaete Capitella teleta (Genbank, ELU06641.1), using CLUSTALX (Fig. 1). The sequences with more conservation were used to make the primers (Table 1).
TABLE 1. Primers used in this study
| Primer name | Forward: 5ˈto 3ˈ | Reverse: 5ˈto 3ˈ |
|---|---|---|
| L1 | AAGAAGTTACGGTGAAAGGTTCAG | TTCTCCCCATCCCACTGG |
| L2 | TGTCGACCGTAAAGTCCTCT | TGTCGGAATGCCCTCTTCAT |
| L3 | GAAGAAGGGCAGATTTGCTG | GTGACCTTCGCCTTGACAGT |
| L4 | GAGAGGCTGGGAATGTTCAA | GCCATTGCTTGAAGAGGAGT |
Lumbricin L1-L4 are the putative lumbricin-like peptides |
||
qPCR was run using the selected primers and β-actin as a housekeeping gene. All qPCR reactions were performed in 25 µL volume, using Quantitec SYBR green PCR 1X (Qiagen), 10 μM of each primer, 30 ng of cDNA, and final concentration. Thermocycler Corbett® was programed at 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, 52°C or 58°C for 15 s, and 72°C for 30 s. Each reaction was performed in triplicate. The product size was 245 bp.
Relative expression and statistical analysis
Relative expression levels among each sample through the experimental groups were determined by the Pfaffl equation (2001). The E value was calculated with the formula; 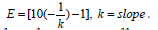 E target represents lumbricin gene efficiency, E ref is reference gene efficiency, ΔCq target is Cq value difference between the control and experimental sample for each gene, and ΔCq ref is Cq value difference between the control and experimental sample for the housekeeping gene.
E target represents lumbricin gene efficiency, E ref is reference gene efficiency, ΔCq target is Cq value difference between the control and experimental sample for each gene, and ΔCq ref is Cq value difference between the control and experimental sample for the housekeeping gene.

To determine statistical differences between the groups, we tested one-way ANOVA with a significant level of 0.05. When there were significant differences, a Tukey test was applied to determine which group had the higher expression level. Those analyses were performed with SPSS statistical software version 20.0.
Antibacterial test
Antibacterial activity was determined through antibiograms based on a protein extraction only from salivary glands of bacterial-challenged leeches. Mueller-Hinton agar with 2% NaCl was used for the antibiograms. Concentrates of the two strains of bacteria were prepared at 1.5 × 108 cfu. mL-1. Extracts were analyzed, producing 16 combinations (8 of each bacteria). Triplicates were made for each combination and control, having 54 culture plates with their respective susceptibility disks.
Two synthetic antibiotics (Enrofloxacin and Oxytetracycline 200 mg/ml) were used as positive control and negative control sterile disks. Plates were incubated at 28°C for 24 hours, then the diameters of the inhibition growth were measured in millimeters.
Statistical analysis
A one-way ANOVA was used to evaluate the differences between the inhibition activities of extract and synthetic antibiotics. A Tukey pairwise comparisons test was used to detect specific differences; p<0.05 was chosen as the significance level, and statistical analyses were performed using SPSS statistical software version 20.0.
Antimicrobial peptides
Four synthetic peptides (SC1208, GenScript®) were resuspended in 5% DMSO and diluted to 2 mg. mL-1, 10 mg. mL-1, 20 mg. mL-1, and 100 mg. mL-1, respectively. Then 10 µL from each concentration was used on the sensitivity disks to test against V. alginolyticus, E. faecalis, E. coli, and B. subtilis.
