Antimicrobial screening of quinazolinones, thiazolidinones, azetidinones and oxadizoles bearing quinoline motifs
2 NITI-Aayog, Atal Innovation Mission, New Delhi, India, Email: unnat.pandit@gmail.com
Received: 05-Aug-2017 Accepted Date: Aug 30, 2017; Published: 01-Sep-2017
Citation: Desai N, Dodiya A, Pandit U. Antimicrobial screening of quinazolinones, thiazolidinones, azetidinones and oxadizoles Bearing quinoline motifs. J Pharmacol Med Chem 2017;1(1):6-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
This present article deals with antimicrobial screening of novel quinoline derivatives developed by our research group. The novel compounds include fusion of heterocyclic rings like thiazolidinones, azetidinones and oxadizoles bearing quinoline motif. These compounds were screened for their antibacterial and antifungal activities. The main purpose of article is to provide information on the development of novel quinoline derivatives to the scientific.
Keywords
Quinoline derivatives; Antimicrobial screening; Novel quinolines
The research and development of novel and innovative antimicrobial agents have encountered with huge success over the past seventy years and provided many classes of isolated natural products or synthetic compounds. Amongst them, quinolone derivatives are very important class as antibacterial and antifungal agents. Over and above, quinoline moiety exhibits biological activities like antibacterial [1], anti-malarial [2], anti-TB [3], anti-proliferative [4] and anti-cancer [5]. Anti-tubercular drugs bedaquiline is a core pharmacophore of quinoline motif. Diarylquinoline is present in the bedaquiline [6]. This is the first drug with a novel mechanism of action for TB in more than 40 years. Quinoline containing drugs such as bosutinib, tipifarnib, topotecan and exatecan have been established as one of the most effective classes of anticancer agents in clinical use today with broad applications in the treatment of several leukaemia and lymphomas as well as in combination chemotherapy of solid tumors [7].
In searching for agents with an improved pharmacokinetic properties, potency or spectrum and lower side effects, a large number of quinoline derivatives and related compounds have been prepared and several of these have shown promise in clinical trials [8]. Quinolines and their derivatives occur in numerous natural products or synthesized compounds, many of which possess interesting physiological and biological properties; particularly the 2- chloroquinoline-3-carbaldehyde has been in the focus of interest of medicinal chemists from the many decades because of the outstanding pharmacological properties such as antimicrobial [9], antimalarial [10], and anti-inflammatory [11]. 2-chloroquinoline-3-carbaldehyde is synthesized by using Vilsmeier-Haack reaction [12]. The vital role of 2-chloroquinoline- 3-carbaldehydes in the field of drug chemistry, prompted us to synthesize a series of 2-chloroquinoline-3-carbaldehydes, which is further employed either to fuse with various other heterocyclic moieties like quinazolinone, oxadiazole, thiazolidinone and azetidinones. Our research group had synthesized compounds containing quinoline and screened for antimicrobial activity i.e., 2-(2-chloro-6-methyl(3-quinolyl))-3-[2-(4-chlorophenyl)-4-oxo(3- hydroquinazolin-3-yl)]-5-[(aryl)methylene]-1,3-thiazolidin-4-ones [13] and 3-chloro-1-(aryl)-4-(2-(2-chloroquinolin-3-yl)-5-(pyridin-4-yl)-1,3,4-oxadiazol- 3(2H)-yl)-4-ethyl-azetidin-2-ones [14]. Comprehensive scientific report on these papers has been reported in the manuscript. Structural activity relationship (SAR) studies revealed that the anti-microbial activity of quinolone based heterocyclic class of molecules depend on the nature of the peripheral substituents and their spatial relationship within the skeleton [15].
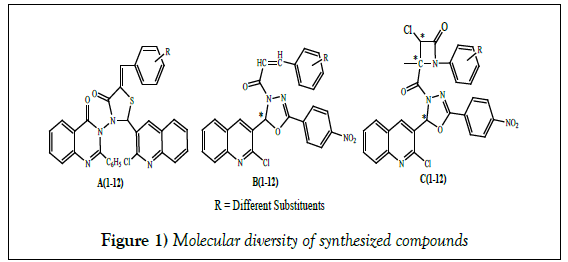
In this paper, we have tried to focus on recent research on quinoline derivatives particularly, 2-chloroquinoline derivatives as antimicrobial agents synthesized by our research group [16-18]. Important features of quinoline based bioactive molecules which included heterocyclic ring as antimicrobial agents or useful as antimicrobial drug (Figure 1).
