Antioxidant, antibacterial/antifungal nanostructures for medical and food packaging applications
2 P. Poni Institute of Macromolecular Chemistry, Romanian Academy, 41A Grigore GhicaVodă Alley, 700487 Iasi, Romania, Email: cvasile@icmpp.ro
Received: 30-Oct-2017 Accepted Date: Nov 15, 2017; Published: 20-Nov-2017
Citation: Munteanu BS, Vasile C. Antioxidant, antibacterial/antifungal nanostructures for medical and food packaging applications J Nanosci Nanomed. November-2017;1(1):15-20.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Electrospinning is a very attractive fibers fabrication technique due to the ability to produce nanoscale materials and structures with outstanding properties. As drug delivery systems it offers nanofiber meshes with high surface/volume ratio, high porosity and high surface exposed to the release media. The biologically active substances/drugs can be encapsulated into the individual polymeric nanofibers by coaxial or monoaxial electrospinning or, by another approach by which the biologically active substances can be entrapped and attached as nanoparticles to the nanofiber mesh. As antimicrobial/antioxidant materials for biomedical applications, the electrospun formulations containing silver nanoparticles are presented. As coating materials for food packaging, electrospun nanofibers containing chitosan formulations with antibacterial/antioxidant/antifungal properties are discussed.
Keywords
Electrospinning; drug delivery; coating; antibacterial; antioxidant; antifungal.
Introduction
Nowadays, nanomaterials and nanoparticles are used for many applications with benefits for our current life [1-3]. One method by which nanostructures can be obtained is electrospinning, a very efficient fibers fabrication technique due to the ability to produce nanoscale materials and structures [4,5] with high porosity and high specific surface area [6]. Also, the three-dimensional nanofibrous network allows the electrospun fibers to resemble native extracellular matrices [7]. By choosing the base polymer they can be easily designed to have enhanced mechanical properties, biocompatibility, and cellular response, making them a good choice to be used in nanocomposites materials applicable in the medical field [8]. The biomedical field is one of the most important application areas that utilizes the technique of electrospinning such as: tissue engineering [9], growth factors [10,11], cardiovascular tissue engineering [12], bone tissue regeneration [13], drug release systems [14], wound healing, etc. [15]. Electrospinning can produce a macroporous scaffold comprising randomly oriented or aligned nanofibers which may incorporate a drug delivery function into the fibrous scaffold. Such electrospun nanofibrous scaffolds may provide also an optimal microenvironment for the seeded cells [16].
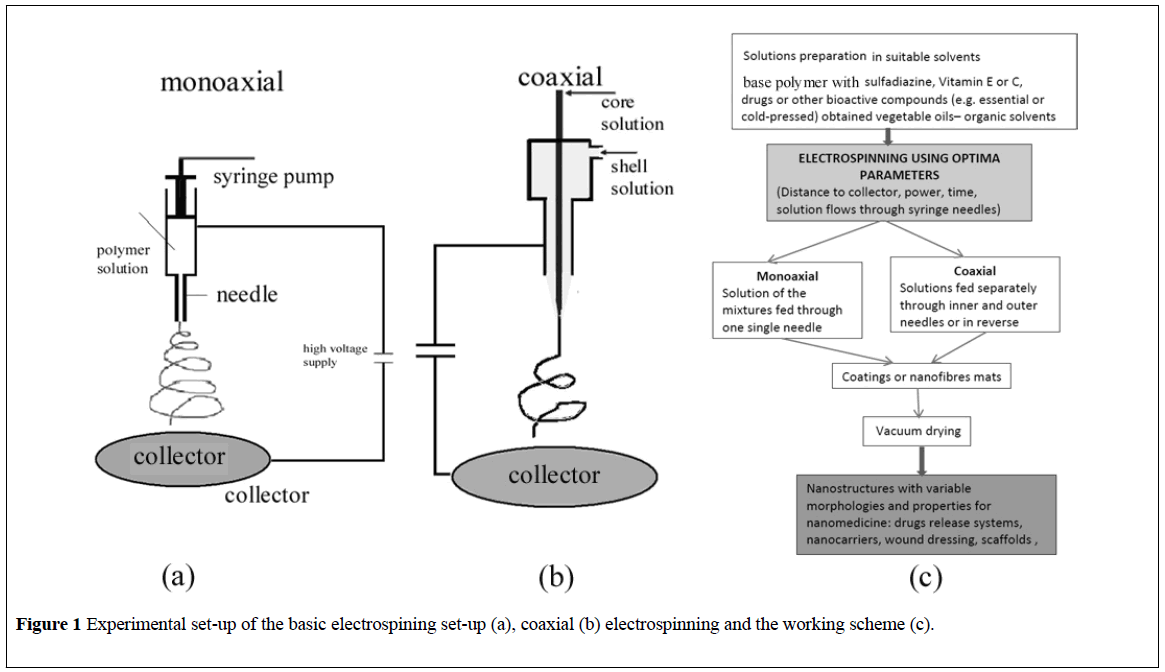
The basic electrospinning set-up is mainly comprised from four main parts: a syringe containing a polymer solution, metallic needle, power supply source, and metallic collector (with a variable design) (Figure 1a) [17]. The high direct voltage (0 to 30 kV) is applied between the metallic collector and the syringe needle. The polymer solution is extruded through the needle tip and at the point of ejection from the needle, a polymer jet is created and extended as a result of the electric charge repulsion outrunning the solution surface tension [18]. The polymeric solution jet flows toward the metallic collector with simultaneous evaporation of the solvent, followed by the deposition of a mat of nanofibers on the collector surface. When nanoparticles are deposited instead of nanofibres the process is usually called electrospraying [19].
