Anti-carcinogenic and therapeutic properties of curcumin
Received: 05-Nov-2018 Accepted Date: Dec 11, 2018; Published: 24-Dec-2018
Citation: Zaminpira S, Niknamian S. Anti-carcinogenic and therapeutic properties of curcumin. J Can Res Metastasis. 2018;1(2):23-34.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
In spite of great progress in therapeutic practices over the past decade, neither the incidence nor the deaths from cancer have changed over the past thirty years. Existing anticancer drugs have limited efficacy, severe complications, and high costs expensive. Hence, identifying pharmaceutical agents lacking these disadvantages is required.
Keywords
Anti-carcinogenic; Curcumin; Therapeutic properties; Screening; Cotonou
Introduction
Cancer is the second most common cause of mortality in human societies today. According to World Health Organization (WHO) reports, by 2012, four million new cases of cancer and 8.2 million deaths due to it will have been reported. According to the latest studies, in 2020, cancer will be the world’s first disease in terms of prevalence [1]. Chemoprevention means the use of natural or synthetic chemical compounds to prevent the onset and progression of cancer. These compounds have little toxicity, side effects, while Curcumin, which is a polyphenolic compound, belongs to both groups [2]. Curcumin has been used in traditional Chinese and Iranian medicine for thousands of years. Traditional treatment with turmeric goes back to around 5000 years ago, which was used to overcome inflammation, infectious diseases and autoimmunity [3,4]. Curcumin has a tremendous potential for treating human diseases like metabolic and infectious diseases, diabetes, psoriasis, rheumatoid arthritis, neurodegenerative diseases, arthritis, atherosclerosis, Parkinson’s disease, Alzheimer’s disease, heart disease, digestive disorders such as indigestion, flatulence, gastric ulcer, duodenal ulcer and cancer [5]. Curcumin has preventive chemical effects, induces sensitivity to cancerous cells against chemotherapy, anti-inflammatory, anti-oxidant, anti-aging, antitumor and anti-inflammatory. The anticancer effects of curcumin are important because the overdose of it prevents the proliferation of cancer cells but does not harm healthy cells [6].
Literature Review
What is curcumin
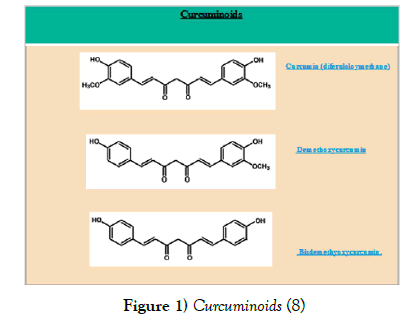
Turmeric is the underground stem of plant from Zingiberacea with the scientific name of Curcuma longa Linn. Turmeric powder is yellow and contains compounds called curcuminoids. Curcumin (77%), Dimethoxycurcinum (17%), and bisdemethoxycurcumin (BDMC) (3%) are the most important curcuminoid (Figure 1) [7]. Diferuloylmethane, chemically known as 1,7-Bis (4-hydroxy-3-methoxyphenyl) 1,6-heptadiene- 3,5-dione, is a yellow phenolic antioxidant, extracted for the first time in an impure form by Vogel et al. The curcumin structure was found by Milobedeska et al. and synthesized by Lamp et al. [8,9]. Curcumin can have at least two forms of keto and enol tautomerism forms.
Figure 1) Curcuminoids [8]
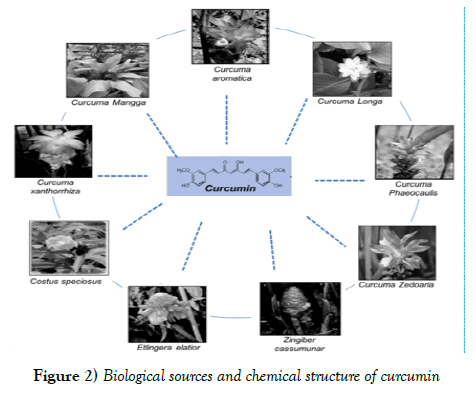
The enol form is more stable energetically in the solid and solution phases. Curcumin contains several functional groups. The aromatic ring systems, which are polyphenols, are bonded together by the two groups of unsaturated carbonyl α and β and form two carbonyl diketone groups. Diketone forms stable enzymes, or can easily be deprotonated and form enolate, whereas the two groups of unsaturated carbonyl α and β are good Michael acceptors and are subjected to nucleophilic attack. Curcumin biosynthetic pathway has been very difficult for researchers. In 1973, Roughly and Whiting suggested two mechanisms for curcumin biosynthesis. The first mechanism involves a chain reaction between cinnamic acid molecules and 5 malonyl-coa, which ultimately leads to the formation of curcuminoid. The second mechanism is the binding of two cinnamic acid molecules by Malonyl-Cove. In both mechanisms, cinnamic acid, which is derived from phenylalanine, is used as the starting point. This is significant because the plant biosynthesis of cinnamic acid as a starting point is rare compared to the common use of P-kumaric acid [10]. In addition, turmeric contains a number of volatile oils (e.g. zingiberone, atlantone and tumerone), sugar, resin and protein. However, except for curcumin, turmeric contains no known agents with anti-inflammatory and anti-proliferative activities [11]. Several sources of curcumin and its analogues have been reported from other species of turmeric, such as Curcuma mangga, Curcuma zedoaria, Costus speciosus, Curcuma xanthorrhiza, Curcuma aromatic, Curcuma phaeocaulis, Etlingera elatior and Zingiber cassumunar.
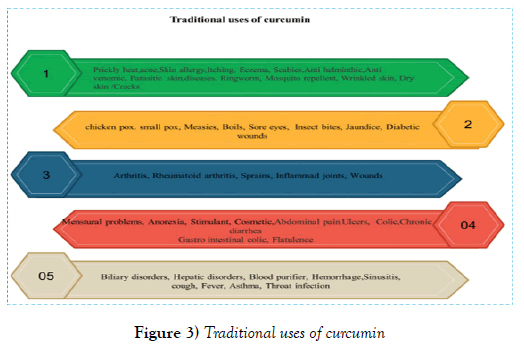
Figure 2 shows different biological sources of curcumin [12]. As part of the Indian medical system, Turmeric Ointment is used to treat common eye infections, bites, burns, acne and various skin diseases. Powdered turmeric is used in conjunction with the milk to treat cough and respiratory diseases. This traditional treatment is also used to treat dental diseases, digestive disorders such as indigestion and acidity, bloating, ulcers, and to reduce the illusions of cannabis and other psychotropic drugs, too. Curcumin is used in perfumes as a natural yellow color and in food as a food additive due to its taste.
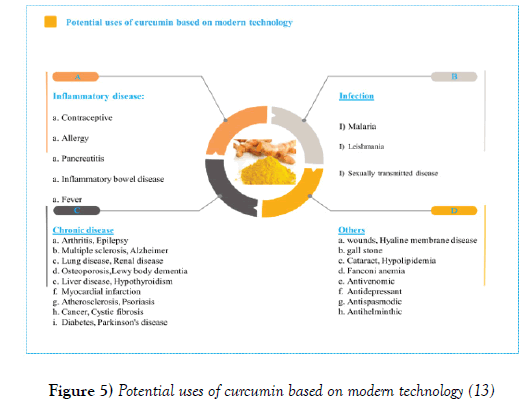
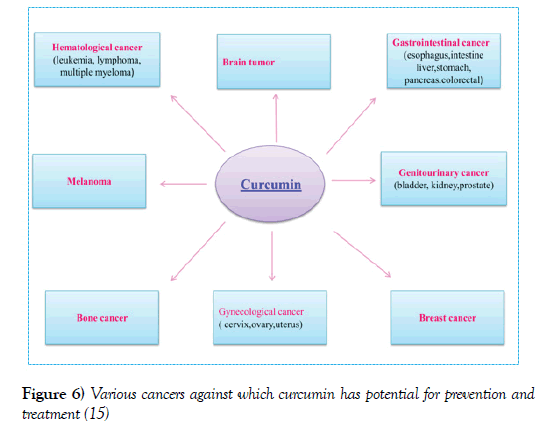
Figure 3 shows the traditional uses of curcumin [9]. In recent years, several studies have been conducted on the biological effects of curcumin. These studies have shown that curcumin has anti-oxidant, anti-bacterial, antiviral, anti-inflammatory, anti-proliferative, pro-apoptotic and other effects, and has tremendous therapeutic potential against human diseases such as metabolic and infections, diabetes, psoriasis, rheumatoid arthritis, neurodegenerative diseases, arthritis, atherosclerosis, Parkinson’s disease, Alzheimer’s disease, heart disease, digestive disorders such as indigestion, flatulence, gastric ulcers, duodenal ulcer, Kidney, depression, and cancer [5]. Figure 4 shows the potential uses of curcumin according to the modern technology [10]. The multiple and multifaceted effects of curcumin have attracted researchers’ interest for specifying the cellular goals and mechanisms involved in the curcumin action paths. Molecular curcumin is highly polythropic or multilateral with many therapeutic effects. The multi-aspect effects of curcumin are numerous given its capacity to interact with different molecules and to regulate multiple molecular targets and pathways. Many molecules and mechanisms are involved in every biologic and pathological events and curcumin, with inhibitory or activating effects on these molecules, overcomes pathological conditions. The molecular goals of curcumin are shown in (Figures 4 and 5) [13,14]. Directly or indirectly interacting with these molecules, curcumin regulates their function and effects its changes. More than 30 different proteins interact directly with curcumin. Due to the extent of the scope of the effects of curcumin and its wide-ranging mechanisms of functioning in different pathological conditions, further discussion is focused on the anticancer mechanisms of curcumin and its effect on signaling pathways associated with cancer. The anticancer potential of curcumin against a variety of cancers has been shown, including leukemia, lymphoma, gastrointestinal tract, urinary tract, breast, uterus, ovaries, lung, melanoma, colon, sarcoma, brain tumors, and so on (Figure 6). The mechanisms by which curcumin inhibits tumor growth include a combination of antioxidant, anti-inflammatory, anti-angiogenic, anti-neoplastic, cell-cycle inhibition, and pro-apoptotic properties and through the regulation of genes and molecules involvement in these pathways, it induces its inhibitory effects on cancer [15,16].