• Design of 2-chloroquinoline based hybrids as novel and inventive antimicrobial agents.
• Antimicrobial activity of 2-chloroquinoline based compounds.
In this paper, we have discussed the antimicrobial activity of compounds like 2-(2-chloroquinolin-3-yl)-5-((aryl)benzylidene)-3-(4-oxo-2-phenylquinazolin-3- (4H)-yl)-thiazolidin-4-ones (A1-12), 1-[2-(2-chloro(3-quinolyl))-5-(4- nitrophenyl)(1,3,4- oxadiazolin-3-yl)]-3-(aryl)-prop-2-en-1-ones (B1-12) and 3-chloro-1-(aryl)-4-(2-(2-chloro-6- methylquinolin-3-yl)-5-(pyridine-4-yl)-1,3,4- oxadiazol-3(2H)-yl)-4-ethyl-azetidin-2-ones (C1-12). The data of antimicrobial activity is reported in Table 1. Details of experimental are already given in our published papers and therefore it not worth to discuss in the present paper [16-18].
| S. No. |
C. No. | -R | MIC for bacteria μg/ml  ± SD | MIC for fungi μg/ml  ± SD | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gram Positive | Gram Negative | ||||||||
| E. coli | P. aeruginosa | S. aureus | S. pyogen. | C. albicans | A. niger | A. clavatus | |||
| MTCC-443 | MTCC-1688 | MTCC-96 | MTCC-442 | MTCC-227 | MTCC-282 | MTCC-1323 | |||
| 1 | A1 | 2-Cl | 250 ± 2.64 | 250 ± 3.05 | 250 ± 3 | 250 ± 3.05 | 200 ± 4.16 | 500 ± 3.60 | 500 ± 3.21 |
| 2 | A2 | 3-Cl | 100 ± 3 | 250 ± 3.51 | 500 ± 4.04 | 100 ± 3.51 | 250 ± 3.05 | 1000 ± 2.6 | 100 ± 3.24 |
| 3 | A3 | 4-Cl | 150 ± 2.30 | 25 ± 1 | 100 ± 3.60 | 200 ± 4.16 | 500 ± 4.04 | 100 ± 3.05 | 1000 ± 3.2 |
| 4 | A4 | 2-NO2 | 100 ± 3.05 | 200 ± 4 | 250 ± 2.64 | 250 ± 2.64 | 250 ± 2.08 | 500 ± 3.51 | 500 ± 3.46 |
| 5 | A5 | 3-NO2 | 100 ± 3.51 | 250 ± 2.08 | 500 ± 3.05 | 500 ± 4.58 | 500 ± 4.04 | 100 ± 3.60 | 1000 ± 3.5 |
| 6 | A6 | 4-NO2 | 200 ± 2.64 | 250 ± 2.51 | 250 ± 3.60 | 250 ± 2.51 | 100 ± 4.04 | 100 ± 3.05 | 500 ± 3.21 |
| 7 | A7 | 2-OH | 500 ± 3 | 50 ± 2.64 | 500 ± 4.16 | 250 ± 3.05 | 100 ± 3.51 | 500 ± 2.08 | 100 ± 3.05 |
| 8 | A8 | 3-OH | 100 ± 3.51 | 100 ± 4.50 | 200 ± 4.16 | 100 ± 4.93 | 500 ± 3 | 500 ± 3.05 | 100 ± 3.51 |
| 9 | A9 | 4-OH | 50 ± 2.64 | 200 ± 2.64 | 100 ± 3.60 | 100 ± 4.72 | 500 ± 3.51 | 100 ± 3.51 | 500 ± 3 |
| 10 | A10 | 4-CH3 | 500 ± 4.72 | 50 ± 2.51 | 250 ± 3.21 | 250 ± 3 | 500 ± 3.78 | 1000 ± 3 | 100 ± 3.60 |
| 11 | A11 | 4-OCH3 | 500 ± 4.04 | 100 ± 4.61 | 500 ± 3.51 | 500 ± 4.04 | 200 ± 4 | 1000 ± 3.5 | 1000 ± 2.3 |
| 12 | A12 | 3,4,5-(OMe)3 | 250 ± 4.04 | 250 ± 2.51 | 500 ± 3.05 | 1000 ± 3.51 | 500 ± 3.46 | 1000 ± 3 | 100 ± 4.16 |
| 13 | B1 | 2-Cl | 500 ± 2.34 | 500 ± 3.35 | 500 ± 3.6 | 500 ± 3.34 | 250 ± 4.36 | 500 ± 3.50 | 500 ± 3.11 |