In this review, recent drug delivery nanostructures based on electrospun nanofibers (such as eletrospun meshes containig sulfadiazine modified chitosan nanoparticles) are presented together with recent applications of electrospun formulations containing silver nanoparticles as antimicrobial/ antioxidant materials for biomedical applications. Also, recent electrospun coatings based on chitosan fomulations for antibacterial/antioxidant/ antifungal food packagings are presented.
Experiment
Drug releasing systems
The requirements of the controlled drug release involve the delivery of controlled amounts of a drug, over a specified period of time and target, with a predictable and controllable rate [20-22].
Compared with other dosage forms, several advantages of the use of the electrospun polymer nanofibers have been recognized. Therapeutic compounds such as lipophilic and hydrophilic drugs, proteins or antimicrobial agents [23] can be incorporated into the nanocarrier polymers using monoaxial or coaxial electrospinning. Electrospun scaffolds have gained an exponentially increasing popularity because of their ultrathin fiber diameter, high surface-volume ratio, high porosity and high surface exposed to the release media [21]. Thus, the electrospun/ electrosprayed nanoporous structures provide very short diffusion length [24,25] and more rapid substance transfer [26] for drug release in comparison with drug-loaded films or capsules. The drug release profile can be tailored by controlling the morphology and the porosity of the nanofibers and also the composition of the fibers [6]. Additionally, electrospun nanofibers can be coated [27] onto various substrates and medical devices [28].
Nanofibers offer an option for the treatment of skin damages as tissueengineered skin substitutes which can help skin reconstruction [29]. In this case, the drug enclosed into the nanofibers mesh will be released by different mechanisms when the nanofibrous mesh is swollen, biodegraded [30] and/or absorbed by the human body. An effective wound dressing system will give a large initial burst release of the drug [31] which is important to stop the growth of the bacteria especially in the early stage of the wound healing process [32]. The burst must be followed by a long term release at inhibitory level [31]. The continued low release rate should keep the wound free from infections for days or weeks [33].
According to literature data, the biologically active substances/drugs can be encapsulated into the individual polymeric nanofibers by (a) coaxially [34] or (b) monoaxially [35] electrospinning of the active substance and the polymer [36]. In another approach the biologically active substances can be (c) entrapped and attached as nanoparticles to the nanofiber mesh.
(a) Coaxial electrospinning – (Figure 1b) can be used to encapsulate drugs or biologically active substances inside the individual polymer nanofibers [37]. In a common process, two (or more) polymer solutions are electrospun through different coaxial capillary channels (needles) (Figure 1b), resulting in a core–shell-structured composite nanofiber. The shell polymer, after the electrospinning, acts as a barrier to control the release of the loaded molecules [38]. If the shell fluid is able to be processed by electrospinning, the core fluid can either be or not be electrospinnable. An advantage of this method is the possibility to enclose almost any drugs (especially hydrophobic ones) in the core regardless of drug–polymer interactions. Hence, drugs [39], proteins [38] and growth factors [24] and even genes [40] can be incorporated into nanofibers simply by dissolving them in the core solutions. A drawback of the coaxial electrospinning comes from the differences in the physical properties of the core and shell solutions conductivities and viscosities of the two solutions.