Figure 4) Molecular targets of curcumin [15]
Figure 5) Potential uses of curcumin based on modern technology [13]
Figure 6) Various cancers against which curcumin has potential for prevention and treatment [15]
New scientific studies show that curcumin is a highly polyotropic molecule that interacts with multiple molecular targets. Curcumin may be directly coupled to modulate the activity of these molecules or indirectly regulate their function. More than 30 different proteins have been found that interact directly with curcumin, including DNA polymerase [17], Focal adhesion kinase (FAK) [18], thioredoxin [19] reductase [20], protein kinase (PK) C [21], lipoxygenase (LOX) and tubulin [22]. It has also been shown that curcumin can be bonded to certain metallic bivalent capacities such as iron, copper, manganese and zinc [23,24]. As shown in Figure 4, curcumin strongly inhibits the activation of some transcription factors, including nuclear factor- κB (NF-κB)[25,26], activated protein-1 (AP-1), signal transducer and activator of transcription (STAT) proteins [26,27], (hypoxia-inducible factor-1 (HIF- 1) [28], Notch-1 [29], (early growth response-1 (Egr-1) [30], β- Catenin [31]. However, on the other, it activates some transcription factors such as aryl hydrocarbon receptor (AhR) [32], activating transcription factor (ATF) [33], C/EBP homologous protein (CHOP) (34), electrophile Response Element (EpRE) [35], peroxisome preoliferator-activated receptor-gamma (PPAR-γ) [36], NF-E2-related factor (Nrf2) [37]. It has been shown that nuclear factors, AP-1, NF-κB, STAT-3, β-catenin, Egr-1, HIF-1, and Notch-1 have a role in cell proliferation, cell survival, invasion, angiogenesis, tumorigenicity and inflammation. In most of the cancers, these transcription factors have expression increase. NF-kB represents a family of eukaryotic transcription factors playing an important role in regulating the expression of a wide range of vital genes for inherent and acquired immunity, inflammation and cell survival [38,39]. Non-regulated NF-kB activity happens in a number of diseases, especially cancer, and acute and chronic inflammatory diseases. In un-stimulated cells, NF-kB in cytosol is used as a heterodimer in physical collaboration with a protein called the inhibitor κB (IκB) [40,41]. Various pathogenic stimuli, including bacterial products, carcinogens, cancer promoters, cytokines, radiation, ischemia/reperfusion, and oxidants can activate NF-kB through several signal transmission pathways. After activation, NF-kB is transmitted to the nucleus, where it induces the expression of more than 200 target genes that induce cell proliferation, invasion, metastasis, resistance to treatment, and/or inflammation [42]. The constant expression of active NF-κB has been reported in many cell lines and tumors, including in breast cancer [43], gynecologic cancer [44], gastrointestinal cancer [45], head and neck squamous cell carcinoma [46], hematological cancer [47], melanoma [48]. Curcumin prevents the activation of NF-kB in cell types though inhibiting the transfer of P65 to the nucleus and suppressing the breakdown of IκBα (49). By inhibiting the activation of NF-kB, curcumin suppresses the expression of various survival cells and proliferation genes, including Bcl-2, BCL-XL and Cyclin D1, IL-6, cyclooxygenase 2 (COX-2) and matrix metallopeptidase (MMP) -9. It then stops the cell cycle, inhibits the cellularity of the cell and induces apoptosis later on [50]. AP-1, known for the first time as an induction transcription factor of 12-O-tetradecanoylphorbyl- 13-acetate (TPA), is another transcription factor that expresses genes responsible for cell proliferation, survival, differentiation, apoptosis, cell migration, and adjusts transformation [51]. AP-1 is a dimeric complex consisting of many different proteins belonging to the family of C-FOS, c-Jun, ATF and Jun proteins [52]. These AP-1 factors can respond to the element TPA binding and increase the expression of the target gene [53]. It has been shown that curcumin prevents the activation of AP-1 through preventing the AP-1 binding to its binding motive to DNA in the tumor promoter [54]. Curcumin increases the expression of glutamate-cysteine ligase (GCL) and other enzymes of phase II, due to the increased content of JunD and C-jun in the AP-1 complex and MafG/MafK and reduction in the EpRE complex [55]. As already stated, curcumin can activate some transcription factors such as AhR, ATF3, CHOP, EpRE and NRF2. The induction of ATF3 contributes to the pre-apoptotic effects of this compound [33]. The activation of Nrf2 by curcumin is associated with induction of hemeoxygenase-1 (HO-1) and increased expression of the activity of the aldose reductase promoter [56,57].
Growth factors and protein
Growth factors and their receptors play an important role in the natural process of growth and differentiation. Unregulated expression of these molecules can end in the abnormal growth and malformation [58]. In addition, increased expression of growth factors, such as transforming growth factor-α (TGF-α), could end in non-neoplastic disorders such as psoriasis [59,60]. Curcumin has shown to modify the expression and activity of these growth factors, so showing anti-proliferative, anti-invasive and anti-angiogenic effects (Figure 5). Epidermal Growth Factor Receptor (EGFR; ERBB-1; HER1 in humans) is a plasmid membrane integrin tyrosine kinase protein with a dominant connection to the cysteine-rich extracellular ligand, a transmembrane hydrophobic membrane, and a C-terminal semiconductor containing tyrosine with kinase function and various positions of autophosphorylation of tyrosine [61,62]. This is one of the members of the family of ErbB receptors linked to the subfamilies of four receptor tyrosine kinases: EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her3 (ErbB-3), Her4 (ErbB-4) [61]. EGFR activation occurs mainly through liganddependent mechanisms yet can also occur through pathways independent of the ligand, as well as by enhancing receptor expression [61]. EGFR and its family members are stimulated by multiple ligands, including EGF, EGF, TGF-α, amphiregulin, betacellulin, epigen, epiregulin, and the growth factor of EGF-binding to heparin [61,63]. The ligand induces the binding of the receptor’s extracellular domain, forming the hemorrhage receptor and the heterodimer. The formation of this receptor dimer complex, auto- and/or cross-phosphorylation of the tyrosine resin stimulates the receptor terminal C at the tail of the receptor which can trigger phosphorylation/signaling cascade through interlocking with proteins with the dominant SH2- and the terminal bond to phosphotyrosin [61]. Furthermore, it has shown that EGFR can be moved to the nucleus where it can act as a vector for cyclin D1 [61,64] and as an activating aid for STAT3 [65] and E2F1 [66], Unregulated signaling pathway of EGFR is an important contributing factor to many types of cancers such as breast [67], lung [68], colorectal [69] and head/neck [70]. EGFR has been reported as a potential curcumin target [71]. Curcumin blocks EGFR signaling pathway by blocking EGFR tyrosine phosphorylation and inhibiting EGFR gene expression by interacting with PPAR-γ activation [72]. Curcumin significantly inhibits the proliferation and survival of PC- 14 adenocarcinoma of the lung and P34 adenocarcinoma of the pancreas, associated with inhibiting the extracellular kinase receptor phosphorylation (ERK 1/2) and decreasing the expression of COX-2 and EGFR protein [72]. Likewise, curcumin has shown that tyrosine kinase activity inhibits the neu/HER2 receptor and discharges the receptor protein. The suppression of HER2/neu and EGFR activities is one of the mechanisms by which curcumin suppresses the growth of breast cancer cells [73]. Angiogenesis is a physiological process of the growth of new blood vessels from preexisting vessels. In cancer, angiogenesis is generally considered as an important step in the growth and metastasis of the tumor. Growth factors produced by the tumor can stimulate vascular formation [74]. Curcumin might directly inhibit angiogenesis and reduce the expression of various pro-angiogenic growth factors such as VEGF, FGF and EGF [75]. Estrogen and alpha and beta receptors (ERα ERβ) play an important role in the development and development of breast cancer [76]. As in many receptors in breast cancers, ER moderation is a promising tool for controlling breast cancer. Curcumin has inhibited the growth of both ER-positive MCF-7 and T47D cells, as well as ER-negative cells MDA-MB231, suggesting that curcumin may exert its chemical precursor effects independently of the occurrence of estrogen receptor [77]. The effects of curcumin are mediated through the inhibition of other protein kinases, including autophosphorylation-activated protein kinase (AK) [20], Ca2+ -dependent protein kinase (CDPK) [20], FAK [18], IL-1 receptor-associated kinase IRAK) [78], Janus Kinase (JAK) [79], mitogen-activated protein kinases (MAPKs) [80,81], the mammalian target of rapamycin (mTOR) [82,83], phosphorylase kinase (PhK), protamine kinase (cPK), PKA, pp60c-src [20], cytosolic PKB/Akt [84], PKC [81], spleen tyrosine kinase (Syk) [85] (Figure 5).