Results
To obtain the sequences of the synthetic peptides, we used a search matrix with lumbricine sequence as an anchor with the draft genome for Hirudo medicinalis and to the completed genomic library for the medicinal leech http://genomes.ucsd.edu/hirmed1/ and then the resultant sequences were corroborated using the algorithm created by [11]. The DNA sequences obtained by the algorithms were translated to the protein and then aligned to C. teleta and L. rebellus, where we found highly conserved amino acids (Figure 1). We identified four putative lumbricin-like in H. medicinalis. (RQKDKRSYGERFSMFTGPQFISPPERIKP; RQKDKRTYVDRKVLYTGPQFTSPSERIKP;
RQTDKREYSEKKGRFAGPQFVYPVDKIRP;
RQKDKRDYVERLGMFKGPQFSYPPERIRP). According to Cho [12] and determined that our positive control (lumbricin I (6-34) should have had effects, as reported in the paper. The full-length lumbricin I, was extracted from animals. The shorter, slightly more potent version was synthesized (amino acids 6-34 of lumbricin I). So the sequences that we have in our hypothesis are the most powerful parts (peptide number 6-34) in the correlation of Lumbricin I [12] and not the entire peptide (1-76).
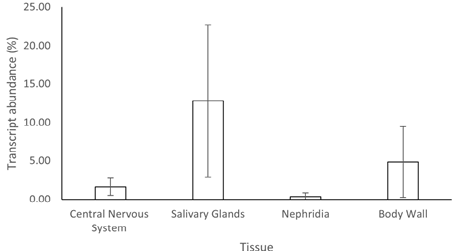
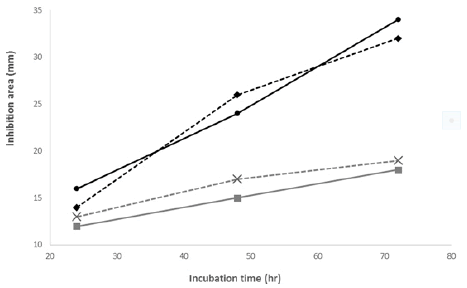
The expression from the four putative AMPs from the salivary gland, nephridia, central nervous system, and body wall (muscle) of H. medicinalis was measured by qPCR (Figure 2). It was found that the synthetic lumbricin-like peptides had higher abundances in the salivary gland than in the other tissues and control samples. So we decided to work only with salivary gland extracts for the antibiograms. After 24 hours of inoculation, the antibacterial test showed an inhibitory effect in all four bacterial strains. However, more inhibition was found with protein extracts from organisms dissected 72 hours after inoculation with bacteria. The inhibition zones created by the protein extract were significantly more extensive than commercial antibiotics (Figure 3,4). This extract inhibited gram-positive and gram-negative bacteria and maintained their effectiveness over two weeks (Table 2). It was observed that the antibiotic managed to inhibit the two bacteria. Both individually and together. It was left for a few weeks and unlike other samples with commercial drugs such as ampicillin G, ampicillin, and amoxicillin at concentrations of 50 ug/ml, the protein extract containing the family of AMPs of H. medicinalis, manages to keep the Sen disc intact for more than 2 weeks (Figures 5 and 6). The antibacterial extract from the salivary gland had a greater effect on the Gram-positive bacteria but reacted very well with both. None synthesized peptides inhibit separately at 2 mg. mL-1 or 10 mg. mL-1 concentration, even using the four peptides as a mixture. We increase to 20 mg. mL-1 individually, and only peptide no. 3 showed a slight antibacterial activity against V. alginolitycus. However, remarkable antimicrobial activity was observed when the concentration was at 100 mg. mL-1. Peptides 1, 3, and 4 presented significant inhibition in the antibiograms against the same bacteria.
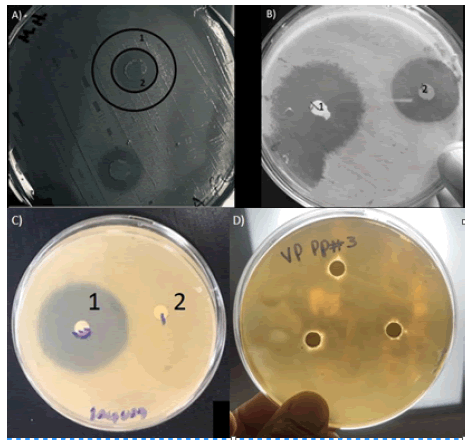
Figure 3: Sensitivity disks test using V. aliginolyticus. A) inhibition zones using the tissue extract from salivary glands tested by duplicate; B) effect from enrofloxacin, one and two oxytetracycline commercial antibiotics; C) Inhibition zone registered with enrofloxacin, one and two saliva gland extract without effect. D) Peptide three without effect
Figure 4: Sensitivity disks test using V. alginolyticus. A) Inhibition of 11- 16 mm with protein extract; B)-D) inhibition of synthetic peptides 1, 3 and 4 at 100 mg/ml each one.