| 14 | B2 | 3-Cl | 100 ± 3.65 | 200 ± 3.55 | 100 ± 4.04 | 100 ± 3.43 | 100 ± 3.55 | 100 ± 2.44 | 500 ± 3.20 |
| 15 | B3 | 4-Cl | 200 ± 3.30 | 100 ± 2.13 | 1000 ± 3.3 | 500 ± 4.13 | 100 ± 4.34 | 200 ± 3.02 | 1000 ± 3.2 |
| 16 | B4 | 2NO2 | 50 ± 3.23 | 50 ± 4.57 | 500 ± 3.23 | 200 ± 2.53 | 500 ± 2.48 | 500 ± 3.5 | 100 ± 3.36 |
| 17 | B5 | 3-NO2 | 1000 ± 3.5 | 500 ± 2.18 | 500 ± 3.04 | 100 ± 4.55 | 100 ± 4.24 | 100 ± 3.4 | 500 ± 3.41 |
| 18 | B6 | 4-NO2 | 100 ± 4.45 | 500 ± 2.58 | 100 ± 2.56 | 500 ± 2.53 | 500 ± 4.25 | 1000 ± 3.5 | 100 ± 3.31 |
| 19 | B7 | 2-OH | 500 ± 3.32 | 250 ± 2.69 | 500 ± 4.13 | 250 ± 3.05 | 1000 ± 3.2 | 500 ± 2.02 | 500 ± 3.25 |
| 20 | B8 | 3-OH | 25 ± 3.56 | 50 ± 4.57 | 200 ± 3.08 | 100 ± 4.93 | 500 ± 3.4 | 200 ± 3.12 | 100 ± 3.35 |
| 21 | B9 | 4-OH | 250 ± 3.12 | 50 ± 2.66 | 100 ± 3.45 | 500 ± 4.73 | 500 ± 3.53 | 100 ± 3.55 | 500 ± 3.3 |
| 22 | B10 | 4-CH3 | 200 ± 4.35 | 100 ± 2.42 | 200 ± 3.36 | 250 ± 3.6 | 250 ± 3.75 | 100 ± 3.4 | 1000 ± 3.4 |
| 23 | B11 | 4-OCH3 | 100 ± 4.24 | 500 ± 3.45 | 100 ± 3.53 | 100 ± 4.02 | 500 ± 4.8 | 1000 ± 3.2 | 100 ± 2.3 |
| 24 | B12 | 3,4,5-(OMe)3 | 200 ± 4.36 | 200 ± 3.55 | 500 ± 3.36 | 500 ± 3.53 | 100 ± 3.43 | 100 ± 3.54 | 100 ± 4.15 |
| 25 | C1 | 2-Cl | 200 ± 4.04 | 100 ± 3.78 | 500 ± 4 | 500 ± 4.16 | 500 ± 3.78 | 100 ± 2.35 | 500 ± 2.51 |
| 26 | C2 | 3-Cl | 100 ± 4.72 | 500 ± 3.05 | 50 ± 3.05 | 200 ± 3.51 | 500 ± 2.51 | 1000 ± 3 | 100 ± 4.08 |
| 27 | C3 | 4-Cl | 100 ± 3.78 | 50 ± 2.64 | 100 ± 4.55 | 25 ± 2.21 | 100 ± 4.04 | 100 ± 1.24 | 500 ± 2.35 |
| 28 | C4 | 2NO2 | 62.5 ± 4.0 | 100 ± 4.55 | 200 ± 3.78 | 200 ± 4 | 500 ± 2.35 | 500 ± 3.24 | 100 ± 1.32 |
| 29 | C5 | 3-NO2 | 100 ± 3.65 | 50 ± 4.04 | 100 ± 3.05 | 50 ± 1.24 | 200 ± 4 | 1000 ± 2.0 | 100 ± 3.60 |
| 30 | C6 | 4-NO2 | 200 ± 4.51 | 100 ± 2.51 | 50 ± 2.51 | 100 ± 3.51 | 1000 ± 4.5 | 1000 ± 4.5 | 200 ± 4 |
| 31 | C7 | 2-OCH3 | 50 ± 4.93 | 500 ± 4 | 500 ± 3.51 | 500 ± 1 | 500 ± 2.30 | 500 ± 2.30 | 500 ± 2.30 |
| 32 | C8 | 3-OCH3 | 100 ± 4.04 | 500 ± 3.34 | 250 ± 4.04 | 200 ± 1.32 | 1000 ± 3.5 | 500 ± 3.05 | 500 ± 4.04 |
| 33 | C9 | 4-OCH3 | 250 ± 3.78 | 100 ± 4 | 100 ± 3.21 | 100 ± 4.04 | 200 ± 3.51 | 500 ± 3.55 | 500 ± 3.55 |
| 34 | C10 | 4-CH3 | 500 ± 3.21 | 50 ± 3.55 | 200 ± 3.54 | 1000 ± 4.16 | 100 ± 230 | 100 ± 3.78 | 500 ± 4.04 |
| 35 | C11 | 2-F | 100 ± 3.05 | 250 ± 3.78 | 100 ± 3.05 | 250 ± 2.08 | 500 ± 3.05 | 500 ± 4.21 | 500 ± 2.08 |
| 36 | C12 | 4-F | 100 ± 4.55 | 100 ± 3.51 | 500 ± 3.01 | 500 ± 3.60 | 200 ± 3.78 | 500 ± 3.05 | 100 ± 3.46 |