Inflammatory cytokines
During severe infection or after severe injury, excessive synthesis and production of inflammatory cytokines, including TNF-α, IL-1β and IL-6, play a major role in the development of topical and systemic inflammation, resulting in severe pathophysiological impairment or defects in the limbs [86,87]. The cytokine gene and the expression of the protein in the producing cells are heavily controlled and one of the important steps in this gene transcription setting. Therefore, inhibiting the production of proinflammatory cytokines by regulating transcription factors, such as NF-kB, is a potential strategy for controlling inflammatory reactions [87,88]. Some studies have shown that curcumin can modulate the production of various inflammatory cytokines, resulting in strong anti-inflammatory activities [89,90]. TNF-α plays an important role in regulating immune cells and systemic inflammation [91]. Disruption of TNF-α production is shown in a variety of inflammatory diseases (such as rheumatoid arthritis, Crohn’s disease, multiple sclerosis, psoriasis) and cancer [92]. In vitro and in vivo studies have proven curcumin’s strong inhibitory effects on TNF-α production. In monocytes and alveolar macrophages, curcumin inhibits the production of stimulated PMA or lipopolysaccharide (LPS) mediated TNF-α [89]. In diabetic rats, chronic treatment with curcumin reduces serum TNF-α levels, cognitive impairment, oxidative stress, and inflammation significantly [93]. Interleukin is another group of inflammatory cytokines with an important role in regulating inflammatory response. In addition, signaling pathways such as NF-kB and STATs have a role in tumor invasion and angiogenesis [94]. In HaCaT-cells treated with TNF-α, curcumin deters the expression of IL-1β and IL-6 by inhibiting NF-kB and the MAPK pathway [95]. In human lymphocytes stimulated with cancanavaline A, phytohemagglutinin and PMA, curcumin inhibits the synthesis of IL-2 and this effect may interfere with NF-kB inhibition [96].
Enzymes
Some types of enzymes associated with inflammation and cancer have shown to be modified by curcumin. These enzymes are COX-2 inducible nitric oxide synthase (iNOS), 5-LOX phospholipases A2 (PLA2). COX-2, an induction form of COX, can selectively be induced by mitochondrial and inflammatory stimuli, ending in an increase in the synthesis of prostaglandins in inflamed and neoplastic tissues [97,98]. Evidence shows that COX-2 is increasing in a wide range of cancers in humans, such as colon, liver, pancreas, breast, lung, bladder, skin, stomach, head and neck [98]. Curcumin can reduce the expression and the activity of COX-2 in vitro and in vivo [99,100]. In TPAtreated mouse, curcumin inhibits the expression of COX-2 protein strongly along activating TPA-stimulated NF-kB [99]. In gastrointestinal cell lines (SKGT- 4, SCC 450, IEC-18 HCA -7,), curcumin suppresses the COX-2 protein induced by chenodeoxycholate or PMA and its mRNA expression [101]. HO-1 is an enzyme catalyzing degradation to bileuridine, iron, and carbon monoxide [102]. HO-1 induction is involved in inflammatory response in the lung [103], liver [104] and kidney [105], as well as systemic response to hemorrhagic shock [106]. Curcumin inhibits glomerular fibrosis through HO-1 induction [107]. The induction of HO-1 by curcumin is connected with the production of reactive oxygen species (ROS), activation of P38 and inhibition of phosphatase [108]. Other important enzymes whose expression is reduced by curcumin are arylamine N-acetyltransferase [109], ATPase [110], desaturase [111], DNA polymerase [17], farnesyl protein transferase (FPTase) [112], iNOS (113), 5-LOX [114], MMP [115], NAD (P) H dehydrogenase quinine oxidoreductase 1 [116], ornithine decarboxylase (ODC) [117], PLA2 [73], telomerase [118,119], and xanthine oxidase (XO ) [120,121]. Conversely, the enzymes enhanced by expression curcumin are GCL [122], and 2 domain-containing tyrosine src homology [123] (Figure 5).
Adhesion molecules
Cell adhesion molecules (CAMs) are glycoproteins at the cell surface needed for binding other cells or extracellular matrix in a process called cell adhesion [124]. The expression of cell surface expression of various adhesion molecules such as intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), endothelial leukocyte adhesion molecule-1 (ELAM-1) play an important role in inflammatory diseases and Neoplastics [125,126]. It is reported that TNF-α-expressed expression in ICAM-1, VCAM-1, and E-selectin is inhibited by inhibiting NF-kB, which shows the expression of CAMs is partially regulated by NF-kB [127]. The most recent studies have shown that curcumin inhibits the expression of VCAM-1 in human intestinal microvascular endothelial cells by suppressing AKT, MAPK, P38, and NF-kB [128].
Apoptosis-associated proteins
Apoptosis, or planned cell-death, defined as a cellular suicide mechanism after serious cell damage is essential for the development and maintenance of cell homeostasis in single cell and porcelain organisms [129]. Uncontrolled apoptosis can lead to cancer, autoimmune disease, and degenerative diseases [130]. Hence, increased interest has been on clarifying apoptotic pathways for causing disease and identifying compounds that can induce apoptosis. Studies have shown that curcumin can induce apoptosis in a number of human cancer cells and inhibit the onset of tumor onset and growth in animals [131-134]. Curcumin chemical prevention action may lie in its ability to induce apoptosis by several pathways [135]. A microarray study showed apoptotic genes regulated by curcumin in tumor cells. The results were indicative of the fact that among the 214 genes associated with apoptosis, expression of 104 genes was altered by curcumin. The genes expressed by curcumin are HIAP1, CRAF1, TRAF6, CASP1, CASP2, CASP3, CASP4, HPRT, GADD45, MCL-1, NIP1, BCL2L2, TRAP3, GSTP1, DAXX, PIG11, UBC, PIG3, PCNA, CDC10, JNK1, RBP2 [134]. The genes that are expressed by curcumin are TRAIL, TNFR, AP13, IGFBP3, SARP3 PKB, IGFBP, CASP7, CASP9, TNFSF6, TRICK2A, CAS, TRAIL-R2, RATS1, hTRIP, TNFb TNFRSF5 [136].
Nano-formulations of curcumin
The use of curcumin for various diseases is mainly due to its active biological functions, such as anti-inflammatory, antioxidant, antimicrobial, anti-Alzheimer’s, anti-tumor, anti-diabetic and anti-rheumatic activities [12,137]. Moreover, curcumin has shown to be a blood glucose-lowering agent, neuromuscular, cardio and nervous protective molecule [138]. More importantly, this molecule suppresses thrombosis and protects against heart attacks as well. Over the past two decades, the publication of about 8,000 dreams, articles, reports, comments, patents and clinical trials has proven that curcumin, which is actually a potential therapeutic molecule. Moreover, the molecule is considered to be “generally recognized as safe (GRAS) by the American Food Drug Administration (USFDA)” [139]. Like many other small molecules of drug-rich drugs, curcumin is also restricted for its efficient use in clinical situations for the treatment of disease. These limitations are low water content and inherent dissolution rate, low physical-chemical instability, rapid metabolism, low bioactive absorption, pharmacokinetics and low bioavailability, targeting efficiency and low penetration [140-142]. All of these factors affect the effective use of curcumin as a therapeutic molecule significantly. Thus, different formulations like natural, modified, and micro/nano-curcumin formulations, emulsions, creams, solutions, pills, gels, wound adhesives, and so on are used for conventional or exploratory injections to achieve optimal results in different pathologic conditions [143-145]. Curcumin shows much strength, like traditional use for centuries, excellent biological activity, extensive pre-clinical, animal, clinical, and human use that promotes the rapid development of curcumin or curcumin formulations in medicine. These positive indicators promote nanotechnologists to design and formulate nanocorcinom formulations to enhance dissolution, sustainability, cellular uptake/internalization, attribute, tolerance, and therapeutic index [145,146]. During the past decade, several methods have been developed according to nano-materials to increase the use of curcumin in vitro, in vivo and in the field of preclinical studies, like the use of conjugates/polymer conjugates, lipid/liposomes hydro/micro/ nanogel, and nanoparticles (NPs) [146]. Specific roles and the benefits of using any delivery system are presented in Table 1. Many of these efforts have initially improved bioavailability, yet newer formulations have stressed the efficient targeting of curcumin in the site with the help of antibodies, aptamers, and peptides [145]. Effective delivery of curcumin through using nanotechnology not only helps overcome solubility, rapid drug metabolism, decomposition and sustainability issues, but also nanoscience. Thus, it is necessary to diffuse or target tissue debris, while the unwanted toxicity around the normal cells/minimize the texture [139]. The applications of curcumin nano-materials in the treatment of cancer In many in vitro and in vivo conditions, curcumin nanomaterials have shown superior therapeutic benefits over free curcumin [139]. In this section, we check the use of different curcumin formulations for cancer treatment. After heart disease, cancer is the second leading cause of death in humans. The most commonly used therapies are surgery, chemotherapy, radiotherapy, targeted therapy, immunotherapy, hyperthermia, photothermic therapy, and other alternative therapies. Traditionally, chemotherapy is highly recommended for both solid tumors and metastasis. Nonetheless, the side effects of chemotherapy for normal and healthy tissues/organs are quite harmful. Thus, curcumin and its nano-materials play an important role in increasing sensitivity to radiation/chemotherapy and can act as a therapeutic option to provide a suitable dose at tumor site. Curcumin nano-forms significantly enter the cancer cells through endocytosis or receptor mediators in the presence of endocytosis and curcumin is released into active form to induce its biological effects [139].