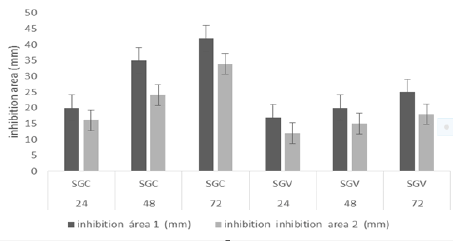
TABLE 2. Bacteria inhibition diameter growth for each extracts in the time after inoculation
| Sample ID | Time post inoculation (h) | Inhibition diameter (mm) Zone 1 | Inhibition diameter (mm) Zone 2 |
|---|---|---|---|
| SGC | 24 | 20 | 16 |
| SGC | 48 | 35 | 24 |
| SGC | 72 | 42 | 34 |
| CNSC | 24 | 17 | 14 |
| CNSC | 48 | 30 | 26 |
| CNSC | 72 | 39 | 32 |
| SGV | 24 | 17 | 12 |
| SGV | 48 | 20 | 15 |
| SGV | 72 | 25 | 18 |
| CNSV | 24 | 19 | 13 |
| CNSV | 48 | 20 | 17 |
| CNSV | 72 | 27 | 19 |
SGC: Salivary Glands, CNSC: Central Nervous System inoculated with E. faecalis; SGV: Salivary Glands, CNSV: Central Nervous System inoculated with V. alginolyticus |
|||
Discussion
Increased antibiotic resistance means that the effectiveness of antibiotics used to treat infections is diminished or non-existent. Millions of people acquire antibiotic-resistant to different pathogens, and thousands will die directly from antibiotic-resistant diseases each year. That's why searching for novel antibacterial substances in natural products is essential since human beings have been constantly exposed to microorganisms. Invertebrates, in nature, have strategies to survive and digest different kinds of food as leeches that feed on human blood produce several antibacterial substances that are excellent antimicrobials. To look for these substances, we first search through bioinformatics analysis inside the genomic sequences of H. medicinalis, looking for multiple families of antimicrobial effects and being homologous to lumbricin. In our way to answer, why are various members of these families? Are they possibly tuned to different target organisms? Do they all have antimicrobial activity.
This study shows a family of lumbricin-like peptides in H. medicinalis. The four putative lumbricin-like showed an antimicrobial effect on different bacteria. They were found in all tissues tested, with different abundances, where the salivary glands showed the higher quantity. Twenty-four hours after bacterial challenge with gram-negative or gram-positive bacteria, gene expression of lumbricin-like genes 1, 2, 3, and 4 were significantly up-regulated in the salivary glands. We found that all tissues express the four AMPs; however, only the salivary glands extract was used for the antimicrobial test since it was the principal AMPs producer under our conditions. Other studies on earthworm and lumbricin I showed the most potent action within the amino acids 6-34 of the peptide, so we focus on this small sequence from the four AMPs.
AMPs give us other advantages when used as a family of peptides since they reduce the possibility that the bacteria will mutate to create resistance, as well as to find out if single doses of AMPs can be applied as treatments without generating resistant bacteria, so we must consider these benefits. AMPs can be applied as treatments that generate zero-resistant bacteria. We could include protein extracts of this type in our diet as food supplements that would replace current antibiotics. These protein extracts must be encapsulated and protected from stomach acids to reach their target. Using protein extracts would reduce the cost of synthesizing or purifying peptides, but we can ensure this by verifying them in future trials.
