Association of high serum triglycerides and triglycerides/ HDL ratio with raised HbA1c, creatinine, microalbuminuria and development of diabetic kidney disease and diabetic renal failure. Mathematical and statistical regression models of 10,370 diabetic patients
Received: 27-Nov-2017 Accepted Date: Dec 18, 2017; Published: 22-Dec-2017
Citation: Aziz KMA. Association of high serum triglycerides and triglycerides/ HDL ratio with raised HbA1c, creatinine, microalbuminuria and development of diabetic kidney disease and diabetic renal failure. Mathematical and statistical regression models of 10,370 diabetic patients. Clin Nephrol Res. 2017;1(1):17-25.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Renal disorders and chronic kidney disease (CKD) in diabetics, or the diabetic kidney disease (DKD), renal failure and end stage renal disease (ESRD) still remains most important complications of diabetes with high prevalence. Apart from hyperglycemia, causes and risk factors must be analyzed to reduce the economic burden. Current research was conducted to study the triglycerides and triglycerides to HDL-C ratio (TG/HDL) and their involvement in the development of proteinuria/nephropathy, raising creatinine levels (CKD/DKD). 10,370 Diabetic Patients were recruited in the study for more than 12 years, from 2005 until 2017. 6201 (59.8% were males and 4169 (40.2%) females. 3940 (38%) subjects demonstrated nephropathy while 1348 (13%) demonstrated DKD/CKD. HbA1c was significantly correlated with triglycerides and TG/HDL ratio and microalbumin (p<0.0001, 0.021 and <0.0001, respectively). Triglycerides and TG/HDL were also highly correlated with creatinine and micoalbumin (p=0.006, p=0.002, p<0.0001 and p<0.0001 respectively). Levels of HbA1c, triglycerides, TG/HDL and creatinine were elevated among the patients with nephropathy (p-values: 0.001, 0.001, 0.004, <0.0001, respectively). Similarly, Levels of HbA1c, triglycerides, TG/HDL and microalbuminuria were elevated among the patients with DKD (p-values: 0.03, 0.018, 0.009, <0.0001. Regression models were also developed to demonstrate the effect of dyslipidemia or elevated serum triglycerides in raising the serum creatinine and urine microalbumin levels and development of DKD; all regression models were significant (p<0.0001). For diagnostic statistics, Receiver Operating Curve (ROC) was constructed. The ROC for triglycerides and nephropathy demonstrated area under the curve (AUC) 0.559 (95% CI 0.530 to 0. 588; p-value<0.0001). By this observation and data analysis, for the detection of the development of nephropathy, a triglyceride cutoff point of 138 mg/dl with 65% sensitivity and 55% specificity was observed. Similarly, ROC for detection of DKD, a triglyceride cutoff point of 153 mg/dl with 60% sensitivity and 61% specificity was found (AUC 0.581; 95% CI 0.522 to 0.640; p-value<0.0001). Study findings concluded and recommended that all diabetic patients should be screened for the dyslipidemia and nephropathy to prevent DKD and ESRD.
Keywords
Microvascular disease, triglycerides, cardiovascular, diabetes, microalbuminuria
Introduction
Diabetes mellitus is major cause of microvascular disease, including neuropathy, retinopathy, nephropathy. The landmark trial, DCCT (diabetes control and complication trial) has demonstrated that reductions in HbA1c levels will reduce the risk of microvascular and macrovascular complications [1-3]. Elevated levels of HbA1c or uncontrolled diabetes is usually associated with increased levels of serum triglycerides [4,5]. Additionally, it was found that dyslipidemia in the diabetic state is also associated with renal disease [6].
Chronic involvement of kidney in diabetes, also called chronic kidney disease (CKD) and now better termed, the diabetic kidney disease (DKD), leads to microalbuminuria and gross proteinuria and ultimately to end stage renal disease (ESRD). Atherosclerotic cardiovascular disease (ASCVD) is one of the major leading causes of morbidity and mortality among diabetic patients, which usually coexist with DKD [7].
Hyperglycemia and hypertension are the main risk factors for the development and progression of DKD [8]. However, it has been observed that in spite of the achievement of recommended targets and goals for glycemic control and blood pressure, the residual risk for diabetic nephropathy remains high among type-2 diabetic patients [9,10]. Hence, in other words, diabetic dyslipidemia still remains one of the major risks for the DKD or ESRD, apart from hypertension [11,12]. Hence, cardiovascular complications, such as hypertension (HTN) or ischemic heart disease (IHD) are also associated with kidney disorders in diabetes, as both of them share a common risk of dyslipidemia. Several epidemiological studies have found an association between lipids diabetic kidney disease and other diabetes related disorders [13-23]. Triglycerides and triglyceride to HDL-C ratio (TG/HDL), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were studies as well. HDL-C is considered good cholesterol, and have also cardiovascular protective effect; however, elevated levels of other lipids are considered harmful and are considered risk factors for cardiovascular and renal system. This effect is augmented when the patient is diabetic [24-28].
TG/HDL ratio can be considered an excellent tool and strategy for estimating risk of atherosclerosis among diabetes patients. Furthermore, DKD/CKD is also associated with atherosclerosis [29,30]. Hence, association of lipids, especially triglycerides, with the development of renal impairment must be studied.
Under this background and literature review, our main objective was to study involvement of serum lipids (triglycerides and TG/HDL ratio) in the development of diabetic renal disease or disorders. Until date there are no studies which have demonstrated direct association and involvement of triglycerides with raised serum HbA1c, creatinine, and microalbuminuria with consequent development of DKD. Furthermore, studies are lacking for the statistical regression models which can predict renal impairment by the given triglyceride or triglyceride to HDL-C ratio (TG/HDL). These were the objectives of the current research, to study how these risk factors can contribute to the development of DKD under the influence of dyslipidemia. It was our aim also to develop statistical regression models for triglyceride and triglyceride to HDL-C ratio to demonstrate the effect of dyslipidemia or elevated serum triglycerides in raising the serum creatinine and urine microalbumin levels and development of DKD.