| Type of nanoparticles | Significance and comments | |
|---|---|---|
| Liposomes [147-150] | Liposomes are generated from phospholipid bilayers. This is the second most widely used vehicle to solubilize/encapsulate curcumin. | |
| Cyclodextrins [151-154] | Cyclodextrins are cyclic oligosaccharides that can solubilize curcumin in a lipophilic cavity, and the hydrophilic outer surface helps in greater dispersion of the formulation. | |
| Micelles [155-159] | Micelles or polymeric micelles are composed of amphiphilic block copolymers that spontaneously form 20–100 nm micelles in aqueous solution at the above critical micellar concentration. The hydrophobic core of micelles can effectively house curcumin for solubilization and targeted delivery. | |
| Dendrimers [160-164] | Dendrimers are composed of highly branched and star-shaped networks of macromolecules. Typically, dendrimers are formed symmetrically around the core at nanometer-scale dimensions and are three-dimensionally spherical in morphology. These carriers are highly suitable for conjugation and loading of curcumin. | |
| Nanogels [165-167] | Nanogels are hydrogel nanoparticles of swollen physical/chemically cross-linked networks composed of hydrophilic or amphiphilic polymer chains. These carriers can be designed to transport various drug molecules including curcumin. These carriers mimic human tissues due to higher hydrophilicity in the system due to swollen nature. | |
| Gold nanoparticles [168, 169] | Gold nanoparticles are emerging as a novel platform as photothermal agents, contrast agents, and radiosensitizers. In addition, current literature supports their use in the delivery of curcumin. | |
| Polymers [170-173] | Polymers have been exploited to improve solubility and bioavailability of curcumin. Polymeric carriers have been widely studied for efficient delivery of curcumin. | |
| Conjugates [174, 175] | Conjugation of curcumin to small molecules and hydrophilic polymers is a known practice to increase aqueous solubility. | |
| Lipid nanoparticles [176-179] | Lipid nanoparticles are typically spherical in shape with a lipid core matrix that can solubilize curcumin. The lipid core is usually stabilized by surfactant molecules. | |
| Magnetic nanoparticles [180-182] | Magnetic nanoparticles are a class of nanoparticles that can be used for multifunctional purposes including delivery of drugs (curcumin), magnetic resonance imaging, and hyperthermia. | |
Table 1: Commonly used curcumin delivery systems and their specific advantages over conventional Systems
Curcumin emulsion formulation
Micro-emulsions are isotropic nanostructures as stable solutions of surfactants, oils, and water. Curcumin-based microemulsion is expected to enhance the delivery of curcumin via topical and transdermal routes for systemic sclerosis, psoriasis, and skin cancer. Curcumin microemulsion is highly permeable to eucalyptol and is fluctuated with the moderate solubility of curcumin compared to many microemulsions based on esteem oil and oleic acid [147-183]. Simultaneous administration of curcumin and paclitaxel nanoemulsions can defeat the multi-drug resistance in human ovarian cancer cells (SKOV3) by inhibiting the activity of NF-kB, reducing the expression of P-gp, and accelerating apoptosis [184]. In addition, curcumin nano-emulsion increases the bioavailability of paclitaxel by 5.2 times. In addition, oral administration of paclitaxel to models of transgenic goofy mice that carries the SKOV3 tumor causes a 3.2-fold increase in accumulation of paclitaxel in the tumor site. This is due to a decrease in the expression level of the proteins of the P-gp of the intestines and of the cytochrome P450 3A2 (CYP3A2) [185].
Curcumin liposomes formulation
Liposomes are composed of synthetic phospholipid vesicles, which appear to be bio-safe and bio-compatible and protect medications from external stimuli. Given the presence of both hydrophilic and hydrophilic groups in the structure, liposomes are an interesting carrier for delivery of the drug. The hydrophobic layer mainly contains phospholipids and cholesterol molecules. This fat-based carrier is suitable for delivering water-insoluble chemical preventive agents like curcumin, resveratrol, oryzanol and N-acetylcysteine. Based on the drug’s lipophilicity, the drug can be placed between the two layers of phospholipids or in the interior of the liposome. Liposomes are specifically designed to regulate drug release, permeability, cellular assimilation, targeting, and distribution [186]. It has been determined that a more developed absorption capacity of curcumin can be obtained by dissolving, mixing or mixing it with different types of phospholipid [146]. Encapsulated curcumin based on DMPC- (dimyristoylphosphatidylcholine) inhibited 70-80% cellular activity in prostate cancer cells LNCaP and C4- 2B. Curcumin loading liposomes were undeniably more effective than crude curcumin as the concentration of 10 times more crude curcumin was required to produce similar cellular responses. These data emphasized bioremediation and higher absorption of curcumin [187]. Sou et al. successfully formulated lipid quercine with 1,2-dimyristoyl-sn-glycero-3- phosphocholine (DMPC) and an ammonium anion L-glutamic acid, N- (3-carboxy-1-oxopropyl) 1.5-dihexadecyl ester (SA). Intravenous injection of this formulation in rat did not show any acute responses in circulating blood cells and more curcumin accumulated in the bone marrow and spleen tissue [188]. The recent pharmacokinetic study of solid curcumin lipid nanoparticles in patients with osteosarcoma was reported in 4 hours of oral administration of 2000-4000 mg of curcumin, up to about 31.42 to 41.15 ng/ ml of curcumin. Most importantly, patients experienced no side effects [189]. A comparative study was conducted to examine the absorption of curcumin loaded in liposome and serum albumin in normal spleen lymphocytes and EL4 lymphoma cells, respectively, through liquid phase peenocytosis and membrane fusion. Liposomal formulations containing curcumin were better carriers and more fluorescence and absorption levels were observed in lymphoma cells compared to normal cells [190]. Li et al. evaluated the ratio of lipid curcumin (10: 1 wt./Wt) on various pancreatic cancer cells, such as ASPC-1, BxPC-3, Capan-1, Capan-2, HS766-T and MiapaCa2, and the concentration IC50 inhibitory activity was at 2.0-37.8 μM, whereas IC90, which was evaluated as cytotoxicity, was 6.75-94.5 μM [191]. Narayanan et al. presented an interesting insight into the use of curcumin and resveratrol in liposomes to examine their combined effects on:
1) Cell growth, apoptosis and cell cycle.
2) Activation of p-activated proteins, cyclin D1, mammalian target of rapamycin (m-TOR) and androgen receptor (AR) involved in prostate tumor progression PTEM-CaP8.
In general, this compound formula significantly reduced prostate adenocarcinoma and its incidence in the body (p<0.001) [192]. A new phospholipid-curcumin disk nanoparticle was successfully designed with a diameter of less than 50 nm and a two-layer phospholipid thickening with apolipoproteins as a stabilizer. The anti-proliferative activity of this curcumin nanoparticle in hepatoma and Jeko lymphoma cells was significantly higher than that of crude curcumin. Moreover, this nanoparticle induces more apoptosis in cancer cells [193]. Positive encoding nano-liposome curcumin was designed using PEG and polyethylene glycol (PEI) cations. Although this method decreased inclusions (45%), its cellular toxicity was significantly different from that of different cancer cell lines, like mouse cancer cells (B16F10 melaoma LL2 lung carcinoma, CT26 colorectal adenocarcinoma, JC breast adenocarcinoma) and human cancer cells HepG2 hepatocellular carcinoma, A549 lung carcinoma, HT-29 colorectal adenocarcinoma, cervical carcinoma about 20 times higher than crude curcumin. In vivo administration of this formula reduces tumor growth in mice with CT26 and B16F19 cancer cells [194] Encapsulation of curcumin with co-anticancer agents is another formulation method used to cure cancer, as the synergistic effect of several drugs increases the anticancer effects, along with reducing cell cytotoxicity to non-malignant [195]. Furthermore, a mixture of curcumin-based liposomes and oxaliplatin showed a higher inhibition in the growth of colorectal cancer compared with oxaliplatin alone [196].