(b) Monoaxial electrospinning – (Figure 1a) simply encapsulates the biologically active substances/drugs within the individual nanofibers by dispersing/mixing them into the polymer solution. The obtained mixture is further electrospun through a single needle system [41]. Using this method, electrospun nanofibers of chitosan/polyethylene oxide [42] and chitosan/polyurethane [43] were obtained, containing silver sulfadiazine with good antibacterial activity against both Gram-negative and Grampositive bacteria. In acidic medium the release of silver sulfadiazine from chitosan beads is governed by chitosan erosion [44] or even disintegration [45]. The release of silver sulfadiazine from chitosan/chondroitin sulfate films at neutral pH occurred by a sustained release (over a period of days) [46].
Dimensions of the electrospun fibres are comparable with those of natural collagen and vary with type of electrospun material and parameters of electrospinning – Table 1 [23].
| Sample | Average nanofibres diameter (nm) | Reference |
|---|---|---|
| Chitosan | ||
| Sulfadiazine modified chitosan inner needle | 32 ± 10 nm | [42] |
| Sulfadiazine modified chitosan outer needle | 30 ± 10 nm | [42] |
| Sulfadiazine modified chitosan/chitosan mixture uniaxial | 35 ± 10 nm | [42] |
| PLA/Vitamin E/silver | 140 ± 60 nm | [43] |
| PCL/ THF:DMF (1:1) | 500-900 nm | [18] |
| PU /DMF, 3.8-12.8wt% | ∼60-800nm | |
| pHEMA/Monomer | 315 ± 140nm | |
| Vancomycin-Loaded Electrospun Rana chensinensis Skin Collagen/Poly(L-lactide) Nanofibers | 500 ± 800 nm | [30] |
| Poly(lactic acid-co-glycolic acid) (PLGA) | 1000-1800 nm | [14] |
| Core/sheath structured composite nanofibers with a core of blended salicylic acid (SA) and poly(ethylene glycol) (PEG) and a sheath of poly(lactic acid) PLA) | 300-3000 nm | [34] |
Table 1: Dimensions of some morphological units identified in SEM images (some comparable with those of natural collagen [14,18,30,34,42,43].
(c) Besides encapsulating the biologically active substances/drugs into the individual polymeric nanofibers by coaxially or monoaxially electrospinning, there is another approach by which the active substances can be entrapped and attached as nanoparticles to the polymeric nanofiber mesh. This can be achieved by simultaneous electrospinning the polymer and electrospraying the active substance [49,50], spraying the active substance into the electrospinning jet so that the particles containing the active substance are attached to the fiber surface prior to deposition on the mat [51], electrospraying the suspension containing the active substance nanoparticles onto the previously electrospun nanofiber scaffold [30]. By monoaxial electrospinning of the mixture of polymer and active substance suspension through a single nozzle [52] or by coaxial electrospinning with the nanofiber polymer solution flowing through the central nozzle and of the colloidal suspension of the active substance through the outer nozzle which generates a nanofibers mesh covered with active nanoparticles, codeposited from colloidal suspension during the process of electrospinning [53] can be obtained.
An advantage of the nanostructures with active substances entrapped and attached as nanoparticles to a polymeric nanofiber mesh is the improved availability of the active substances to the targeted medium, in comparison with the corresponding systems with fibers containing inside the active nanoparticles [54,55]. As was previously shown, the required burst release can be easily achieved when a major part of the active nanoparticles entrapped into the nanofibrous mesh is exposed at the fiber surface [56].