Methods
This is a prospective cross sectional analytical and cohort study, conducted at the diabetology clinic of Aseer Diabetes Center of Aseer Central Hospital, Ministry of Health, Saudi Arabia. Study duration was more than 12 years, from August 2005 until September 2017. The study recruited 10,370 diabetic patients (after exclusion criteria) who, were followed up in this clinic. Study included both type-1 and type-2 diabetic patients. Children (less than 13 years of age), patients with severe liver or hepatic disorders, patients demonstrating urinary tract infection, known cases of nephrotic syndrome before the onset of diabetes, patients with end stage renal disease (ESRD) or dialysis and pregnant women were excluded from the study.
Patients demonstrating levels of serum creatinine>1.5 were defined as chronic renal/kidney disease (CRD/CKD) and these diabetic subjects were also considered “diabetic kidney disease” (DKD). Furthermore, patients demonstrating microalbuminuria or gross proteinuria were labeled as “nephropathy”.
Laboratory methods
All samples were collected in fasting state of 12 hours, early in the morning. Serum triglyceride (mg/dl) was measured by an enzymatic procedure; the sample is incubated with lipoprotein lipase (LPL) enzyme reagent that converts triglycerides to free glycerol and fatty acids. These are further oxidized to dihyhroxyacetone phosphate and hydrogen peroxide (H2O2) which is again converted to quinoneimine, absorbance of which is directly proportional to the total amount of glycerol. Absorbance is measured by bichromatic (510,700 nm) endpoint technique.
HDL-C (mg/dl) was measured directly in plasma by Automated High Density Lipoprotein (AHDL) method by the Dimension® clinical chemistry system and analyzer (Siemens healthcare diagnostics Inc. Newark, DE 19714, U.S.A), in vitro diagnostic test intended for quantitative determination of HDL-C. Triglycerides and HDL ration was measured as TG/HDL.
Serum creatinine (mg/dl) was quantitatively measured by CREA methodology by Dimension® clinical chemistry system and device (Siemens Healthcare Diagnostics Inc. Newark, DE 19714, USA). The technique for the measurement of creatinine in plasma and urine involved picrate which, in the presence of a strong base NaOH, chemically reacts with creatinine to form a red chromophore. The rate of increasing absorbance at 510nm due to the formation of this chromophore is directly proportional to the creatinine concentration in the sample of blood or urine and which, is measured using by a bichromatic (510,600 nm) rate methodology. Hence, creatinine in the plasma was determined quantitatively [31-33].
HbA1c was measured by A1c Flex® Reagent by the Dimension® clinical chemistry system, in vitro diagnostic assay for the quantitative determination of both percent hemoglobin A1c and total hemoglobin, based on a turbidimetric inhibition immunoassay (TINIA) principle, and the measurement of total hemoglobin is based on a modification of the alkaline hematin reaction, an NGSP certified methodology (Siemens healthcare diagnostics Inc. Newark, DE 19714, USA). The percentage of total hemoglobin that is glycated was calculated and reported as %HbA1c (in g/dL), and final result has been standardized to the results obtained in DCCT.
For the detection of nephropathy and presence of albumin or protein in urine, fasting urine samples were examined for the presence of microalbuminuria, macroalbuminuria or proteinuria. All urine samples were first examined for the presence of gross proteinuria by QuikCheck™ urinalysis reagent strips (ACON biotech, Co., Ltd.) to rule out macroalbumin in urine. This technique is based on the phenomenon of pH indicators which releases hydrogen ions to the protein. Samples which demonstrated macroalbuminuria (in mg/dl) or gross proteinuria by the color indicator of the reagent strips (ranging from 1+ to 4+) were defined/ labeled as “nephropathy”. Samples with negative albumin were further examined for the presence of microalbumin in urine by MALB method used by Dimension® clinical chemistry system and device, in vitro diagnostic test for quantitative measurement of albumin (mg/L) in human urine by particle-enhanced turbidimetric inhibition immunoassay (PETINIA) methodology (Siemens Healthcare Diagnostics Inc. Newark, DE 19714, USA). Samples demonstrating microalbuminuria (albumin excretion in urine in the range of 30-300 mg/L) were also labeled and defined as nephropathy.
All laboratory sample requests were entered in a computer software and results retrieved by Natcom Hospital Information System (NATCOM HIS; National Computer System Co. Ltd [34].
Statistical methods
Patients' data were analyzed by IBM® SPSS® statistics, version 20, for Microsoft Windows. All statistical tests were applied according to the available standard medical statistical methods. Data were summarized as percentages with mean ± SD and 95% CI for the variables.
Independent t-test was used to test the significance between the groups of variables. For Pearson's correlation analysis and regression model development, all standard statistical assumptions were taken into account that variables must show linear relationship.
Predictive regression models were used to develop relationship of triglycerides (and TG/HDL ratio) and other variables (creatinine, microalbumin), and it was then estimated by mathematical linear equations to confirm that how serum lipids contribute to the development of high or increased levels of serum creatinine, urine microalbumin and development of DKD. To maximize the likelihood ratio, ROC curve was used for the estimation of sensitivity and specificity for triglycerides with detection of cutoff points for diagnosing DKD. Statistical power of 90% was built for detection of significance and p-values (two-sided) of less than 0.05 were considered significant.
