Antitumor property of the venom of snakes of the genus Coluber
Received: 28-Feb-2020 Accepted Date: Mar 12, 2020; Published: 18-Mar-2020
Citation: Rakhmatullaev EA, Iriskulov BU. Antitumor property of the venom of snakes of the genus Coluber. Clin Pharmacol Toxicol Res 2020;3(1):1-3
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
This article presents data on histological, electron microscopic, and biochemical methods for studying the venom of snakes of the genus Coluber. The effect of Coluber snake venom on antitumor activity was studied. We studied the parameters of T-helper activity and indicators of the chemokine RANTES. The results of the study suggest that the poisons of the Coluber genus have antitumor properties, which is confirmed by the results of the studies.
Keywords
Vascular anastomosis; Renal transplantation; Cold ischemia; Kidney transplant
Introduction
According to the World Health Organization, about 1,000,000 people suffer from snake bites annually on Earth, 50,000 of them die, some of the remaining become disabled. This determines the need for a thorough study of the nature of the damaging effects of zootoxins.
Snake venom is a complex of biologically active compounds, which is a valuable raw material for pharmaceutical science and industry. Snake venom is used in the manufacture of antidote sera, as well as in the composition of medicines as a painkiller, anti-inflammatory and local irritant in diseases of the peripheral nervous system [1].
Separate components of poisons (endonuclease, phospholipase A2, phosphodiesterase) are used as chemical reagents for the diagnosis of blood diseases and the nervous system, for modeling a number of pathological syndromes and studying the mechanism of blood coagulation.
Some components of poisons can also be used as antitumor agents. A potential target for selective immunomodulation is voltage-gated potassium channels that are inhibited by β-bungarotoxin.
Proteolytic enzymes from Coluber snake venom are of interest as natural potassium channel modulators in the practice of treating cardiac pathologies and oncological diseases.
The poison is a complex mixture of hydrolytic enzymes, non-enzymatic low and high molecular weight proteins and a small number of organic and inorganic molecules (pigments, mineral components and trace elements) [2].
Snake venoms have both common features: the presence of phospholipase A2, hyaluronidase, L-amino acid oxidase, and distinctive features. For example, venom snakes of aspids and sea snakes contain toxic polypeptides (neurotoxins) that disrupt the transmission of excitation in neuromuscular synapses and thereby cause paralysis of skeletal and respiratory muscles. Viper venoms contain proteolytic enzymes with trypsin, thrombin and kallikrein-like action. As a result of poisoning with these poisons, hemorrhagic edema develops, due to increased vascular permeability, as well as disturbances in the blood coagulation system.
In the study of Mukhamedova D.G. (1996) indicated that the poison of Central Asian cobra can increase the activity of the complement system, the synthesis of lysozyme, and stimulate the phagocytic activity of neutrophilic leukocytes. At the same time, all snake venoms can regulate the functional relationship between the cytoplasmic membranes of body cells and their receptors and exhibit a cytostatic effect. This is based on the common ability of all poisons to modulate cyclase activity and to separate the processes of respiration and oxidative phosphorylation. These properties of poisons make them potential immunomodulators.
Many studies have shown that chemokines play a key role in organizing all stages of the T cell response, from recruiting naive T cells to inflamed tissue, migrating T cells in lymphoid organs to moving activated T cells from lymphoid tissues to effector sites, and effector movements T cells in nonlymphoid tissues.
One of the chemokines that has been shown to play a role in immune responses to viral infections is the beta chemokine RANTES (regulated by the activation of normal T cells expressed and secreted). Although RANTES was initially thought to be a T-cell-specific chemokine, it is now known to be expressed by a number of other cell types, including epithelial cells and platelets, and acts as a potent chemoattractant for many cell types, such as monocytes, NK cells [3]. Memory T cells [4], eosinophils [5] and DC [6]. The RANTES receptor, CCR5, is a G protein bound receptor that, in addition to be the main receptor for RANTES, can also bind MIP1α (CCL3) and MIP β (CCL4).
At the same time, the antitumor effect of the poison of some snakes, snakes of the genus Coluber, has not been fully considered, which determines the relevance of the study [7].
Research Interest
To study the antitumor activity of Coluber genus snake venoms by modulating the primary immune response in vivo and in vitro.
Materials and Methods
To achieve this goal, poisons of the Coluber genus, as well as blood serum of experimental animals, and liver biopsy samples of experimental mice were studied. 100 white mice of the Vistar genus were used for researching.
The following research methods were used: histochemical, electronmicroscopic and biochemical methods.