Polymerization of curcumin
Nano-encapsulation of curcumin with polymers is a promising approach that simultaneously improves the biological efficiency of curcumin and reduces the rate of decomposition of curcumin in the body. So far, many natural and synthetic biodegradable polymers are used for encapsulation, like poly (vinyl alcohol) (PVA), poly (lactic-co-glycolic acid) (PLGA), N-isopropylacrylamide (NIPAAM), N-vinyl pyrrolidone, Polyethylene glycol monoacrylate (NIPAAM VP/PEG A), silk fibroin, chitosan. Overall, these polymers have common characteristics, including biocompatibility, biodegradability, easy physicschemical properties, and potential for moderated release of drugs g [197- 199]. Poly (lactic-co-glycolic acid) (PLGA) is a common choice in the production of various biomedical carriers due to biocompatibility and biodegradability. In an effort to produce a safe carrier, a variety of PLGA nanoparticles is discovered for encapsulation of curcumin. A simple solidoil- water solvent evaporation method is used to curcumin incorporation in PLGA nanoparticles. Particle size can be controlled by concentration of surfactant and sonication time [200]. Then, Yallapu et al. designed the solvent evaporation method for increasing the encapsulation of curcumin in PLGA nanoparticles through less particle size, cellular absorption, and anticoagulation properties [201]. A recent study showed that the encapsulation of curcumin nanoparticles by PVA and PLGA increases the fecundity of cancer cells. The authors of the study reported that curcumin-coated nanoparticles by controlling the NFKB nuclear factor in killing various cancerous cell lines from leukemia (K-562), human colon cancer (HCT-116), pancreatic cancer (PANC-1 and MIA PaCa- 2) are more efficient than curcumin free [186]. The functionalization of the surface of the PLGA nanoparticle having curcumin by bis (sulfosuccinimidyl) suberate (BS3) eased the binding of anxin A2 and led to the effective treatment of curcumin in cancer cells of MDA-MB-231 positive anxin A2 [202]. Shahani and Panyam developed a stable and injectable microparticular formulation of curcumin (i.e., 38.1 mg/100 mg of particles, 76.2% encapsulation efficiency) with a higher loading capacity compared to many formulations. Improved glutathione-s-transferase (GST) activity in the liver was observed with the injectable microparticles, and this phenomenon was consistent for four weeks. GST activity represents a powerful endogenous defense mechanism against carcinogens [203]. Dextran sulfate-chitosan-based nano-formulations are biocompatible materials that can be used for oral, intravenous and controlled delivery purposes. Anitha et al. measured cell absorption of encapsulated curcumin particles in dextran sulfate-chitosan nanoparticles using spectrophotometric method in cell lines of L929, MCF7, PC3, and MG 63 cells. Moreover, the study of cytotoxicity and fluorescence-activated cell sorting (FACS) suggested that the anticancer activity of this formula in the MCF-7 cell line was higher than the other cancer cells [204]. Copolymers such as NIPAAM, N-vinyl- 2-pyrrolidone, polyethylene glycol monoacrylate (NIPAAM [VP/PEG A]) are used to encapsulate curcumin. It was found that curcumin coated nanoparticles coated with NIPAAM (VP/PEG A) (VP/PEG A) were very effective in controlling the viability of medulloblastoma and glioblastoma cells. The initial result showed that curcumin expresses the expression of the IGF pathway that is important for the formation and growth of brain tumors [170,171]. Encapsulation of curcumin and doxorubicin by treating polymorphic nanoparticles treated multifocal resistant cancer cells more effectively (K562 cells). The initial release of curcumin from nanoparticles reduced the expression of Bcl-2 and MDR1, suppressing the mechanism of exertion of drug from cancer cells. Consequently, the release of doxorubicin was induced by cancer cell death [205].
Curcumin self-assemblies
Several various methods have been developed for the complexation of curcumin or the self-assembly of curcumin with β-cyclodextrin and their derivatives. Moreover, several β-cyclodextrin and curcumin complexes are reported recently. Cyclodextrins are oligosaccharides with a hydrophilic outer layer and a lipophilic core. The complexation and incorporation of hydrophobic drugs (e.g., curcumin) can occur in the central nucleus of cyquelocastrin. Cyclodextrin increases stability, bioavailability, decompression of curcumin and inhibition of non-malignant cells toxicity [151]. Yallapu et al. developed a delivery system for beta-cyclodextrin (CD) mediated curcumin drug via incubation. Measurement of cell proliferation and colonization revealed that self-assembly of curcumin-CD increased the delivery of curcumin and the therapeutic effect of CD-curcumin on prostate cancer cells has been improved compared with free curcumin [152]. The selfassembly of cyclodextrin-curcumin has more toxic effects compared to free curcumin in (human chronic myeloid leukemia) KBM-5, neck squamous cancer (SSC-4) human head, and (Caco-2) (human colonic carcinoma and, Caco-2 (human colonic carcinoma) and Panc-28 (pancreatic cancer). a tumor necrosis factor (TNF, activation of NF-kB, inhibition of the genes involved in cyclin D1, invasion and angiogenesis). This formulation controls tumor necrosis factor (TNF), activation of NF-kB, inhibition of the genes involved in cyclin D1, invasion and angiogenesis. On the other hand, the formulation expresses the expression of death receptors (DR4, DR5) in cancer cells of KBM- 5 increased [152]. A similar pattern of curcomin encapsulation in cyclodextrin and poly (ciclodextrin) led to self-assembly formation whose anti-cancer potential is enhanced by reducing the expression of the family of BCL2 pro-survival genes, Bax, Bcl-xL, and induction of apoptosis. Moreover, it is shown that the cellular toxicity of this formula is better than other formulations of ciclodlockestrin-curcumin. Treatment with this formulation showed a significant cut in the poly [ADP-ribose] polymerase [PARP] protein, as an indicator for cell death through apoptosis [206]. Curcumin hydration in the presence of poly (ethylene) -cholesteryl ether (PEG- Chol) showed a synergistic effect on myeloma cell lines (RPMI 8226, U266 and 5TGM1) to produce uniform nanoparticles (10 nm) [207]. A complex of liposomes, PEG, and polyethylene glycols were used to encapsulate curcumin. This complex showed inhibitory effects of 5-fold and 20-fold resistance on HepG2, HT- 29, HeLa, A549, CT26/cur-r and B16F10/cur-r cells. In rats with CT-26 or B16F10 cells, with this nan-formulation of curcumin, 60-90% inhibition in tumor growth was observed [208].
Mycelium curcumin formulation
Polymylcellulose micelles (PM) are macromolecular communities of amphiphilic copolymers in aqueous solutions that form a spherical core and internal shell formed by hydrophobic interactions with insoluble parts in water. Micelle should not be confused with liposomes; the liposomes are composed of two lipid layers, whereas micelle is made of single-layer lipid. Advantages of using polymer micelles as carriers for hydrophobic drugs are improving the stability and solubility of the drug, reducing toxicity to healthy cells, prolonging circulation time and increasing tissue penetration. Several biodegradable and biocompatible amphiphilic copolymers are used in the manufacture of PM, including pluronic, poly (ethylene glycol) -b-poly (D, L-lactide) (PEG-PDLLA), poly (ethylene glycol) -b-PCL (PEGPCL), poly (ethylene glycol) -b-poly (lactide-co-glycolic acid) (PEG-PLGA), and poly (κ-caprolactone). Pluronic, consisting of poly (ethylene oxide) PEO hydrophilic blocks and poly (propylene oxide) PPO are the most commonly used polymer for micelle systems based on hydrophilic/hydrophilic interactions for micellisation [209]. Sahu et al. showed that Pluronic (F127) has more molecular weight to trap curcumin compared to Pluronic (F68) with less molecular weight, although the release of curcumin is reversed. After 10 days, 80% of the curcumin was released from Pluronic, whereas only 60% of curcumin is released from Pluronic (F127). Pluronic (F127) has cytotoxic activity on HeLa cells in vitro, whereas IC50 for free curcumin, pluronic F68, and pluronic F127 are 14.32 16.01, 17.45 μM respectively [210]. Micelle systems based on polymer materials, widely used for delivery of curcumin to cancer cells, have been studied. Methoxy poly (ethylene glycol)- b-poly (ԑ-caprolactone-co-p dioxanone) (MPEG-P[CL-co-PDO]), amphiphilic, micelle polymer nano-particles were used for delivery of curcumin to PC-3 human prostate cancer cell. The mixed micellar copolymers had high incubation efficacy (95%<) had release profile of the resistant drug and dose-dependent cytotoxic effect on cancer cells relative to crude curcumin. In addition, no toxicity was found in the empty micellar carrier [211]. In another study, PEO was mixed with hydrophobic nuclei PCL to form a copolymer with curcumin-bound incubation properties. As expected, as PCL molecular weight increased, the interaction between hydrocortisone curcumin and PCL increased, resulting in improved incubation efficiency. Interestingly, the curcumin solubility reached 626.98 μg/ml from 11 ng/ml, whereas its anticancer activity was retained, which was confirmed with in vitro cytotoxicity studies on rat’s melanoma cancer cell (B16-F10) and human cancer cells human lymphoma cell lines (Mino and SP-53) and human mantle cell lymphoma cell lines (JeKo-1) [212].
Formulation of curcumin nano-gel
Drug delivery with the use of hydrogel and nano-gel is not something new, yet there are only few studies available on the delivery of curcumin to cancer cells and relevant biological interactions. Nano-gel, a miniature version of hydrogel, has the same characteristics as its micro-counterpart, including high stability in aqueous solutions, high aqueous adsorption and inflammation ratios. In addition to other properties of nano-carriers, nano-gel has additional unique features such as large total surface and porous structure, respectively, for conjugation of drawers and storage of drugs [165,213].