It is known that the chitosan enhances the wound healing [57] favoring fibroblast attachment [58] and re-epithelialization [59] of the wound. Thus, a chitosan nanofiber mesh is a good candidate for wound healing systems also due to chitosan biocompatibility, antibacterial, and antifungal [60] activities. Considering these excellent properties of the chitosan, high molecular weight chitosan nanofibrous structures having attached active nanoparticles of sulfadiazine (a well-known antibacterial agent [61] used in the treatment of wound infections [62]) or sulfadiazine modified chitosan (which was found to have enhanced antibacterial properties [63-65]) were obtained [47] by mono-axial and coaxial electrospinning. The sulfadiazine or sulfadiazine modified chitosan nanoparticles loosely attached at the surface of the nanofibers, could provide a burst release in the first 20 min (in phosphate buffer solution of pH 6 at 37°C ) which is important to stop the possible initial infection in a wound, while the sulfadiazine or sulfadiazine modified chitosan from the nanoparticles which are better stuck (or even encapsulated) into the chitosan nanofibers were slowly released with releasing mechanism governed by the erosion/ disruption of the chitosan nanofiber mesh. Thus, the fiber forming high molecular weight chitosan [66] assured the formation of the nanofibrous mesh while the sulfadiazine or sulfadiazine modified chitosan both in the form of a relatively stable suspension assured the formation of the active nanoparticles attached to the chitosan nanofiber mesh.
Electrospun formulation containing silver nanoparticles as antimicrobial coatings for biomedical applications
Silver nanoparticles (AgNPs) can be used as a broad-spectrum antibacterial agent for both Gram positive and Gram negative bacteria in biomedical and food packaging applications. Because of their high reactivity originating from the large surface to volume ratio, the AgNPs can effectively eliminate bacteria and yeasts even at rather low concentrations [67]. Furthermore, antibacterial activity of silver nanoparticles was found to be dependent on the size of silver particles, since the only nanoparticles that present a direct interaction with the bacteria preferentially have a diameter of approximately 1-10 nm [68]. The smaller particle size provides improved antibacterial activity [69].
Antibacterial property of electrospun nanofibers containing AgNPs was reported by numerous studies such as polylactic acid/AgNPs fibers against Staphylococcus aureus and Escherichia coli [70], poly(ethylene oxide)/ AgNPs fibers intermixed with polyurethane fibers against Escherichia coli [71], polyacrylonitrile/ AgNPs fibers against Gram positive Bacillus cereus and Gram negative Escherichia coli micro-organisms [72], and Nylon-6/AgNPs nanofibers against both Gram negative Escherichia coli and Gram positive Staphylococcus aureus [73].
Antioxidant activity of vitamin E, a fat soluble antioxidant [74] was combined with antibacterial property of AgNPs in electrospun polylactic nanofibers in order to obtain multifunctional biomaterials. The polylactic acid/ AgNPs /vitamin E nanofibers inhibited growth of Escherichia coli, Listeria monocytogenes and Salmonella typhymurium up to 100%. The release rate of silver ions from the nanofibers immersed in aqueous solution was kept approximately constant even after 10 days of immersion. The polylactic acid/ AgNPs /vitamin E nanofibers had antioxidant activity and the results of the tests on fresh apple and apple juice indicated that the polylactic acid/ AgNPs /vitamin E nanofiber membrane actively reduced the polyphenol oxidase activity. These materials could find application in food industry as a potential preservative packaging for fruits and juices [48].
As was previously shown due to their intrinsic flexibilty, the electrospun nanofibers can be coated onto various medical devices [28] which require the flexibility of the coated layer. Bioactive formulations containing polyurethane and small amounts of biocompatible polymers (hydrolyzed collagen, elastin, hyaluronic acid or chondroitin sulfate, and silver nanoparticles) were coated by electrospinning onto pure polyurethane membrane in order to study the possibility of improving the antibacterial properties of the polyurethane urinary catheters. The obtained coated polyurethane membranes had good antimicrobial activity against Escherichia coli, Salmonella typhymurium, and Listeria monocytogenes [75]. The same authors have shown that the AgNPs improved the electrospinability of the polyurethane bioactive formulations. At low content of AgNPs (less than 0.3%) the coated formulations had high cell proliferation and good biocompatibility having the advantage of adding low amounts of bioactive and biocidal components [76].
Antibacterial/antioxidant/antifungal electrospun coatings for food packaging
Efforts are being made to increase the storage and shell life of food by using active antimicrobial packagings. The antibacterial agents may be coated onto the packaging material [77,78]. Due to its known antibacterial activity chitosan was also used to obtain antibacterial coatings [79] or used to encapsulate/incorporate another antibacterial agent [80] in order to improve the antimicrobial activity of the coating [81]. Various procedures have been proposed for coating the antimicrobial agent: spraying (nebulisation) [82], lamination [79], immersion, etc. [83,84].