| Ampicillin | 100 ± 4.57 | 100 ± 4.12 | 250 ± 4.15 | 100 ± 3.55 | --- | --- | --- | ||
| Griseofulvin | --- | --- | --- | --- | 500 ± 2.64 | 100 ± 3 | 100 ± 3.46 | ||
± SD = Standard Deviation and p ≤0.0001 for all compounds
Table 1: Antibacterial and antifungal activities of bioactive compounds A1-12, B1-12 and C 1-12
Material and Method
Anti-microbial screening
Minimum inhibitory concentration for bacteria (MICb) of all the synthesized compounds was determined against four different strains of bacteria and minimum inhibitory concentration for fungi (MICf) of all the synthesized compounds was determined against three strains of fungi. The results were compared with standard drug ampicillin and griseofulvin respectively. Broth dilution testing allows the option of providing both quantitative (MIC) and qualitative (category interpretation) results. MIC can be helpful in establishing the level of resistance of a particular bacterial strain and can substantially affect the decision to use certain antimicrobial agents. Broth dilution can again be performed by 2 ways:
Macrodilution: Uses broth volume of 1 ml in standard test tubes.
Microdilution: Uses about 0.05 to 0.1 ml total broth volume and can be performed in a microtiter plate or tray. The procedure for both macro and microdilution are same except the volume of the broth.
Determination of anti-bacterial screening
Antibacterial studies of the compounds reported in this paper were tested against the representative panel of Gram-positive (Staphylococcus aureus (MTCC-96), Streptococcus pyogenes (MTCC-442)) and Gram-negative (Escherichia coli (MTCC-443) and Pseudomonas aeruginosa (MTCC-1688)) bacteria using conventional broth dilution method [19,20]. All MTCC cultures were obtained from the Institute of Microbial Technology, Chandigarh. The activity of compounds was determined as per National Committee for Clinical Laboratory Standards (NCCLS) protocol using Mueller Hinton Broth (Becton Dickinson, USA). “Primary antibacterial activity screening was carried out in six sets against E. coli, S. aureus, P. aeruginosa and S. pyogenes at different concentrations of 1000 μg/mL, 500 μg/mL, 250 μg/mL. The compounds found to be active in primary screening were similarly diluted to obtain 200 μg/mL, 125 μg/mL, 100 μg/mL, 62.5 μg/mL, 50 μg/mL, 25 μg/mL and 12.5 μg/mL concentrations for secondary screening to test in a second set of dilution against all microorganisms. Inoculum size for test strain was adjusted to 106 CFU/mL (Colony Forming Unit per milliliter) by comparing the turbidity (turbidometric method).