Electron microscopic research method
The material was fixed in a buffered 2.5% solution of glutaraldehyde, and then additionally fixed in a 1% solution of osmium tetroxide. Semi-thin sections were stained with toluidine blue solution. Ultrathin sections were contrasted with lead citrate. The study of objects was carried out on an electron microscope EM-200.
Antitumor immunity was evaluated by increasing the T-helper activity of experimental animals, as well as by the content of Rantes protein related to β-chemokines.
Transplantable mouse lymphosarcoma was used as a tumor model. The experiment was carried out in 2 stages. At stage 1, the animals were implanted with a different number of tumor cells derived from ascites. On day 10, mice were inoculated with a suspension of tumor cells in physiological saline with a volume of 0.1 ml at a dose of 100,000 cells/ individual. To assess the dynamics of the tumor node every other day, the length, width and height of the tumor were measured.
At stage 2, the animals were divided into 2 groups: group 1 (50 animals) were injected with Coluber snake venom at a dosage of 1/500 LD50, animals were not administered to animals of group 2 (50 animals).
Results
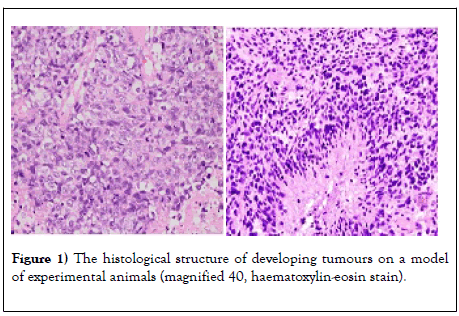
The experimental tumor model initially leads to the appearance in the liver of mice of signs of inflammation, fatty degeneration, and fibrosis (Figure 1).
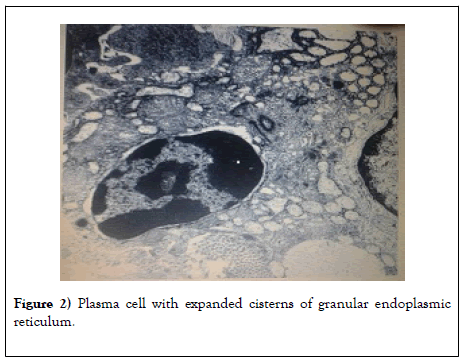
So, in Figure 2 it is seen that the granular endoplasmic reticulum occupies almost the entire cytoplasm. The core is large (10 μm) with a typical chromatin arrangement. Mature plasma cells complete the stages of differentiation and proliferation of the B-cell series.
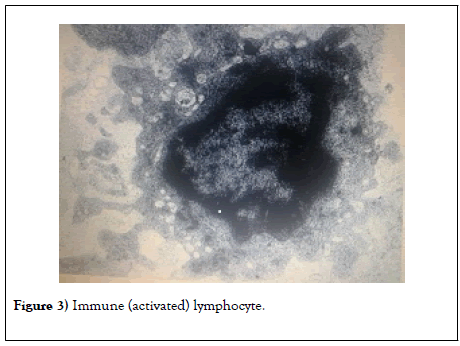
After the introduction of Coluber genus snake venom, a pronounced immune response in the form of activated lymphocytes is observed in laboratory animals already on the 4th-5th days (Figure 3).
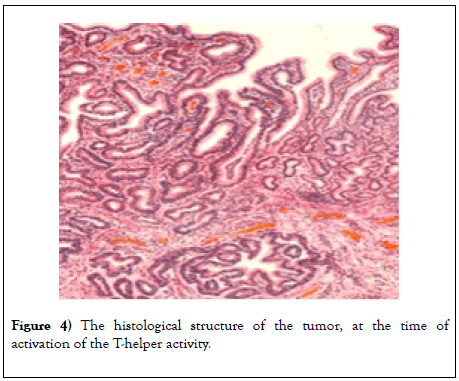
So, in the cytoplasm, the number of ribosomes, large mitochondria, lysosomes and vacuoles increases, which indicates a pronounced immune response of the experimental animal (Figure 4).
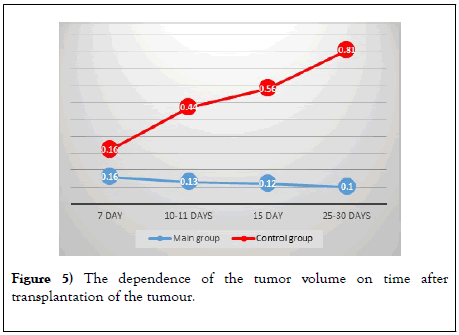
On the 7th day of the therapy with Coluber genus snake venom, an improvement in dynamics was noted in the form of a decrease in the size of the tumor by 0.05 cm3, and then the sizes gradually decreased. In animals of the control group, the death of animals was already recorded on days 8– 10 (Figure 5).