One way to perform the coating of the active agent is the electrospinning method due to several advantages: besides the high specific surface area, the very thin thickness of the coated (deposited) layer which can be easily control by the changing of the deposition time or of the flow rate with the possibility to obtain very thin coatings which in some cases are enough to obtain the desired antibacterial effect [27,85].
Plasticizers are added to the coatings to overcome the brittleness exhibited during packaging formation [80] and to improve the flexibility and processability. It is known that polymer nanofibers and have higher yield strength and especially higher ductility than the corresponding bulk material [86,87] due to low nanofiber crystallinity resulting from rapid solidification of the ultrafine electrospun jets [88,87]. Thus it is expected that the nanofibrous electrospun coating layers will have the needed flexibility during the package formation/life time without the plasticizer addition.
Polyethylene films chitosan-coated by electrospinning had good antimicrobial activity against food pathogen microorganisms as Grampositive (Listeria monocytogenes) or Gram-negative (Escherichia coli, Salmonella) [26]. The addition of vitamin E to the coatings improved the aspect, smell, pH, reaction with H2S and total number of germs for minced poultry meat packaging [85]. Polylactic acid films coated by electrospnning with formulation containing chitosan had excellent antifungal activities against Aspergillus brasiliensis, Fusarium graminearum, Penicillium corylophilum [89].
REFERENCES
- Zinatloo-Ajabshir S, Mortazavi-Derazkola S, Salavati-Niasari M. Simple sonochemical synthesis of Ho 2 O 3-SiO 2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminant. Ultrasonics Sonochemistry 2017;39:452-60.
- Zinatloo-Ajabshir S, Morassaei MS, Salavati-Niasari M. Facile fabrication of Dy 2 Sn 2 O 7-SnO 2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminants. Journal of Colloid and Interface Science 2017;497:298-308.
- Zinatloo-Ajabshir S, Mortazavi-Derazkola S, Salavati-Niasari M. Schiff-base hydrothermal synthesis and characterization of Nd2O3 nanostructures for effective photocatalytic degradation of eriochrome black T dye as water contaminant. Journal of Materials Science: Materials in Electronics 2017:1.
- Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances 2010;28:325-47.
- Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Engineering 2006;12:1197-211.
- Eichhorn SJ, Sampson WW. Relationships between specific surface area and pore size in electrospun polymer fibre networks. Journal of The Royal Society Interface 2010;7:641-9.
- Bhowmick S, Rother S, Lee PS, et al. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application–The role of chondroitin sulfate and sulfated hyaluronan. Materials Science and Engineering:C 2017;79:15-22.
- Baptista AC, Ferreira IM, Borges JP. Electrospun fibers in composite materials for medical applications. Journal of Composites and Biodegradable Polymers 2013;1:56-65.
- Ma B, Xie J, Jiang J, et al. Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine 2013;8:1459-81.
- Zhang Y, Ouyang H, Lim CT, et al. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2005;72:156-65.
- Zhao P, Jiang H, Pan H, et al. Biodegradable fibrous scaffolds composed of gelatin coated poly (ϵ-caprolactone) prepared by coaxial electrospinning. Journal of Biomedical Materials Research Part A 2007;83:372-82.
- Sell SA, Garg K, Wolfe PS, et al. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Advanced drug delivery reviews 2009;61:1007-19.
- Linh B, Thuy N, Lee KH, et al. Functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate for bone regeneration in rat calvaria defects. Journal of Biomedical Materials Research Part A 2013;101:2412-23.
- Maleki M, Latifi M, Mathur S, et al. Electrospun core–shell nanofibers for drug encapsulation and sustained release. Polymer Engineering & Science 2013;53:1770-9.
- Chen H, Peng Y, Wu S, et al. Electrospun 3D fibrous scaffolds for chronic wound repair. Materials 2016;9:272-3.
- Jakobsson A, Ottosson M, Zalis MC, et al. Three-dimensional functional human neuronal networks in uncompressed low-density electrospun fiber scaffolds. Nanomedicine: Nanotechnology, Biology and Medicine 2017;13:1563-73.
- Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arabian Journal of Chemistry 2015.
- Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer 2008;49:2387-425.
- Alehosseini A, Ghorani B, Tucker N, et al. Principles of Electrospraying: A New Approach in Protection of Bioactive Compounds in Foods. Critical Reviews in Food Science and Nutrition 2017.
- Salehi R, Irani M, Rashidi MR, et al. Stimuli-responsive nanofibers prepared from poly (N-isopropylacrylamide-acrylamide-vinylpyrrolidone) by electrospinning as an anticancer drug delivery. Designed Monomers and Polymers 2013;16:515-27.
- Vasile C, Oprea AM, Nistor MT, et al. Drug delivery and release from polymeric. In: Nanomaterials in Nanotechnology and Drug Delivery, Nanoplatforms in Drug Delivery, ed. J.L. Arias. Boca Raton 2015;1:28-81.
- Vasile C, Nistor MT, Cojocariu AM. Nano-sized Polymeric Drug carrier systems. Chapter 3 In: Nanotechnology and Drug Delivery, Nanoplatforms in Drug Delivery, ed. Arias, J.L. Boca Raton 2015;1:81-141.
- Chew SY, Wen Y, Dzenis Y, et al. The role of electrospinning in the emerging field of nanomedicine. Current pharmaceutical design 2006;12:4751-70.
- Ji W, Sun Y, Yang F, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharmaceutical research 2011;28:1259-72.
- Teng Y, Qiu Z. Fluid bed coating and granulation for CR delivery. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice 2010:115-27.
- Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. International Journal of Nanomedicine 2013;8:2997-3017.
- Munteanu BS, Paslaru E, Sdrobis A, et al. Chitosan coatings applied to polyethylene surface to obtain food-packaging materials. Cell Chem Technol 2014;48:565-75.
- Chao YK, Lee CH, Liu KS, et al. Sustained release of bactericidal concentrations of penicillin in the pleural space via an antibiotic-eluting pigtail catheter coated with electrospun nanofibers: results from in vivo and in vitro studies. International Journal of Nanomedicine 2015;10:3329-36.
- Sheikh FA, Ju HW, Lee JM, et al. 3D electrospun silk fibroin nanofibers for fabrication of artificial skin. Nanomedicine: Nanotechnology, Biology and Medicine 2015;11:681-91.
- Bae H, Lee J. Encapsulated particles attached on electrospun fibers by in situ combination of electrospinning and coaxial electrospraying. Journal of nanoscience and nanotechnology 2014;14:7574-80.
- Alhusein N, Blagbrough IS, Paul A. Electrospun matrices for localised controlled drug delivery: release of tetracycline hydrochloride from layers of polycaprolactone and poly (ethylene-co-vinyl acetate). Drug Delivery and Translational research 2012;2:477-88.
- Leung V, Hartwell R, Yang H, et al. Bioactive nanofibres for wound healing applications. Journal of Fiber Bioengineering and Informatics 2011;4:1-14.
- Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006;27:2450-67.
- Wang C, Yan KW, Lin YD, et al. Biodegradable core/shell fibers by coaxial electrospinning: processing, fiber characterization, and its application in sustained drug release. Macromolecules 2010;43:6389-97.
- Zhang M, Li Z, Liu L, et al. Preparation and Characterization of Vancomycin-Loaded Electrospun Rana chensinensis Skin Collagen/Poly (L-lactide) Nanofibers for Drug Delivery. Journal of Nanomaterials 2016.
- Jiang H, Wang L, Zhu K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. Journal of Controlled Release 2014;193:296-303.
- Braghirolli DI, Steffens D, Pranke P. Electrospinning for regenerative medicine: a review of the main topics. Drug Discovery Today 2014;19:743-53.
- Tiwari SK, Venkatraman S. Electrospinning pure protein solutions in core–shell fibers. Polymer International 2012;61:1549-55.
- Nguyen TT, Ghosh C, Hwang SG, et al. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. International journal of pharmaceutics 2012;439:296-306.
- Saraf A, Baggett LS, Raphael RM, et al. Regulated non-viral gene delivery from coaxial electrospun fiber mesh scaffolds. Journal of Controlled Release 2010;143:95-103.
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, et al. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. International Journal of Pharmaceutics 2013;452:333-43.