Mangalathil et al. designed a biocompatible, biodegradable, chitin nano-gel encapsulating curcumin for treating skin cancer through transdermal routes. Chitin nano-gel had, 70-80 nano-meters, a specific toxicity to human skin melanoma (A375), but was less toxic to human skin fibroblasts (HDF) cells. The flow cytometric results showed that curcumin-encapsulating chitin nanogel had apoptosis effect compared with crude curcumin, indicating that the anticancer activity of curcumin was retained even after being incorporated into the gel [212]. In another study, alginate-chitosan-pluronic nanogel was synthesized through the polycation interactions process to encapsulate curcumin to test cancer in vitro. Although the encapsulation efficiency was higher (about 5-10 times), the effect of encapsulated curcumin cell cytotoxicity was not statistically superior to HeLa cells [4]. A study by Wei et al., a nano form of curcumin was designed that sustained and significantly increased cell permeability and anticancer activity in standard oral administration. Curcumin as an ester binds to a nano-gel hyaluronic cholesterol acid (CHA) that is capable of delivering targeted therapies to cancer-resistant cancerous cells expressing a drug-resistant CD44. The CHA-CUR nano-gel shows excellent solubility and stable release of the drug in physiological conditions. CHA-CUR nano-gel with suppressing the expression of NF-kB, TNF-α and ah COX-2 dough, similar to free curcumin, induces apoptosis in cancerous cells. CHA-CUR effectively inhibited tumor growth in the adenocarcinoma of the human pancreas MiaPaCa-2 and the anthropogenic invasive animal models of 4T1 mouse breast cancer [166].
Discussion and Conclusion
Curcumin is an inexpensive polyphenolic compound extracted from curcuma longa, which is widely available, non-toxic, with pharmaceutical opportunities. Some in vitro and in vivo conditions and clinical trials have provided evidence for the active role of curcumin in preventing and treating various human diseases, including cancer. At the molecular level, multiple paths of curcumin targets have highlighted its ability in controlling cancer at various levels, and so potentially have bypassed the development of resistance. However, there is little information available to explain the underlying mechanism of curcumin activity. Clinical trials show safety, tolerance, nontoxicity (even up to 8000 mg/day), and the effectiveness of curcumin. These studies provide a solid base for well-controlled studies in larger groups as well as open up ways for future drug development. Nevertheless, curcumin activity is limited due to its poor bioavailability and some complications. The development of curcumin formulations in the form of nanoparticles, liposomes, micelles or phospholipid complexes to enhance bioavailability and its efficacy is still in its infancy. Most of these studies have only been conducted in pre-clinical animal models, so a major disadvantage is the lack of understanding of the dangers of curcumin nanoparticles in humans. Thus, testing these formulations as therapeutic approaches is highly desirable and it is very important for future clinical trials and their use in humans. However, curcumin has proven itself as a safe and promising molecule for not only cancer prevention, but also inflammation-controlled diseases.
REFERENCES
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):359-86.
- Duvoix A, Blasius R, Delhalle S, et al. Chemo-preventive and therapeutic effects of curcumin. Cancer Lett. 2005;223(2):181-90.
- Maheshwari RK, Singh AK, Gaddipati J, et al. Multiple biological activities of curcumin: A short review. Life sciences. 2006;78(18):2081-7.
- Sharma R, Gescher A, Steward W. Curcumin: The story so far. Eur J Cancer. 2005;41(13):1955-68.
- Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int. 2014.
- Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133-64.
- Perrone D, Ardito F, Giannatempo G, et al. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med. 2015;10(5):1615-23.
- Shanmugam MK, Rane G, Kanchi MM, et al. The multifaceted role of curcumin in cancer prevention and treatment. Mol. 2015;20(2):2728-69.
- Naksuriya O, Okonogi S, Schiffelers RM, et al. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365-83.
- Mullaicharam A, Maheswaran A. Pharmacological effects of curcumin. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2012;2(2):92.
- Sandur SK, Pandey MK, Sung B, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765-73.
- Aggarwal BB, Sundaram C, Malani N, et al. Curcumin: The Indian solid gold. Adv Exp Med Biol. 2007;595:1-75.
- Mullaicharam A, Maheswaran A. Pharmacological effects of curcumin. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2012;2(2):92-9.
- Zhou H, Beevers C, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12(3):332-47.
- Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267(1):133-64.
- H Sarkar F, Li Y, Wang Z, et al. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des. 2010;16(16):1801-12.
- Takeuchi T, Ishidoh T, Iijima H, et al. Structural relationship of curcumin derivatives binding to the BRCT domain of human DNA polymerase lambda. Genes Cells. 2006;11(3):223-35.
- Leu TH, Su SL, Chuang YC, et al. Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem Pharmacol. 2003;66(12):2323-31.
- Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280(26):25284-90.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994;341(1):19-22.
- Skrzypczak-Jankun E, Zhou K, McCabe NP, et al. Structure of curcumin in complex with lipoxygenase and its significance in cancer. Int J Mol Med. 2003;12(1):17-24.
- Gupta KK, Bharne SS, Rathinasamy K, et al. Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. FEBS J. 2006;273(23):5320-32.
- Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J Alzheimers Dis. 2004;6(4):367-77.
- Ishihara M, Sakagami H. Re-evaluation of cytotoxicity and iron chelation activity of three β-diketones by semiempirical molecular orbital method. In Vivo. 2005;19(1):119-23.
- Shin HK, Kim J, Lee EJ, et al. Inhibitory effect of curcumin on motility of human oral squamous carcinoma YD‐10B cells via suppression of ERK and NF‐κB activations. Phytother Res. 2010;24(4):577-82.
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP‐1 and NFκB transcription factors. J Neurochem. 2007;102(2):522-38.
- Bhattacharyya S, Mandal D, Saha B, et al. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007;282(22):15954-64.
- Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15(6):1557-62.
- Wang Z, Zhang Y, Banerjee S, et al. Retracted: Notch‐1 down‐regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106(11):2503-13.
- Chen A, Xu J, Johnson A. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25(2):278.
- Prasad CP, Rath G, Mathur S, et al. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/β-catenin signaling. Chem Biol. Interact. 2009;181(2):263-71.
- Rinaldi AL, Morse MA, Fields HW, et al. Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits (-)-benzo (a) pyrene-7R-trans-7, 8-dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Research. 2002;62(19):5451-6.
- Yan C, Jamaluddin MS, Aggarwal B, et al. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4(2):233-41.
- Jung EM, Lim JH, Lee TJ, et al. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis. 2005;26(11):1905-13.
- Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin: The molecular targets and therapeutic uses of curcumin in health and disease. Springer. 2007;595:127-48.
- Chen A, Xu J. Activation of PPARγ by curcumin inhibits moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):447-56.
- Yang C, Zhang X, Fan H, et al. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Research. 2009;1282:133-41.
- Hayden M, West A, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25(51):6758.
- Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132(3):344-62.
- Thompson RC. NF-kappa B-dependent regulation of the diagnostic marker CD10 and role of BCL-2 activity in histone deacetylase inhibitor-induced apoptosis in human B-lymphoma cell lines. Boston University. 2013.
- Verma IM, Stevenson JK, Schwarz EM, et al. Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev. 1995;9(22):2723-35.
- Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18(49):6853.
- Sovak MA, Bellas RE, Kim DW, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100(12):2952-60.
- Lu T, Sathe SS, Swiatkowski SM, et al. Secretion of cytokines and growth factors as a general cause of constitutive NFκB activation in cancer. Oncogene. 2004;23(12):2138.
- Wang W, Abbruzzese JL, Evans DB, et al. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5(1):119-27.
- Jackson-Bernitsas D, Ichikawa H, Takada Y, et al. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-κB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 2007;26(10):1385.
- Kordes U, Krappmann D, Heissmeyer V, et al. Transcription factor NF-κB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia. 2000;14(3):399.
- Yang J, Richmond A. Constitutive IκB kinase activity correlates with nuclear factor-κB activation in human melanoma cells. Cancer Research. 2001;61(12):4901-9.
- Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). J Biol Chem. 1995;270(42):24995-5000.
- Aggarwal S, Takada Y, Singh S, et al. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor‐κB signaling. Int J Cancer. 2004;111(5):679-92.
- Karin M, Liu Z-g, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol.1997;9(2):240-6.
- Vesely PW, Staber PB, Hoefler G, et al. Translational regulation mechanisms of AP-1 proteins. Mutation Research/Reviews in Mutation Research. 2009;682(1):7-12.
- Angel P, Imagawa M, Chiu R, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49(6):729-39.
- Bierhaus A, Zhang Y, Quehenberger P, et al. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77(4):772-82.
- Dickinson DA, Iles KE, Zhang H, et al. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17(3):473-5.
- Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371(3):887-95.
- Kang ES, Kim GH, Kim HJ, et al. Nrf2 regulates curcumin-induced aldose reductase expression indirectly via nuclear factor-κB. Pharmacol Res. 2008;58(1):15-21.
- De Jong JS, Van Diest PJ, Van Der Valk P, et al. Expression of growth factors, growth factor receptors and apoptosis related proteins in invasive breast cancer: relation to apoptotic rate. Breast Cancer Res Treat. 2001;66(3):201-8.