Figure 5: The dependence of the tumor volume on time after transplantation of the tumour.
Throughout the experiment, one can observe the picture that persistent tumor regression is observed in the main group (Table 1).
| Indicator | 7-day | 8-10 day | 11-15 day | 20-30 day |
|---|---|---|---|---|
| RANTES | 1700 ng/ml | Main group 1600 ng/ml |
1400 ng/ml | 1000 ng/ml |
| RANTES | 1800 ng/ml | Control group 1200 ng/ml |
850 ng/ml | 770 ng/ml |
Table 1: Indicators of the rantes protein related to β- chemokines with the use of coluber snake venom.
The analysis showed that under the influence of the venom of snakes of the genus Coluber, type 2 T-helpers are activated in comparison with type 1 Thelpers, as indicated by the amount and ratio of RANTES protein.
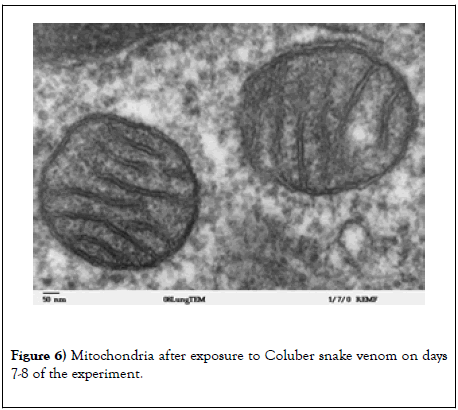
After the treatment with Coluber snake venoms, there is a clear dynamic in improving the well-being of experimental animals, which is confirmed by electron microscopic research methods (Figure 6).
It has been shown that after the action of Coluber snake venom, the number of CD4 CD8 T cells of antiviral memory increases. Moreover, the nature of the differentiation of memory T cells changed after exposure to Coluber snake venom, which may serve as a basis for assuming the role of the influence of the chemokine RANTES. Moreover, virus-specific CD4 CD8 memory T cells, after exposure to Coluber snake venom, are capable of producing multiple cytokines in large quantities; we concluded that RANTES β-chemokines, which are involved in the “helping” memory of T cells, can cause the so-called anamnestic answer. A key feature of T-cells is CD8 memory. In addition, RANTES memory cells formed secondary effector CD8 T cells, which were phenotypically and functionally like secondary effector T cells.
In contrast to the main group, in the control group CD8 T cells lose their ability to effectively perform effector functions. This “ depletion ” is hierarchical and progressive: virus-specific CD8 T cells gradually lose their ability to produce IL-2, multiply rapidly, efficiently kill, produce TNF α and, when severely depleted, produce IFN γ. These depleted CD8 T cells also express inhibitory receptors such as PD-1, LAG-3, 2B4, and CD160. These receptors are actively involved in inhibiting the function of CD8 T cells during chronic infection and blocking these pathways can stimulate antiviral T cell responses.
Discussion and Conclusion
The results of histological, electron microscopic, and biochemical research methods allow us to conclude that the use of Coluber snake venom positively affects the state of the tumor caused by the experimental living model. So, the size of the tumor decreased already on the 7th day by 0.02 cm3 (p ≤ 0.001) and continued to decrease and by the end of the experiment reached 0.10 cm3 (-0.06 cm3), p ≤ 0.001.
The results allow us to conclude that Coluber snake venom is able to modulate the functional activity of the immune system in the form of an increase in T-helper activity and normalization of RANTES protein related to β-chemokines, which indicates an increase in the non-specific resistance of the animal’s body.
This venom of the snake of the genus Coluber makes promising in the search for immunomodulators of narrowly targeted action and opens new possibilities for their use in oncology.
REFERENCES
- Cooper SS, Terekhov IV. Influence of low-intensity microwave radiation with a frequency of 1 ghz on the functional status of mononuclear leukocytes of whole blood in practically healthy young faces. Int J App Res. 2016;5:1083-87.
- Amanda P. Human mutant chemokine rantes and the use of mutant chemokines for the treatment of multiple sclerosis. J Invest Dermatol. 2019;12:312-17.
- Moskalev AV. The role of chemokines in the development of an antiviral immune response. Bulletin of the Russian Military Medical Academy. 2017;3:183-88.
- Esche C, Stellato C, Beck LA. Chemokines: Key players in innate and adaptive immunity. J Invest Dermatol. 2005;125:615-28.
- Loetscher P, Seitz M, Clark-Lewis I, et al. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322-27
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593-620.
- Schall TJ, Bacon K, Toy KJ, et al. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669-71.