Mueller Hinton Broth was used as a nutrient medium to grow and dilute the compound suspension for test organisms. 2% DMSO was used as a diluent/ vehicle to obtain the desired concentration of synthesized compounds and standard drugs to test upon standard microbial strains. Synthesized compounds were diluted to 1000 μg/mL concentration, as stock solution. The control tube containing no antibiotic was immediately sub-cultured [before inoculation] by spreading a loopful evenly over quarter of a plate of medium suitable for the growth of test organisms. The culture tubes were then incubated for 24 h at 37°C and the growth was monitored visually and spectrophotometrically. Ten μg/mL suspensions were further inoculated on an appropriate media and growth was noted after 24 h and 48 h. The lowest concentration (highest dilution) required to arrest the growth of bacteria was regarded as control. Standard drug used in the present study is as follows [21,22].
The ensuing conditions must be following for the screening of antimicrobial activity [23-25].
(i) There should be intimate contact between the test organisms and substance to be evaluated.
(ii) Required conditions should be provided for the growth of microorganisms.
(iii) Conditions should be same through the study.
(iv) Aseptic/sterile environment should be maintained.
The evaluation can be done by the following methods:
(i) Turbidometric method.
(ii) Agar streak dilution method.
(iii) Serial dilution method.
(iv) Agar diffusion method.
Following techniques are used as agar diffusion method
i. Agar cup method.
ii. Agar ditch method.
iii. Paper disc method.
We have used the Broth Dilution Method to screen the antimicrobial activity. It is one of the non-automated in vitro microbial susceptibility tests. This classic method yields a quantitative result for the amount of antimicrobial agents that is needed to inhibit growth of specific microorganisms. It is carried out in:
(a) Microdilution Method in Tubes
(b) Microdilution format using plastic trays.
Determination of Antifungal activity
“The newly prepared compounds 5a-m were screened for their antifungal activity as primary screening in six sets against C. albicans, A. niger and A. clavatus at various concentrations of 1000, 500, 250 μg/mL. The primary active compounds were similarly diluted to obtain 200, 125, 100, 62.5, 50, 25 and 12.5 μg/mL concentrations for secondary screening to test in a second set of dilution against all fungi. The fungal activity of each compound was compared with griseofulvin as a standard drug, which showed 500, 100 and 100 μg/mL MIC against C. albicans, A. niger and A. Clavatus respectively. For fungal growth, in the present protocol, we used Sabourauds dextrose broth at 28 °C in aerobic condition for 48 h. DMSO and sterilized distilled water were used as negative controls while griseofulvin (1 U strength) was used as a positive control” [23-25].
Results and Discussion
Antibacterial activity
From the antibacterial screening results, it has been observed that final compounds A2, A4, A5, A8, B2, B6, B11, C2, C3, C5, C8, C11 and C12 were good active against E. Coli, while compounds A9, B4, C4 and C7 possess very good activity against E. Coli and compound B8 possesses excellent activity against E. Coli as compared to the standard drug Ampicillin. In the same way, final compounds A8, A11, B3, B10, C1, C4, C6, C9 and C12 possess good activity against P. Aeruginosa, while compounds A7, A10, B4, B8, B9, C3, C5 and C10 possess very good activity against P. Aeruginosa and compound A3 possesses an excellent activity against P. Aeruginosa as compared to the standard drug Ampicillin. In same way, final compounds A1, A4, A6, A8, A10, C4, C8 and C10 possess good activity against S. Aureus, while compounds A3, A9, B2, B6, B9, B11, C3, C5, C9 and C11 possess very good activity against S. Aureus and compounds B3, B10, C2 and C6 possess an excellent activity against S. Aureus as compared to standard drug Ampicillin. Final compounds A2, A8, A9, B2, B5, B11, C6 and C9 were good active against S. Pyogenus, compounds B6 and C5 possess very good activity against S. Pyogenus and compound C3 possesses an excellent activity against S. Pyogenus as compared to the standard drug Ampicillin. The remaining compounds of the entire series possesses only moderate to poor antibacterial activity.