- Kohsari I, Shariatinia Z, Pourmortazavi SM. Antibacterial electrospun chitosan–polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydrate Polymers 2016;140:287-98.
- Lee SJ, Heo DN, Moon JH, et al. Chitosan/polyurethane blended fiber sheets containing silver sulfadiazine for use as an antimicrobial wound dressing. Journal of Nanoscience and Nanotechnology 2014;14:7488-94.
- Li J, Jiang C, Lang X, et al. Multilayer sodium alginate beads with porous core containing chitosan based nanoparticles for oral delivery of anticancer drug. International Journal of Biological Macromolecules 2016;85:1-8.
- Bodmeier R, Paeratakul O. Spherical agglomerates of water insoluble drugs. Journal of Pharmaceutical Sciences 1989;78(11):964-7.
- Fajardo P, Martins JT, Fuciños C, et al. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. Journal of Food Engineering. 2010;101:349-56.
- Munteanu BS, Dumitriu RP, Profire L, et al. Hybrid nanostructures containing sulfadiazine modified chitosan as antimicrobial drug carriers. Nanomaterials 2016;6:207.
- Munteanu BS, Aytac Z, Vasile C, et al. Polylactic acid (PLA)/Silver-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. Journal of Nanoparticle Research 2014;16:2643-4.
- Vitchuli N, Shi Q, Nowak J, et al. Multifunctional ZnO/Nylon 6 nanofiber mats by an electrospinning–electrospraying hybrid process for use in protective applications. Science and technology of advanced materials. 2011;12.
- Braghirolli DI, Zamboni F, Acasigua GA, et al. Association of electrospinning with electrospraying: a strategy to produce 3D scaffolds with incorporated stem cells for use in tissue engineering. International Journal of Nanomedicine 2015;10:5159-70.
- Xuyen, NT, Kim TH, Geng HZ, et al. Three-dimensional architecture of carbon nanotube-anchored polymer nanofiber composite. Journal of Materials Chemistry. 2009;19:7822-5.
- Ávila HA, Reboredo MM, Castro M, et al. Nanofibers obtained by electrospinning of BaTiO3 particles dispersed in polyvinyl alcohol and ethylcellulose. Materials Research 2013;16:839-43.
- Krupa A, Jaworek A, Sundarrajan S, et al. Mechanical properties of an electrospun polymer fibre-metal oxide nanocomposite mat. Fibres & Textiles in Eastern Europe 2012;91:22-7.
- Gupta D, Venugopal J, Mitra S, et al. Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials 2009;30:2085-94.
- Jaworek A, Krupa A, Lackowski M, et al. Nanocomposite fabric formation by electrospinning and electrospraying technologies. Journal of Electrostatics 2009;67:435-8.
- Chang HI, Lau YC, Yan C, et al. Controlled release of an antibiotic, gentamicin sulphate, from gravity spun polycaprolactone fibers. Journal of Biomedical Materials Research Part A 2008;84:230-7.
- Kumar MN. A review of chitin and chitosan applications. Reactive and Functional Polymers 2000;46:1-27.
- Hilmi AB, Halim AS, Hassan A, et al. In vitro characterization of a chitosan skin regenerating template as a scaffold for cells cultivation. Springerplus 2013;2:79.
- Azad AK, Sermsintham N, Chandrkrachang S, et al. Chitosan membrane as a wound‐healing dressing: characterization and clinical application. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2004;69:216-22.
- Tikhonov VE, Stepnova EA, Babak VG, et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2 (3)-(dodec-2-enyl) succinoyl/-derivatives. Carbohydrate Polymers 2006;64:66-72.
- Heyneman AL, Hoeksema HE, Vandekerckhove D, et al. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns 2016;42:1377-86.
- Cuttle L, Pearn J, McMillan JR, et al. A review of first aid treatments for burn injuries. Burns 2009;35:768-75.
- Dragostin OM, Samal SK, Lupascu F, et al. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. International Journal of Molecular Sciences 2015;16:29843-55.
- Dumitriu RP, Profire L, Vasile C, et al. Sulfadiazine—Chitosan Conjugates and Their Polyelectrolyte Complexes with Hyaluronate Destined to the Management of Burn Wounds. Materials 2015;8:317-38.