- Elder JT, Fisher GJ, Lindquist PB, et al. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989;243(4892):811-4.
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: A model for targeted therapy. Clin Cancer Res. 2006;12(18):5268-72.
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-37.
- Burgess AW, Cho HS, Eigenbrot C, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12(3):541-52.
- Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802-8.
- Lo HW, Hsu SC, Ali-Seyed M, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7(6):575-89.
- Hanada N, Lo HW, Day CP, et al. Co-regulation of B-Myb expression by E2F1 and EGF receptor. Mol Carcinog. 2006;45(1):10-7.
- Ahmed KM, Cao N, Li JJ. HER-2 and NF-kappaB as the targets for therapy-resistant breast cancer. Anticancer Res. 2006;26(6):4235-43.
- Dancey JE, Freidlin B. Targeting epidermal growth factor receptor--are we missing the mark? Lancet. 2003;362(9377):62-4.
- Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med. 2011;11(57):95-105.
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666-72.
- Korutla L, Cheung JY, Mendelsohn J, et al. Inhibition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin. Carcinogenesis. 1995;16(8):1741-5.
- Chen A, Xu J. Activation of PPAR {gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):447-56.
- Hong RL, Spohn WH, Hung MC. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin Cancer Res. 1999;5(7):1884-91.
- Gasparini G. Biological and clinical role of angiogenesis in breast cancer. Breast Cancer Res Treat. 1995;36(2):103-7.
- Strimpakos AS, Sharma RA. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10(3):511-46.
- Herynk MH, Fuqua SA. Estrogen receptors in resistance to hormone therapy. Adv Exp Med Biol. 2007;608:130-43.
- Verma SP, Goldin BR, Lin PS. The inhibition of the estrogenic effects of pesticides and environmental chemicals by curcumin and isoflavonoids. Environ Health Perspect. 1998;106(12):807-12.
- Jurrmann N, Brigelius-Flohe R, Bol GF. Curcumin blocks interleukin-1 (IL-1) signaling by inhibiting the recruitment of the IL-1 receptor-associated kinase IRAK in murine thymoma EL-4 cells. J Nutr. 2005;135(8):1859-64.
- Rajasingh J, Raikwar HP, Muthian G, et al. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem Biophys Res Commun. 2006;340(2):359-68.
- Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174(12):8116-24.
- Rafiee P, Nelson VM, Manley S, et al. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): Role of PKC, MAPKs, and NF-kappa B. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):388-98.
- Johnson SM, Gulhati P, Arrieta I, et al. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29(8):3185-90.
- Shinojima N, Yokoyama T, Kondo Y, et al. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3(6):635-7.
- Kizhakkayil J, Thayyullathil F, Chathoth S, et al. Modulation of curcumin-induced Akt phosphorylation and apoptosis by PI3K inhibitor in MCF-7 cells. Biochem Biophys Res Commun. 2010;394(3):476-81.
- Gururajan M, Dasu T, Shahidain S, et al. Spleen tyrosine kinase (Syk), a novel target of curcumin, is required for B lymphoma growth. J Immunol. 2007;178(1):111-21.
- Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163(2):316-21.
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12(1):141-79.
- Tak PP, Firestein GS. NF-kappa B: A key role in inflammatory diseases. J Clin Invest. 2001;107(1):7-11.
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39(1):41-7.
- Chen D, Nie M, Fan MW, et al. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology. 2008;82(4):264-9.
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104(4):487-501.
- Aggarwal BB, Shishodia S, Takada Y, et al. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006(56):161-86.
- Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: Behavioral and biochemical evidences. Eur J Pharmacol. 2007;576(1-3):34-42.
- Dinarello CA. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 2006;25(3):307-13.
- Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19(3):469-74.
- Ranjan D, Chen C, Johnston TD, et al. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappa-B activation, and IL-2 signaling. J Surg Res. 2004;121(2):171-7.
- Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4(1):17-23.
- Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: A molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24(2):96-102.
- Chun KS, Keum YS, Han SS, et al. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24(9):1515-24.
- Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67(8):3853-61.
- Zhang F, Altorki NK, Mestre JR, et al. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20(3):445-51.
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61(2):748-55.
- Otterbein LE, Kolls JK, Mantell LL, et al. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103(7):1047-54.
- Amersi F, Buelow R, Kato H, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104(11):1631-9.
- Vogt BA, Shanley TP, Croatt A, et al. Glomerular inflammation induces resistance to tubular injury in the rat: A novel form of acquired, heme oxygenase-dependent resistance to renal injury. J Clin Invest. 1996;98(9):2139-45.
- Tamion F, Richard V, Bonmarchand G, et al. Induction of heme-oxygenase-1 prevents the systemic responses to hemorrhagic shock. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1933-8.
- Gaedeke J, Noble NA, Border WA. Curcumin blocks fibrosis in anti-Thy 1 glomerulonephritis through up-regulation of heme oxygenase 1. Kidney Int. 2005;68(5):2042-9.
- McNally SJ, Harrison EM, Ross JA, et al. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19(1):165-72.
- Chen YS, Ho CC, Cheng KC, et al. Curcumin inhibited the arylamines N-acetyltransferase activity, gene expression and DNA adduct formation in human lung cancer cells (A549). Toxicol In Vitro. 2003;17(3):323-33.
- Shimizu S, Jareonkitmongkol S, Kawashima H, et al. Inhibitory effect of curcumin on fatty acid desaturation in Mortierella alpina 1S-4 and rat liver microsomes. Lipids. 1992;27(7):509-12.
- Kohl NE, Omer CA, Conner MW, et al. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995;1(8):792-7.
- Camacho-Barquero L, Villegas I, Sanchez-Calvo JM, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7(3):333-42.
- Hong J, Bose M, Ju J, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: Effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25(9):1671-9.
- Mun SH, Kim HS, Kim JW, et al. Oral administration of curcumin suppresses production of matrix metalloproteinase (MMP)-1 and MMP-3 to ameliorate collagen-induced arthritis: Inhibition of the PKCdelta/JNK/c-Jun pathway. J Pharmacol Sci. 2009;111(1):13-21.
- Tsvetkov P, Asher G, Reiss V, et al. Inhibition of NAD(P)H: Quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc Natl Acad Sci USA. 2005;102(15):5535-40.
- Liao YF, Hung HC, Hour TC, et al. Curcumin induces apoptosis through an ornithine decarboxylase-dependent pathway in human promyelocytic leukemia HL-60 cells. Life Sci. 2008;82(7-8):367-75.
- Lee JH, Chung IK. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010;290(1):76-86.
- Chakraborty SMN, Ghosh U, Bhattacharyya NP, et al. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol Cell Biochem. 2007;297(1-2):31-9.
- Pauff JM, Hille R. Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J Nat Prod. 2009;72(4):725-31.
- Shen L, Ji HF. Insights into the inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett. 2009;19(21):5990-3.
- Balamurugan AN, Akhov L, Selvaraj G, et al. Induction of antioxidant enzymes by curcumin and its analogues in human islets: Implications in transplantation. Pancreas. 2009;38(4):454-60.
- Kim HY, Park EJ, Joe EH, et al. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171(11):6072-9.
- Elangbam CS, Qualls CW Jr, Dahlgren RR. Cell adhesion molecules--update. Vet Pathol. 1997;34(1):61-73.
- Bruijn JA, De Heer E. Adhesion molecules in renal diseases. Lab Invest. 1995;72(4):387-94.
- Haapasalmi K, Makela M, Oksala O, et al. Expression of epithelial adhesion proteins and integrins in chronic inflammation. Am J Pathol. 1995;147(1):193-206.
- Rajan S, Ye J, Bai S, et al. NF-kappaB, but not p38 MAP kinase, is required for TNF-alpha-induced expression of cell adhesion molecules in endothelial cells. J Cell Biochem. 2008;105(2):477-86.
- Binion DG, Heidemann J, Li MS, et al. Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: Inhibitory role of curcumin. Am J Physiol Gastrointest Liver Physiol. 2009;297(2):259-68.
- Burz C, Berindan-Neagoe I, Balacescu O, et al. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48(6):811-21.
- Singh N, Anand S. Apoptosis in health and disease. Indian J Physiol Pharmacol. 1995;39(2):91-4.
- Conney AH, Lysz T, Ferraro T, et al. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv Enzyme Regul. 1991;31:385-96.
- Huang MT, Smart RC, Wong CQ, et al. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941-6.
- Huang MT, Wang ZY, Georgiadis CA, et al. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13(11):2183-6.
- Jee SH, Shen SC, Tseng CR, et al. Curcumin induces a p53-dependent apoptosis in human basal cell carcinoma cells. J Invest Dermatol. 1998;111(4):656-61.
- Weir NM, Selvendiran K, Kutala VK, et al. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6(2):178-84.
- Ramachandran C, Rodriguez S, Ramachandran R, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25(5):3293-302.
- Maheshwari R, Singh A, Gaddipati J, et al. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081-7.
- Trujillo J, Chirino YI, Molina-Jijon E, et al. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013;1(1):448-56.
- Yallapu MM, Nagesh PK, Jaggi M, et al. Therapeutic Applications of Curcumin Nanoformulations. Aaps J. 2015;17(6):1341-56.
- Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007;4(6):807-18.