Patient consent
This study was reviewed and approved by the research committee of Aseer Diabetes Center, and all methodologies on subjects reported in current study were in accordance with the Helsinki Declaration of 1975 (revised in 2008).
Results
Demographic data for the patients is presented in table-1. Nephropathy was observed in 38% of patients, while 13% demonstrated DKD/CKD. Descriptive statistics for variables are shown in table-2. Table-3 shows correlations between variables. It can be observed that correlations are significant for triglycerides and TG/HDL. Highest correlations were observed between TG/HDL with creatinine and microalbumin (0.298 and 0.394 respectively; p-values<0.0001 for both).
| Parameters | Description with N (%); Totals=10,370 | |
| Gender | Male | Female |
| 6201 (59.8%) | 4169 (40.2%) | |
| Type of Diabetes | Type-1 | Type-2 |
| 1451 (14%) | 8919 (86%) | |
| Nephropahty | Positive | Negative |
| 3940 (38%) | 6430 (62%) | |
| Diabetic Kidney Disease (DKD/CRD/CKD) status | Positive | Negative |
| 1348 (13%) | 9022 (87%) | |
Table 1: Demographic data of diabetic patients.
| Variables | Mean ± SD |
|---|---|
| Age (years) | 54 ± 14.4 |
| Diabetes duration (years) | 16 ± 9.7 |
| Serum creatinine (mg/dl) | 0.958 ± 0.681 |
| HbA1c %(g/dl) | 7.9 ± 1.48 |
| Triglycerides (mg/dl) | 157 ± 95.7 |
| TG/HDL | 4.32 ± 3.5 |
| Microalbumin in urine (mg/L) | 78.3 ± 107.3 |
Table 2: Descriptive statistics for the variables with mean ± SD.
| Variables | Pearson Correlation (r) | p-value |
|---|---|---|
| HbA1c and triglycerides | 0.205 | < 0.0001 |
| HbA1c and TG/HDL | 0.103 | 0.021 |
| Triglycerides and serum creatinine | 0.269 | 0.006 |
| TG/HDL and serum creatinine | 0.298 | 0.002 |
| Triglyceride and microalbumin | 0.312 | < 0.0001 |
| TG/HDL and microalbumin | 0.394 | < 0.0001 |
| HbA1c and microalbumin | 0.212 | < 0.0001 |
| Microalbumin and serum creatinine | 0.273 | < 0.0001 |
Table 3: Correlations of variables.
Table-4 shows significant t-test among group of variables (HbA1c, triglycerides, TG/HDL and creatinine). Hence, it is evident from the table-4 that levels of HbA1c, triglycerides, TG/HDL and serum creatinine were elevated among the patients with nephropathy, with significant pvalues. Similarly, table-5 demonstrates significant differences of variables (HbA1c, triglycerides, TG/HDL and urine microalbumin) among the groups with and without DKD. It is evident that the levels of these variables are elevated among the patients with DKD.
Table-6 demonstrates the significant correlation and regression models between different variables. The regression models were significantly
| Variables and indicators | Patients Variable Values With or Without Nephropathy | ||
|---|---|---|---|
| Mean ± 95% CI | P-values | ||
| HbA1c % (g/dl) | With Nephropahty | Without Nephropahty | 0.001 |
| 8.2 ± 1.5 | 7.65 ± 1.46 | ||
| 7.97 to 8.26 | 7.53 to 7.77 | ||
| Triglyceride (mg/dl) | With Nephropahty | Without Nephropahty | 0.001 |
| 166.8 ± 105.5 | 149.4 ± 92.2 | ||
| 159 to 175 | 144 to 156 | ||
| TG/HDL Ratio | With Nephropahty | Without Nephropahty | 0.004 |
| 4.62 ± 3.77 | 4.08 ± 3.26 | ||
| 4.32 to 4.93 | 3.85 to 4.31 | ||
| Creatinine (mg/L) | With Nephropahty | Without Nephropahty | <0.0001 |
| 1.15 ± 0.99 | 0.825 ± 0.25 | ||
| 1.1 to 1.23 | 0.80 to 0.84 | ||
Table 4: Significant statistical tests between groups of variables (with and without nephropathy) with mean ± SD and p-values.
| Variables and indicators | Patients Variable Values With or Without DKD | ||
|---|---|---|---|
| Mean ± 95% CI | P-values | ||
| HbA1c % (g/dl) | With DKD | Without DKD | 0.03 |
| 9.3 ± 2.2 | 7.8 ± 1.48 | ||
| 9.1 to 10.2 | 7.75 to 7.94 | ||
| Triglycerides (mg/dl) | With DKD | Without DKD | 0.018 |
| 179 ± 97 | 155 ± 91 | ||
| 159 to 196 | 151 to 160 | ||
| TG/HDL Ratio | With DKD | Without DKD | 0.009 |
| 5.18 ± 3.39 | 4.25 ± 2.9 | ||
| 4.51 to 5.84 | 4.1 to 4.4 | ||
| Urine microalbumin (mg/L) | With DKD | Without DKD | <0.0001 |
| 183 ± 128 | 64.2 ± 113 | ||
| 146 to 221 | 56.5 to 71.8 | ||
Table 5: Significant statistical tests between groups (with and without DKD) of variables with mean ± SD and p-values.