- Dragostin OM, Samal SK, Vasile C, et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydrate Polymers 2016;141:28-40.
- Geng X, Kwon OH, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005;26:5427-32.
- Gangadharan D, Harshvardan K, Gnanasekar G, et al. Polymeric microspheres containing silver nanoparticles as a bactericidal agent for water disinfection. Water Research 2010;44:5481-7.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology 2005;16:2346-53.
- Espinosa-Cristóbal LF, Martínez-Castañón GA, Martínez-Martínez RE, et al. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Materials Letters 2009;63:2603-6.
- Xu X, Yang Q, Wang Y, et al. Biodegradable electrospun poly (L-lactide) fibers containing antibacterial silver nanoparticles. European Polymer Journal 2006;42:2081-7.
- Tijing LD, Ruelo MT, Amarjargal A, et al. One-step fabrication of antibacterial (silver nanoparticles/poly (ethylene oxide))–Polyurethane bicomponent hybrid nanofibrous mat by dual-spinneret electrospinning. Materials Chemistry and Physics 2012;134:557-61.
- Shi Q, Vitchuli N, Nowak J, et al. Durable antibacterial Ag/polyacrylonitrile (Ag/PAN) hybrid nanofibers prepared by atmospheric plasma treatment and electrospinning. European Polymer Journal 2011;47:1402-9.
- Pant B, Pant HR, Kim HY, et al. Characterization and antibacterial properties of Ag NPs loaded nylon-6 nanocomposite prepared by one-step electrospinning process. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2012;395:94-9.
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biology and Medicine 2007;43:4-15.
- Macocinschi D, Paslaru E, Munteanu BS, et al. Polyurethane–extracellular matrix/silver bionanocomposites for urinary catheters. Journal of Bioactive and Compatible Polymers 2015;3:99-113.
- Filip D, Munteanu BS, Vasile C, et al. Polyurethane biocompatible silver bionanocomposites for biomedical applications. Journal of Nanoparticle Research 2014;16:2710.
- Muriel-Galet V, Cerisuelo JP, López-Carballo G, et al. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013;30:137-43.
- Muriel-Galet V, Cerisuelo JP, López-Carballo G, et al. Development of antimicrobial films for microbiological control of packaged salad. International Journal of Food Microbiology 2012;157:195-201.
- Hoagland PD, Inventor; The United States Of America, As Represented By The Secretary Of Agriculture, assignee. Biodegradable laminated films fabricated from pectin and chitosan 1999.
- Hong SI, Lee JW, Son SM. Properties of polysaccharide‐coated polypropylene films as affected by biopolymer and plasticizer types. Packaging Technology and Science 2005;18:1-9.
- Lee CH, An DS, Park HJ, et al. Wide spectrum antimicrobial packaging materials incorporating nisin and chitosan in the coating. Packaging Technology and Science 2003;16:99-106.
- Contini C, Katsikogianni MG, O’Neill FT, et al. Development of active packaging containing natural antioxidants. Procedia Food Science 2011;1:224-8.
- Vasile C, Baican MC, Tibirna CM, et al. Microwave plasma activation of a polyvinylidene fluoride surface for protein immobilization. Journal of Physics D: Applied Physics 2011;44.
- Stoleru E, Dumitriu RP, Munteanu BS, et al. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Applied Surface Science 2016;367:407-17.
- Vasile C, Darie RN, Sdrobis A, et al. Effectiveness of chitosan as antimicrobial agent in LDPE/CS composite films as minced poultry meat packaging materials. Cellul Chem Technol 2014;48:325-36.
- Naraghi M, Arshad SN, Chasiotis I. Molecular orientation and mechanical property size effects in electrospun polyacrylonitrile nanofibers. Polymer 2011;52:1612-8.
- Chew SY, Hufnagel TC, Lim CT, et al. Mechanical properties of single electrospun drug-encapsulated nanofibres. Nanotechnology 2006;17:3880-91.
- Papkov D, Zou Y, Andalib MN, et al. Simultaneously strong and tough ultrafine continuous nanofibers. ACS Nano 2013;7:3324-31.
- Miteluţ AC, Tănase EE, Vasile C, et al. Research on chitosan and oil coated PLA as food packaging material. International Workshop “Progress in antimicrobial materials” Iasi, Romania, March 30, 2017