- Burgos-Moron E, Calderon-Montano JM, Salvador J, et al. The dark side of curcumin. Int J Cancer. 2010;126(7):1771-5.
- Yang CS, Sang S, Lambert JD, et al. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(1):139-51.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: Lessons learned from clinical trials. Aaps J. 2013;15(1):195-218.
- Gupta SC, Sung B, Kim JH, et al. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol Nutr Food Res. 2013;57(9):1510-28.
- Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanomedicine: A road to cancer therapeutics. Curr Pharm Des. 2013;19(11):1994-2010.
- Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov Today. 2012;17(1-2):71-80.
- Basnet P, Hussain H, Tho I, et al. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharm Sci. 2012;101(2):598-609.
- Chen Y, Wu Q, Zhang Z, et al. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17(5):5972-87.
- Dhule SS, Penfornis P, Frazier T, et al. Curcumin-loaded gamma-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8(4):440-51.
- Rogers NM, Stephenson MD, Kitching AR, et al. Amelioration of renal ischaemia-reperfusion injury by liposomal delivery of curcumin to renal tubular epithelial and antigen-presenting cells. Br J Pharmacol. 2012;166(1):194-209.
- Yallapu MM, Jaggi M, Chauhan SC. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79(1):113-25.
- Yadav VR, Prasad S, Kannappan R, et al. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol. 2010;80(7):1021-32.
- Dandawate PR, Vyas A, Ahmad A, et al. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharmaceutical Research. 2012;29(7):1775-86.
- Yadav VR, Suresh S, Devi K, et al. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS Pharm Sci Tech. 2009;10(3):752.
- Adhikary R, Carlson PJ, Kee TW, et al. Excited-state intramolecular hydrogen atom transfer of curcumin in surfactant micelles. J Phys Chem B. 2010;114(8):2997-3004.
- Began G, Sudharshan E, Appu Rao AG. Inhibition of lipoxygenase 1 by phosphatidylcholine micelles-bound curcumin. Lipids. 1998;33(12):1223-8.
- Podaralla S, Averineni R, Alqahtani M, et al. Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol Pharm. 2012;9(9):2778-86.
- Song Z, Feng R, Sun M, et al. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354(1):116-23.
- Yu H, Li J, Shi K, et al. Structure of modified epsilon-polylysine micelles and their application in improving cellular antioxidant activity of curcuminoids. Food Funct. 2011;2(7):373-80.
- Babaei E, Sadeghizadeh M, Hassan ZM, et al. Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int Immunopharmacol. 2012;12(1):226-34.
- Cao J, Zhang H, Wang Y, et al. Investigation on the interaction behavior between curcumin and PAMAM dendrimer by spectral and docking studies. Spectrochim Acta A Mol Biomol Spectrosc. 2013;108:251-5.
- Debnath S, Saloum D, Dolai S, et al. Dendrimer-curcumin conjugate: A water soluble and effective cytotoxic agent against breast cancer cell lines. Anticancer Agents Med Chem. 2013;13(10):1531-9.
- Alizadeh AM, Khaniki AS, Azizian AMA, et al. Mohaghgheghi. Protective effect of dendrosomal curcumin combination on colon cancer in rat. Tehran University Medical Journal. 2012;69(11):678-85.
- Yallapu MM, Ebeling MC, Chauhan N, et al. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int J Nanomedicine. 2011;6:2779-90.
- Mangalathillam S, Rejinold NS, Nair A, et al. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale. 2012;4(1):239-50.
- Wei X, Senanayake TH, Bohling A, et al. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: Synthesis, pharmacokinetics, and tumor growth inhibition. Molecular Pharmaceutics. 2014;11(9):3112-22.
- Reeves A, Vinogradov SV, Morrissey P, et al. Curcumin-encapsulating nanogels as an effective anticancer formulation for intracellular uptake. Mol Cell Pharmacol. 2015;7(3):25-40.
- Gangwar RK, Dhumale VA, Kumari D, et al. Conjugation of curcumin with PVP capped gold nanoparticles for improving bioavailability. Mater Sci Eng. 2012;32(8):2659-63.
- Singh DK, Jagannathan R, Khandelwal P, et al. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: An experimental and density functional theory investigation. Nanoscale. 2013;5(5):1882-93.
- Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin ("nanocurcumin"): A novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5(1):3.
- Lim KJ, Bisht S, Bar EE, et al. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11(5):464-73.
- Chun YS, Bisht S, Chenna V, et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis. 2012;33(11):2242-9.
- Zou P, Helson L, Maitra A, et al. Polymeric curcumin nanoparticle pharmacokinetics and metabolism in bile duct cannulated rats. Mol Pharm. 2013;10(5):1977-87.
- Aravind SR, Krishnan LK. Curcumin-albumin conjugates as an effective anti-cancer agent with immunomodulatory properties. Int Immunopharmacol. 2016;34:78-85.
- Nagahama K, Sano Y, Kumano T. Anticancer drug-based multifunctional nanogels through self-assembly of dextran-curcumin conjugates toward cancer theranostics. Bioorg Med Chem Lett. 2015;25(12):2519-22.
- Kakkar V, Muppu SK, Chopra K, et al. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85(3):339-45.
- Kakkar V, Mishra AK, Chuttani K, et al. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int J Pharm. 2013;448(2):354-9.
- Tiyaboonchai W, Tungpradit W, Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm. 2007;337(1):299-306.
- Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667-77.
- Cheng KK, Chan PS, Fan S, et al. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer's disease mice using magnetic resonance imaging (MRI). Biomaterials. 2015;44:155-72.
- Yallapu MM, Othman SF, Curtis ET, et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine. 2012;7:1761-79.
- Yallapu MM, Othman SF, Curtis ET, et al. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32(7):1890-05.
- Liu CH, Chang FY. Development and characterization of eucalyptol microemulsions for topic delivery of curcumin. Chem Pharm Bull. 2011;59(2):172-8.
- Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928-39.
- Ganta S, Devalapally H, Amiji M. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J Pharm Sci. 2010;99(11):4630-41.
- Mohanty C, Das M, Sahoo SK. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin Drug Deliv. 2012;9(11):1347-64.
- Thangapazham RL, Puri A, Tele S, et al. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32(5):1119-23.
- Sou K, Inenaga S, Takeoka S, et al. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352(1-2):287-93.
- Gota VS, Maru GB, Soni TG, et al. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58(4):2095-9.
- Kunwar A, Barik A, Pandey R, et al. Transport of liposomal and albumin loaded curcumin to living cells: An absorption and fluorescence spectroscopic study. Biochim Biophys Acta. 2006;1760(10):1513-20.
- Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322-31.
- Narayanan NK, Nargi D, Randolph C, et al. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1-8.
- Ghosh M, Singh AT, Xu W, et al. Curcumin nanodisks: Formulation and characterization. Nanomedicine. 2011;7(2):162-7.
- Li X, Nan K, Li L, et al. In vivo evaluation of curcumin nanoformulation loaded methoxy poly (ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012;88(1):84-90.
- Duarte VM, Han E, Veena MS, et al. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKbeta protein of the NFkappaB pathway. Mol Cancer Ther. 2010;9(10):2665-75.
- Li L, Ahmed B, Mehta K, et al. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther. 2007;6(4):1276-82.
- Grabovac V, Bernkop-Schnurch A. Development and in vitro evaluation of surface modified poly(lactide-co-glycolide) nanoparticles with chitosan-4-thiobutylamidine. Drug Dev Ind Pharm. 2007;33(7):767-74.
- Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31(25):6597-611.
- Nair KL, Thulasidasan AK, Deepa G, et al. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425(1-2):44-52.
- Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867-75.
- Yallapu MM, Gupta BK, Jaggi M, et al. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351(1):19-29.
- Thamake SI, Raut SL, Ranjan AP, et al. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology. 2011;22(3):035101.
- Shahani K, Panyam J. Highly loaded, sustained-release microparticles of curcumin for chemoprevention. J Pharm Sci. 2011;100(7):2599-609.
- Anitha A, Deepagan VG, Divya Rani VV, et al. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr Polym. 2011;84(3):1158-64.
- Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly (D, L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8(3):852-66.
- Yallapu MM, Jaggi M, Chauhan SC. Poly(beta-cyclodextrin)/curcumin self-assembly: A novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol Biosci. 2010;10(10):1141-51.
- Sou K, Oyajobi B, Goins B, et al. Characterization and cytotoxicity of self-organized assemblies of curcumin and amphiphatic poly(ethylene glycol). J Biomed Nanotechnol. 2009;5(2):202-8.
- Lin YL, Liu YK, Tsai NM, et al. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine. 2012;8(3):318-27.
- Lee WH, Loo CY, Young PM, et al. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv. 2014;11(8):1183-201.
- Sahu A, Kasoju N, Goswami P, et al. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J Biomater Appl. 2011;25(6):619-39.
- Song L, Shen Y, Hou J, et al. Polymeric micelles for parenteral delivery of curcumin: Preparation, characterization and in vitro evaluation. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2011;390(1–3):25-32.
- Ma Z, Haddadi A, Molavi O, et al. Micelles of poly (ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A. 2008;86(2):300-10.
- Stuart MA, Huck WT, Genzer J, et al. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9(2):101-13.
- Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2010;6(1):153-60.