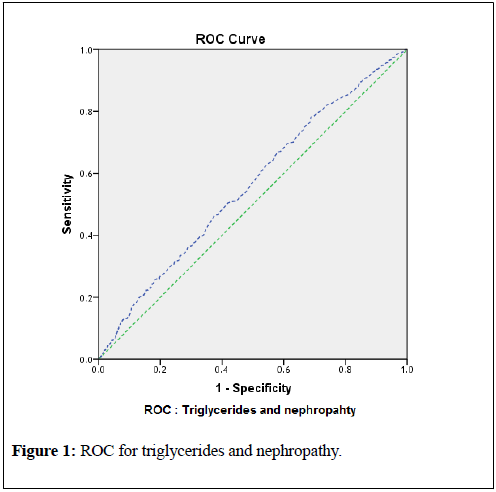
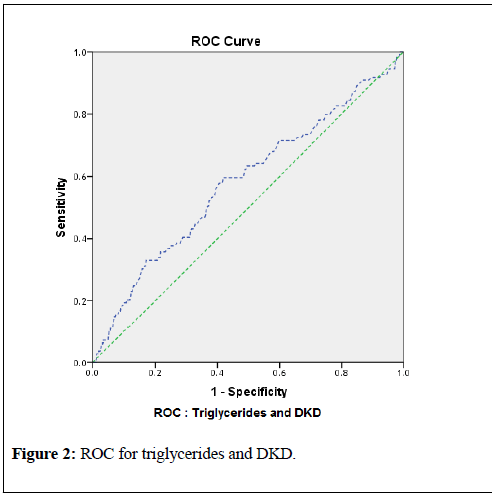
associated, with p-values <0.000 for all variables. This data prove that triglycerides and TG/HDL significantly contributes to the elevated levels of serum creatinine and urine microalbumin and ultimately development of DKD.For diagnostic statistics, and to confirm the regression models, and significant association of triglycerides with the development of nephropathy and DKD, Receiver Operating Curve (ROC) were constructed. The ROC for triglycerides and nephropathy demonstrated area under the curve (AUC) 0.559 (95% CI 0.530 to 0. 588; p-value <0.0001). By this observation and data analysis, for the detection of the development of nephropathy, a triglyceride cutoff point of 138 mg/dl with 65% sensitivity and 55% specificity was observed. Similarly, ROC for detection of DKD, a triglyceride cutoff point of 153 mg/dl with 60% sensitivity and 61% specificity was found (AUC 0.581; 95% CI 0.522 to 0.640; p-value <0.0001). These results are shown in table-7 and graphically in figures-1 and 2.
Discussion
Diabetic dyslipidemia is associated with microvascular and macrovascular complications of diabetes, as has been shown in the studies conducted past few decades [35,36]. Regarding guidelines for diabetic patients provided by American Diabetes Association (ADA) and National Cholesterol Education Program (NCEP), a target for LDL-C is <100 mg/dl, for triglycerides is <150 mg/dl; HDL-C>40 mg/dl is considered protective. [37,38]. Generally, under these guidelines, a normal value of TG/HDL would be <3.75 (150 mg/dl/40 mg/dl).
One of the important and interesting finding demonstrated by recent research studies is the intra-renal accumulation of lipids which ultimately contribute to the glomerular injury via inflammatory pathways involving oxidative stress, pro-inflammatory cytokine and growth factor release [39-45]. Specifically, de novo triglycerides association with diabetes renal complication (DKD) has been observed also [46,47]. Conversely, and as demonstrated by studies, high levels of HDL-C is protective to the cardiovascular and renal systems [48].
High TG/HDL ratio in diabetic state is a significant risk factor for arterial stiffness and carotid atherosclerosis. Hence, atherosclerosis (due to high Triglycerides or TG/HDL) may contribute to the development of DKD [17]. Hence, clinical efforts should be done to reduce triglycerides and to elevate HDL-C to reduce diabetic related complications contributed by dyslipidemia [49-55].
Current study was designed and conducted to observe significant association of triglycerides and TG/HDL ratio with elevated levels of serum creatinine, and microalbuminuria and ultimately development of DKD or diabetic renal disease. Also elevated levels of HbA1c was observed among the patients who demonstrated creatinine > 1.5 mg/dl (DKD) and nephropathy (p=0.001; table-4). This signifies the importance of glycemic control [2]. Our data has demonstrated that 38% of the patients with diabetes developed nephropathy; while 13% demonstrated DKD. This is an alarming figure for the health care policy makers. Both triglycerides and TG/HDL were highly correlated with HbA1c (p <0.0001 and p=0.021, respectively; table-3); HbA1c was also significantly correlated with microalbuminuria (p<0.0001). This signifies that elevated blood glucose elevates TG and ultimately both contribute to the development of DKD. Hence, to control elevated blood glucose should be the primary target. Diabetologist and physicians should make all efforts to reduce HbA1c to the standard goals in order to avoid further diabetes complications and to follow best available guidelines [56-58]. Furthermore, it was observed that both creatinine and urine microalbumin were significantly correlated (r=0.273; p <0.0001). Hence, microalbuminuria (nephropathy) and rising serum creatinine (CKD/DKD) were associated significantly, leading to Diabetic renal disease or renal failure.
Excretion of albumin (or microalbumin) in the urine is an early marker of renal dysfunction or damage in diabetes, causing proteinuria or nephropathy, which may lead to stage renal disease (ESRD) with high incidence (up to 40-50%).
Persistence albumin excretion in urine in the range 30-299 mg/24 h (termed microalbuminuria, and first defined in 1985) is the earliest indicator and marker of incipient diabetic nephropathy and early renal damage in type-1 and type-2 diabetic subjects. Traditionally, the term "diabetic nephropathy" was previously defined as chronic kidney disease (CKD) resulting due to chronic exposure of hyperglycemia in diabetes. However, recently the Diabetes and Chronic Kidney Disease work group of the National Kidney Foundation (NKF) and Kidney Disease Outcomes Quality Initiative (KDOQI) suggested that a diagnosis of CKD presumed to be caused by diabetes should be referred to as “diabetic kidney disease (DKD)” and the term diabetic nephropathy” should be reserved for kidney disease caused by diabetes with histopathological injury demonstrated or proven by renal biopsy. CVD risk is increased to double in diabetic patients with microalbuminuria than those without microalbuminuria. There is inverse relation between microalbumin and GFR. Hence, as albumin excretion in urine increases, GFR decreases and also CVD risk increases progressively. Studies have demonstrated that microalbuminuria ≥ 300 mg/24 h (also called macroalbuminuria) may progress to ESRD if adequate interventions are not taken [11,59-67].