Antifungal activity
From the antifungal screening results, it has been observed that compounds A1, A3, A4, A11, B4, B6, B8, B9, B11, C1, C2, C4, C7 and C11 possess good activity against C. Albecans, while compounds A6, A7, B10, C5, C9 and C12 possesses very good activity against C. Albecans. Compounds B2, B3, B5, B12, C3 and C10 possess excellent activity against C. Albecans as compared to the standard Griseofulvin. Compounds A3, A5, A6, A9, B2, B5, B9, B10, B12, C1, C3 and C10 possessed good activity against A. niger as compared to the standard drug Griseofulvin.
Compounds A3, A7, A8, A10, B4, B6, B8, B12, C2, C4, C5 and C12 possesses good activity against A. Clavatus as compared to Griseofulvin. Remaining compounds of the series possessed moderate to poor antifungal activity.
Structure activity relationship
As discussed above, during preparation of compounds A1-12, we have incorporated quinazolinone and thiazolidinone rings with quinoline nucleus. For the synthesis of compounds B1-12, we have clubbed oxadiazoles ring with quinoline ring and for the preparation of C1-12 compounds, we have clubbed oxadizoles and azetidinones with quinoline nucleus.
We have installed different electron withdrawing groups and electron donating groups on different positions of phenyl ring during synthesis of bioactive molecules.
On the basis of biological activity results, we have observed that when we have introduced electron donating group like hydroxyl group at meta position in compound B8, it has furnished excellent activity against E. coli. In a similar way, it is our observation that the incorporation of electron withdrawing group like chloro group at para position in compound A3, it has yielded an excellent activity against P. aeruginosa. By using chloro and nitro groups at para position in compounds B3, C6, and meta position in C2, an excellent activity against S. aureus is observed. When we have introduced electron donating substituents like methyl and chloro at para position in compound B10 and C3, an excellent activity was observed against S. aureus and S. pyogenus respectively.
In case of anti-fungal screening, we have observed that the introduction of electron withdrawing group like chloro at meta position of B2 and para position in compounds B3 and C3, the excellent activity against C. albicans is observed. In the same way, when we have used electron withdrawing group like nitro at meta position in compound B5, the excellent activity against C. albicans is furnished. In the same way, groups like 3,4,5-(OCH3)3 in compound B12 and methyl group at para position in compound C10, we have observed an excellent activity against C. albicans.
Statistical Analysis
The standard deviation value is expressed in terms of ± SD. On the basis of the calculated value by using ANOVA method, it has been observed that the differences below 0.0001 levels (p ≤ 0.0001) were considered as statistically significant.
Conclusion
2-chloroquinoline-3-carbaldehyde is used as precursor and synthesized a variety of quinoline based thiazolidinone, oxadiazole-chalcone, and oxadiazole azetidinone hybrids scaffolds. We have tried to develop the strategy, which allowed us for the assimilation of three promising bioactive nuclei in a single scaffold in an easy way. All the synthesized compounds were screened for their antimicrobial activity. The results indicates that some newly synthesized compounds exhibited promising antibacterial activity against E. coli, P. aeruginosa, S. aureus, S. pyogenus, while some of the compounds exhibited promising antifungal activity against C. albicans, A. niger and A. clavatus. These results proved that 2-chloroquinoline bearing oxadiazole, thiazolidinone, azetidinones, and quinazolinone heterocyclic compounds are found to be interesting lead molecules for the development of antimicrobial agents.
Acknowledgements
The authors are thankful to Department of Chemistry, M. K. Bhavnagar University, and Bhavnagar for providing research facilities.
REFERENCES
- Guo M, Zheng C, Song M, et al. Synthesis and biological evaluation of rhodanine derivatives bearing a quinolone moiety as potent antimicrobial agents. Bioorg Med Chem Lett 2013;23:4358-61.
- Vandekerckhove S, D’hooghe M Quinoline-based antimalarial hybrid compounds. Bioorg Med Chem 2015 23:5098-119.
- Kanani MB, Patel MP. Design and synthesis of new (bis)trifluoromethyl-promoted N-aryl biquinoline derivatives as antitubercular and antimicrobial agents. Med Chem Res 2015;24:563-75.