In 2010, the Federal Commission for Protection Against Sanitary Risk in Mexico (COFEPRIS) reported that antibiotics were the second best-selling drugs in Mexico, with self-medication accounting for 40% and 60% of total sales. In 2011, it was calculated that as many as 21% of infections among hospital patients were due to healthcare received, being pneumonia the most common (33.2%), followed by urinary tract infections (24.6%) and wound infections (15.6%) [13]. In 2020, we were taught a hard lesson on the fragile nature of human health in the fight against the SAR-Cov2 virus. For some time, we have faced the possibility that the next major global battle will be against bacteria. AMPs also exhibit anti biofilm, antiviral and anticancer activity [14,15]. We must be prepared with antibiotics from non-conventional sources to fight against rapid rates of bacterial growth and resistance to current bactericides, and even better if these peptides have antiviral activity as shown in the literature. AMPs appear to work as a team, group, or collection, making it more difficult for bacteria to build up such a diverse resistance, for example, having a group of antimicrobial molecules so similar that bacteria cannot create resistance because of the variety of molecular structures that are attacking it.
Conclusion
This work contributes to additional knowledge of Hirudo medicinalis to use their protein salivary gland extract as an antibiotic. Annelid-sourced AMPs appear to offer a good line of defense against bacteria in the future. It is worth mentioning that they attack not only pathogenic bacteria but also help select colonizing bacterial symbionts for optimal intestinal flora. We are sure that the following antibacterial treatments will take the form of collections of peptides or RNA that codes for this collection of peptides that will combat bacterial pathogens.
REFERENCES
- Sig AK, Guney M, Guclu AU, et al. Medicinal leech therapy-an overall perspective. Integr. med. res. 2017;6(4):337-43.
[Google Scholar] [Cross Ref ]
- Baker MW, Macagno ER. Gap junction proteins and the wiring (Rewiring) of neuronal circuits. Developmental Neurobiology. 2017 ;77(5):575-86.
[Google Scholar] [Cross Ref ]
- Liu Z, Tong X, Su Y, et al. In-depth profiles of bioactive large molecules in saliva secretions of leeches determined by combining salivary gland proteome and transcriptome data. J. proteom. 2019; 200:153-60.
- Grafskaia E, Pavlova E, Babenko VV, et al. The Hirudo medicinalis microbiome is a source of new antimicrobial peptides. Int j mol sci. 2020 ;21(19):7141.
- Manzoor M, Singh J, Gani A. Exploration of bioactive peptides from various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies. Food Chemistry. 2022; 373:131395.
- Waghu FH, Barai RS, Gurung P, et al. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic acids research. 2016;44(D1): D1094-7.
- Bruno R, Maresca M, Canaan S, et al. Worms’ antimicrobial peptides. Marine drugs. 2019;17(9):512.
- Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacological reviews. 2003;55(1):27-55.
[Google Scholar ] [Cross Ref]
- Grafskaia EN, Nadezhdin KD, Talyzina IA, et al. Medicinal leech antimicrobial peptides lacking toxicity represent a promising alternative strategy to combat antibiotic-resistant pathogens. Eur J Med Chem. 2019; 180:143-53.
- Medicinal leech therapy-an overall perspective
- Beltran JA, Del Rio G, Brizuela CA. An automatic representation of peptides for effective antimicrobial activity classification. Comput struct biotechnol j. 2020 ;18:455-63.
- Cho JH, Park CB, Yoon YG, et al. Lumbricin I, a novel proline-rich antimicrobial peptide from the earthworm: purification, cDNA cloning and molecular characterization. Biochim. Biophys. Acta (BBA)-Mol. basis dis. 1998 ;1408(1):67-76.
- de la Federación DO. Acuerdo por el que se determinan los lineamientos a los que estará sujeta la venta y dispensación de antibióticos. México, DF: DOF. 2010.
- Le CF, Fang CM, Sekaran SD. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob agents chemother. 2017 ;61(4): e02340-16.
- Divyashree M, Mani MK, Reddy D, et al. Clinical applications of antimicrobial peptides (AMPs): where do we stand now?. Protein and peptide letters. 2020; 27(2):120-34.