Our statistical analysis have shown significant correlation among triglycerides, serum creatinine and urine microalbumin (p=0.006 and p<0.0001, respectively; table-3). Similarly TG/HDL ratio was also associated and significantly correlated with serum creatinine and urine microalbumin (p <0.0001 for both; table-3). This implies involvement of lipids (especially triglycerides) in raising creatinine and urine microalbuminuria. It was interested to note that, highest correlations were found between TG/HDL ratio with microalbumin (r=0.394; table-3), indicating increasing proteinuria when triglycerides are elevated and HDL decreases (protective cholesterol).
While comparing variables for the development of nephropathy or proteinuria (i.e., with or without nephropathy), it was observed that HbA1c was significantly higher among the patients who demonstrated nephropathy (8.2 ± 1.5; 95% CI 7.97 to 8.26 and 7.65 ± 1.46; 95% CI 7.53 to 7.77 respectively; p=0.001). Similarly, the group demonstrating nephropathy showed higher levels of Triglycerides (166.8 ± 105.5; 95% CI 159 to 175 and 149.4 ± 92.2 144 to 156 respectively; p=0.001), and raised TG/HDL ratio (4.62 ± 3.77; 95% CI 4.32 to 4.93 and 4.08 ± 3.26 95% CI 3.85 to 4.31 respectively; p=0.004). Our data has also proved that creatinine levels were also higher among the groups demonstrated nephropathy (1.15 ± 0.99; 95% CI 1.1 to 1.23 and 0.825 ± 0.25; 95% CI 0.80 to 0.84 respectively; p<0.0001). All these evidence indicate involvement of higher levels of triglycerides (and low HDL) in the development of nephropathy (proteinuria) and chronic renal failure or diabetic kidney disease (CKD/DKD) by increasing serum creatinine..
When variables were compared with the group of patients with or without DKD, it was observed that HbA1c was higher among the patients with DKD with significant difference (9.3 ± 2.2; 95% CI 9.1 to 10.2 and 7.8 ± 1.48; 95% CI 7.75 to 7.94 respectively; p=0.03). This again demonstrates the significance of good glycemic control. Serum triglyceride levels were higher among the patients with DKD (179 ± 97; 95% CI 159 to 196 and 155 ± 91; 95% CI 151 to 160; p=0.018). Similarly, TG/HDL ratio was higher among DKD patients with significant difference (5.18 ± 3.39; 95% CI 4.51 to 5.84; and 4.25 ± 2.9; 95% CI 4.1 to 4.4 respectively; p=0.009). It was also observed that urine microalbumin levels were also significantly higher among the DKD group (183 ± 128; 95% CI 146 to 221 and 64.2 ± 113; 95% CI 56.5 to 71.8 respectively; p <0.0001). These findings are consistent with previous studies, as mentioned above. However, previous research studies did not analyzed the cohort data in such a manner analyzed in the current study. Similarly, regression models were not developed which shows correlation between triglycerides (or TG/HDL ratio) and serum creatinine with development of proteinuria (nephropathy) and diabetic kidney disease. This was achieved in the current study which has demonstrated that how rising triglyceride levels can contribute to the development of DKD/CKD in diabetic subjects.
Table-6 demonstrates the mathematical regression models and equations. The mathematical equation for triglycerides and serum creatinine is: serum creatinine=0.884 + [ 0.001 × serum triglycerides]. Hence, if Triglycerides are 200 mg/dl, for example, then serum creatinine will be approximately 0.18 mg/dl. However, if triglyceride levels are up to 270, for example, then the predicted serum creatinine would be 1.154 mg/dl. Chronic exposure of tryglycerides with levels of 400 mg/dl will increase the serum creatinine to 1.284 (~1.3). Chronically, this dramatic increase in serum creatinine will lead to DKD. Similarly, and according to this experimental observation, chronic elevations of triglycerides will increase microalbuminuria or proteinuria excretion via kidney. For example, by the given mathematical equation in table-6, if the triglyceride levels are within the normal range, or less than 150 mg/dl, the microalbuminuria levels will be 28 mg/L. However, chronic elevation of triglycerides with the level of 250, for example, will lead to more albumin excretion from the kidney with the levels reaching 35.75 mg/L.
| Data and Variables | Pearson's Correlation (r) | P-Value for Pearson's Correlation | Regression Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | F-Statistic | ANOVA Model P-Value | T-Statistic | P-Value | ||||
| Triglycerides and serum creatinine | 0.269 | 0.006 | 0.072 | 7.59 | 0.006 | 23.3 | <0.0001 | |
| TG/HDL and serum creatinine | 0.298 | 0.002 | 0.088 | 9.4 | 0.002 | 30.9 | <0.0001 | |
| Triglycerides and urine microalbumin | 0.312 | <0.0001 | 0.097 | 16.1 | <0.0001 | 5.56 | <0.0001 | |
| TG/HDL and urine microalbumin | 0.394 | <0.0001 | 0.155 | 17.56 | <0.0001 | 7.03 | <0.0001 | |
| Mathematical / Statistical Regression models and equations | ||||||||
| Triglycerides and serum creatinine | Serum creatinine = 0.884+[0.001 × serum triglycerides] | |||||||
| TG/HDL and serum creatinine | Serum creatinine = 0.906+[0.016 × TG/HDL] | |||||||
| Triglycerides microalbuminuria | Microalbuminuria = 17+[0.075 × serum triglycerides] | |||||||
| TG/HDL and urine microalbumin | Microalbuminuria = 15+[2.9 × TG/HDL] | |||||||
Table 6: Correlation and regression models for the different variables.