- Tseng C, Tzeng C, Yang C, et al. Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinolone derivatives Part 2. J Med Chem 2010;53:6164-79.
- Rogdrigues FA, Bomfim IS, Cavalcanti BC, et al. Mefloquine–oxazolidine derivatives: A new class of anticancer agents. Chem Biol Drug Des 2014;83:126-31.
- Desai NC, Kotadiya GM, Trivedi AR. Studies on molecular properties prediction, antitubercular and anti-microbial activities of novel quinoline based pyrimidine motifs. Bioorg Med Chem Lett 2014;24:3126-30.
- Wakelin I, Waring MJ. DNA intercalating agents. In Comprehensive Medicinal Chemistry; Sammes PG, (Ed); Pergamon: Oxford, UK, 1990;2:725-38.
- Lown JW. Anthracycline and Antracenedione-bases anticancer agents: Bioactive molecules. Elsevier: Amsterdam 1998;8:1579-84.
- Farghaly AM, Habib NS, Khalil MA, et al. Synthesis of novel 2-substituted quinoline derivatives: antimicrobial, inotropic and chronotropic activities. Archiv Der Pharmazie 1990;323: 247-51.
- Sekar M, Prasad KJR. Synthesis of some novel 2-oxopyrano [2,3-b]-and 2-oxopyrido-[2,3-b]-quinoline derivatives as potential antimalarial, diuretic clastogenic and anti-microbial agents. J chem technol biotechnol 1998;72:50-4.
- Bulbule VJ, Deshpande VH, Sathe VT. Heterogeneous henry reaction of aldehydes: Diastereo-selective synthesis of nitroalcohol derivatives over Mg-Al hydrotalcites. Tetrahedron 1999;55:9325-32.
- Abdel-Wahab BF. 2-chloroquinoline-3-carbaldehyde II: Synthesis, reactions and applications. J Chem 2013
- Desai NC, Dodiya AM. Synthesis, characterization and anti-microbial screening of quinoline based quinazolinone-4-thiazolidinone heterocycles. Arabian J chem 2014;7:906-13.
- Desai NC, Dodiya AM, Shihory NR. Synthesis, characterization, and antimicrobial evaluation of quinoline oxadiazole–based azetidinone derivatives. Synth Commun 2012;42:3230–41.
- Gomez CMM, Kouznetsov VV. Microbial pathogens and strategies for combating them: Science, technology and education, A. Méndez-Vilas, Spain, 2013.
- Desai NC, Dodiya AM, Shihori NR. Synthesis and anti-microbial activity of novel quinazolinone-thiazolidine-quinoline compounds. J Saudi chem soc 2013;17:259-67.
- Desai NC, Dodiya AM. Conventional and microwave techniques for synthesis and anti-microbial studies of novel 1-[2-(2-chloro(3-quinolyl))---5-(4-nitrophenyl)-(1,3,4-oxadiazole-3-yl)]-3-(aryl)prop-2-en-1-ones. Med Chem Res 2012;21:1480-90.
- Desai NC, Dodiya AM. Synthesis, characterization and in vitro antimicrobial screening of quinoline nucleus containing 1,3,4-oxadiazole and 2-azetidinone derivatives. J Saudi chem soc 2014;18:425-31.
- Hannan, PC. Guidelines and recommendations for anti-microbial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. Vet. Res 2000;31:373-95.
- Rattan A. Antimicrobials in laboratory medicine. Churchill, B.I., Ed.; Livingstone: India, 2000.
- National Committee for Clinical Laboratory (1993) Standards methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically approved standard. (3rdedn) NCCLS Publication M7-A3: Villanova, PA.
- National Committee for Clinical Laboratory (1992) Standards, reference method for broth dilution antifungal testing of yeasts. Proposed Standard, NCCLS Document M27-P; Villanova, PA.
- Robert C (1970) Medical microbiology. (11th edn), ELBS and E & S; Livingstone, Briton.
- Sujatha GD. Synthesis characterization and biological investigations on metal complexes of 2-[(8-hydroxy-1-quinolin-5-yl) methyl]-1H-isoindole-1, 3 (2H) dione. Ind J Expt Biol 1975;13:286.
- Walksman SA (1947) Microbial antagonism and antibiotic substances. Common Wealth Fund (2nd edn) New York.