| Test variables | Area under the curve (AUC) | Standard error | 95% CI | P-Value | Coordinate triglyceride cutoff points for the detection/ diagnosis of nephropathy and DKD |
|---|---|---|---|---|---|
| Triglycerides and nephropathy status | 0.559 | 0.015 | 0.530 to 0. 588 | <0.0001 | 138 mg/dl (65% sensitivity and 55% specificity) |
| Triglycerides and DKD status | 0.581 | 0.03 | 0.522 to 0.640 | 0.005 | 153 mg/dl (60% sensitivity and 61% specificity) |
Table 7: Results of ROC with AUC, 95% CI, p-values and triglyceride cutoff points.
Regarding TG/HDL ratio, and according to the guidelines, a ratio less than 3.75 is considered normal. According to the data analysis of the current study (table-6), a TG/HDL ratio of 4, for example, will give the value of serum creatinine of 1.04 mg/dl. However, and for example, if HDL-C is low with the value of 35 mg/dl, and triglycerides are high with the levels of 300 mg/dl, then the TG/HDL ratio will be 8.57; this will increase the serum creatinine up to levels of 1.2 mg/dl. Similarly, regarding albumin secretion and TG/HDL ratio, it can be observed from the table-6 that, if TG/HDL ratio is 3.75, the according to the hypothesized mathematical equation, microalbuminuria levels will be up to 25.9 mg/L. However, if this ratio is 8.75, then microalbumin excretion from the kidney will be up to 40.3 mg/L. Hence, elevated triglycerides of TG/HDL ratio will lead to the elevation of serum creatinine, urine micoalbumin, and ultimately to DKD.
To confirm the finding of the current study, and to find the cutoff levels for triglycerides which may lead to the nephropathy and DKD, receiver operating curve (ROC) was used. According to the table-7, it can be observed that the area under the curve (AUC) for relation of triglycerides with the development of nephropathy was 0.559 (95% CI 0.530 to 0. 588; p <0.0001) with triglyceride cutoff point value 138 mg/dl (65% sensitivity and 55% specificity).Similarly, area under the curve (AUC) for relation of triglycerides with the development of DKD (or CKD) was 0.581 (95% CI 0.522 to 0.640; p=0.005) with triglyceride cutoff point value 153 mg/dl (60% sensitivity and 61% specificity). Figures 1 and 2 demonstrate these results graphically.
The diabetologist must screen every patient for the dyslipidemia and microalbuminuria; early treatment is recommended, to prevent development and progression of diabetes related complications.
Three classes of medications are available in the market and appropriate for the management of major triglyceride elevations: fibric acid derivatives (gemfibrozil (Lopid) and fenofibrate (Lipanthyl), niacin, and omega-3 fatty acids. Furthermore, high doses statins (simvastatin, atorvastatin, rosuvastatin) also effective to lower triglycerides by 50%. Niacin (vitamin B-3) decreases triglyceride levels by at least 40% and can raise HDL cholesterol levels by 40% or more. However, it is associated with some side effects such as chemical hepatitis and worsening of glycemic control and increasing the insulin resistance. Omega-3 fatty acids (with a dose of 4 g/day). The triglyceride-lowering effects of fish oils are entirely dependent on the omega-3 content. HDL-C levels can effectively be raised by regular exercise/activity or daily regular walk [68-84].
To prevent nephropathy (and development of microalbuminuria), it is recommended that diabetic hypertensive patients should be treated with ACE inhibitors or ARBs, usually in combination with a diuretic. Target blood pressure for diabetics and those with DKD/CKD stages 1-4 should be <130/80 mmHg [11].
Conclusion and Recommendation
Diabetic nephropathy, DKD and ESRD are renal complications of diabetes. As demonstrated by the current study, triglycerides and TG/HDL ratio were significantly associated with the development of nephropathy and DKD. Hence, with the glycemic control, it is advisable to target diabetic dyslipidemia or hypertriglyceridemia to prevent renal complications. Early detection of blood pressure, microalbuminuria screening and lipid profile analysis is recommended at tertiary care diabetes centers.s
REFERENCES
- King H, Aubert RA, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414-31.
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-9.
- The Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-86.
- Briones ER, Steiger DL, Palumbo PJ, et al. Sterol excretion and cholesterol absorption in diabetics and nondiabetics with and without hyperlipidemia. Am J Clin Nutr. 1986;44:353-61.
- Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983-8.
- Hirany S, O'Byrne D, Devaraj S, et al. Remnant-like particle cholesterol concentrations in patients with type 2 diabetes mellitus and end-stage renal disease. Clin Chem. 2000;46:667-72.
- American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2016;40:S75-S87.
- Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832-39.
- Fioretto P, Dodson PM, Ziegler D, et al. Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol. 2010;6:19-25.
- Molitch ME, DeFronzo RA, Franz MJ, et al. American Diabetes Association. Diabetic nephropathy. Diabetes Care 2003;26(Suppl. 1):S94-S98.
- National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:(Suppl1) S180.
- Mogensen CE. Definition of diabetic renal disease in insulin dependent diabetes mellitus based on renal function tests: The Kidney and Hypertension in Diabetes Mellitus. Kluwer Academic Publishers. 1994:1-14.
- Agrawal RP, Sharma P, Pal M, et al. Magnitude of dyslipidemia and its association with micro and macro vascular complications in type 2 diabetes: a hospital based study from Bikaner (Northwest India). Diabetes Res Clin Pract. 2006;73:211-214.
- Cao C, Wan X, Chen Y, et al. Metabolic factors and micro inflammatory state promote kidney injury in type 2 diabetes mellitus patients. Ren Fail. 2009;31:470-474.
- Cusick M, Chew EY, Hoogwerf B, et al. 3rd; Early Treatment Diabetic Retinopathy Study Research Group. Risk factors for renal replacement therapy in the Early Treatment Diabetic Retinopathy Study (ETDRS), Early Treatment Diabetic Retinopathy Study Report No. 26. Kidney Int. 2004;66:1173-1179.
- Lee PH, Chang HY, Tung CW, et al. Hypertriglyceridemia: an independent risk factor of chronic kidney disease in Taiwanese adults. Am J Med. Sci. 2009;338:185-189.
- Muntner P, Coresh J, Smith JC, et al. Plasma lipids and risk of developing renal dysfunction: the Atherosclerosis Risk in Communities study. Kidney Int. 2000;58:293-301.
- Ritz E, Rychlik I, Locatelli F, Halimi S: End stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795-808.
- Schena FP. Epidemiology of end stage renal disease: international comparisons of renal replacement therapy. Kidney Int Suppl 2000;57:S39-S45.
- Kamran M. A. Aziz. Correlation of Urine Biomarkers: Microalbuminuria and Spot Urine Protein among Diabetic Patients. Application of Spot Urine Protein in Diabetic Kidney Disease, Nephropathy, Proteinuria Estimation, Diagnosing and Monitoring. Recent Pat Endocr Metab Immune Drug Discov. July 2015;9:1.
- Kamran M. A. Aziz. Association between Hypothyroidism, Body Mass Index, Systolic Blood Pressure and Proteinuria in Diabetic Patients: Does treated Hypothyroid with Thyroxin Replacement Therapy prevent Nephropathy/ Chronic Renal Disease? Current Diabetes Reviews, 12(3): 297-306.
- Kamran MA Aziz. Association of Hypothyroidism with High Non-HDL Cholesterol and Ankle Brachial Pressure Index in Patients with Diabetes: 10-Year Results from a 5780 Patient Cohort. A Need for Intervention. Annals Thyroid Res. 2016; 2(2): 53-57.
- Jenkins AJ, Lyons TJ, Zheng D, et al. Lipoproteins in the DCCT/EDIC cohort: Associations with diabetic nephropathy. Kidney Int. 2003;64:817-28.
- Shimizu Y, Sato S, Koyamatsu J, et al. Association of Chronic Kidney Disease and Diabetes with Triglycerides-to- HDL Cholesterol Ratio for a Japanese Population: The Nagasaki Islands Study. Transl Med. 2014;4:124.
- Kamran M. A. Aziz. Association of Microalbuminuria with Ischemic Heart Disease, Dyslipidemia and Obesity among Diabetic Patients: Experience from 5 Year Follow up Study of 1415 Patients. Bioenergetics. 2014;3:118.
- Kamran M. A. Aziz. Association between Non-HDL and HDL Cholesterol with micro albuminuria in patients with Diabetes. Journal of Diabetology. 2013;1:4.
- Kamran M. A. Aziz. Targeting LDL Dyslipidemia for Controlling Progression of Nephropathy in Diabetic Population: A Cross Sectional Analytical Study. Journal of the Dow University of Health Sciences. Karachi 2012;6:7-11.
- Aziz KMA. Association of Serum Lipids with High Blood Pressure and Hypertension among Diabetic Patients. Mathematical Regression Models to Predict Blood Pressure from Lipids. An Experience from 12-year Follow Up of more than 9000 Patients' Cohort. Gen Med. 2017;5:297.
- Shimizu Y, Nakazato M, Sekita T, et al. Association between hemoglobin and diabetes in relation to triglycerides-to-HDL cholesterol ratio for Japanese: The Nagasaki Islands study. Intern Med. 2014;53:837-43.
- Olechnowicz-Tietz S, Gluba A, Paradowska A, et al. The risk of atherosclerosis in patients with chronic kidney disease. Int Urol Nephrol. 45:1605-1612.
- Mitchell RJ. Improved method for specific determination of creatinine in serum and urine. Clin Chem. 1973;19:408-10.
- Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381-7.
- Bishop Michael L. Clinical Chemistry: Principles and Correlations 2nd ed. Philadelphia: Lippincott JB, Company. 1992;441.
- NATCOM Hospital Information System (NATCOM HIS), National Computer System Co, Ltd. http://natcom.com.sa/healthcare and http://natcom.com.sa/clients (Accessed on: October 17, 2017).
- Misra A, Kumar S, Kishore Vikram N, et al. The role of lipids in the development of diabetic microvascular complications: implications for therapy. Am J Cardiovasc Drugs. 2003;3:325-38.
- Russo GT, De Cosmo S, Viazzi F, et al. Plasma Triglycerides and HDL-C Levels Predict the Development of Diabetic Kidney Disease in Subjects With Type 2 Diabetes: The AMD Annals Initiative. Diabetes Care. 2016;39:2278-87.
- Adult Treatment Panel III. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. JAMA. 2001;285:2486-96.
- Brunzell JD, Davidson M, Furberg CD, et al. American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-22.
- Moorhead JF, Chan MK, El-Nahas M, et al. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982;2:1309-11.
- Jandeleit-Dahm K, Cao Z, Cox AJ, et al. Role of hyperlipidemia in progressive renal disease: focus on diabetic nephropathy. Kidney Int Suppl. 1999;71:S31-S36.
- Wang Z, Jiang T, Li J, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 2005;54:2328-2335.
- Sun L, Halaihel N, Zhang W, et al. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919-27.
- Abrass CK. Lipid metabolism and renal disease. Contrib Nephrol 2006;151:106-121.
- Fakhrzadeh H, Ghaderpanahi M, Sharifi F, et al. Increased risk of chronic kidney disease in elderly with metabolic syndrome and high levels of C-reactive protein: Kahrizak Elderly Study. Kidney Blood Press Res. 2009;32:457-463.
- Gr¨one HJ, Hohbach J, Gr¨one EF. Modulation of glomerular sclerosis and interstitial fibrosis by native and modified lipoproteins. Kidney Int Suppl. 1996;54:S18-S22.
- Daousi C, Bain SC, Barnett AH, et al. Hypertriglyceridaemia is associated with an increased likelihood of albuminuria in extreme duration (>50 years) type 1 diabetes. Diabet Med. 2008;25:1234-1236.
- Kim DM, Ahn CW, Park JS, et al. An implication of hypertriglyceridemia in the progression of diabetic nephropathy in metabolically obese, normal weight patients with type 2 diabetes mellitus in Korea. Diabetes Res Clin Pract. 2004;66:S169-S172.
- Molitch ME, Rupp D, Carnethon M. Higher levels of HDL cholesterol are associated with a decreased likelihood of albuminuria in patients with long-standing type 1 diabetes. Diabetes Care. 2006;29:78-82.
- Takamatsu N, Abe H, Tominaga T, et al. Risk factors for chronic kidney disease in Japan: a community-based study. BMC Nephrol. 2009;10:34.
- Wang F, Ye P, Luo L, et al. Association of risk factors for cardiovascular disease and glomerular filtration rate: a community-based study of 4,925 adults in Beijing. Nephrol Dial Transplant. 2010;25:3924-3931.
- Chew EY, Klein ML, Ferris FL 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079-1084.
- Yokoyama H, Sone H, Oishi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant. 2009;24:1212-19.
- Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience): Prospective Cardiovascular Münster study. Am J Cardiol. 1992;70:733-37.
- Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345-1361.
- Benarous R, Sasongko MB, Qureshi S, et al. Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52:7464-9.
- American Diabetes Association Standards of Medical Care in Diabetes - 2017. Diabetes Care 2017;40:S1-S138.
- Kamran M. A. Aziz. Management of Type-1 and Type-2 Diabetes by Insulin Injections in Diabetology Clinics - A Scientific Research Review. Recent Pat Endocr Metab Immune Drug Discov. 2012 May;6:148-170.
- Kamran M. A. Aziz. Unique glycemic and cardio-renal protective effects of metformin therapy among type-2 diabetic patients: a lesson from a five-year cross-sectional observational study of 1590 patients. Research. 2014;1:874<.
- Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
- Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest. 1985;9:85-95.
- Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413-1418.
- Vaur L, Gueret P, Lievre M, et al. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: observations from the DIABHYCAR (type-2 Diabetes, Hypertension, Cardiovascular Events and Ramipril) study. Diabetes Care. 2003;26:855-60.
- Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225-232.
- Miettinen H, Haffner SM, Lehto S, et al. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke. 1996;27:2033-2039.
- Valmadrid CT, Klein R, Moss SE, et al. The risk of cardiovascular disease mortality associated with micro albuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 160:1093-1100.
- Gall MA, Hougaard P, Borch-Johnsen K, et al. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314:783-788.
- Ravid M, Lang R, Rachmani R, et al. Long-term reno-protective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med. 1996;156:286-289.
- University of Michigan Health System. Screening and management of lipids. Ann Arbor, Mich: University of Michigan Health System 2009.
- Klop B, Wouter Jukema J, Rabelink TJ, et al. A physician's guide for the management of hypertriglyceridemia: the etiology of hypertriglyceridemia determines treatment strategy. Panminerva Med. 2012;54:91-103.
- Shimabukuro M, Higa M, Tanaka H, Shimabukuro T, Yamakawa K, Masuzaki H. Distinct effects of pitavastatin and atorvastatin on lipoprotein subclasses in patients with Type 2 diabetes mellitus. Diabet Med. 2011;28:856-64.
- >van der Graaf A, Cuffie-Jackson C, Vissers MN, et al. Efficacy and safety of coadministration of ezetimibe and simvastatin in adolescents with heterozygous familial hypercholesterolemia. J Am Coll Cardiol. 2008;52:1421-9.
- Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6:413-26.
- Abourbih S, Filion KB, Joseph L, et al. Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am J Med. 2009;122:962e1-8
- Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol. 2008;51:2375-84.
- McKenney JM, McCormick LS, Weiss S, et al. A randomized trial of the effects of atorvastatin and niacin in patients with combined hyperlipidemia or isolated hypertriglyceridemia. Collaborative Atorvastatin Study Group. Am J Med. 1998;104:137-43.
- Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc. 2008;83:470-8.
- Roth EM, Bays HE, Forker AD, et al. Prescription omega-3 fatty acid as an adjunct to fenofibrate therapy in hypertriglyceridemic subjects. J Cardiovasc Pharmacol. 2009;54:196-203.
- Lavie CJ, Milani RV, Mehra MR, et al. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585-94.
- Egert S, Kannenberg F, Somoza V, et al. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009;139:861-68.
- Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19-30.
- Kastelein JJ, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa for Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94-106.
- letcher B, Berra K, Ades P, et al. Managing abnormal blood lipids: a collaborative approach. Circulation. 2005;112:3184-209.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-45.
- Spate-Douglas T, Keyser RE. Exercise intensity: its effect on the high-density lipoprotein profile. Arch Phys Med Rehabil. 1999;80:691-5.