Bacteriological assessment of river Jataganga, located in Indian Himalaya, with reference to physico-chemical and seasonal variations under anthropogenic pressure: a case study
2 Vellore Institute of Technology, Vellore 632 014, Tamil Nadu, India
Received: 21-Nov-2017 Accepted Date: Dec 15, 2017; Published: 25-Dec-2017
Citation: Nidhi Aishvarya, Mukesh Kumar Malviya, Ashish Tambe, et al. Bacteriological assessment of river Jataganga, located in Indian Himalaya, with reference to physico-chemical and seasonal variations under anthropogenic pressure: a case study. J Environ Microbiol 2017;1(1):10-16.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Bacteriological water quality of the river Jataganga, located in Indian Himalaya, has been assessed along with the physico-chemical and seasonal variations under anthropogenic activities, in two consecutive years. While the bacteriological analysis included total viable counts (TVC), total coliforms (TC), fecal coliforms (FC) and fecal streptococci (FS), the physicochemical factors included pH, temperature, conductivity, total dissolved solids (TDS), dissolved oxygen (DO), biological oxygen demand (BOD) and chemical oxygen demand (COD). The TVC and TC were estimated to be highest in rainy season and lowest in winter at the sampling points, in both the years. FC fluctuated with respect to the location, season and year. FS also developed higher population in rainy season in both the years. The pure bacterial isolates belonged to the families Enterobacteriaceae, Micrococcaceae, Pseudomonadaceae and Bacillaceae, representing the indicators of water pollution, pathogens responsible for water borne diseases, and plant growth promoters as well. The pH and temperature of water at the sampling sites were about neutral to slightly alkaline and in psychrophilic range, respectively. The TDS was found to be within the minimum prescribed limits at all the study sites. The DO and BOD were assessed to be highest in winter followed by rainy and summer seasons, respectively, while COD was higher in rainy season followed by summer and winter, in both the years. Factorial analysis amongst years, locations and seasons, and their interaction with respect to the bacterial populations and the physico-chemical factors was statistically significant.
Keywords
River Jataganga; anthropogenic activities; physico-chemical factors; bacteriological analysis; seasonal variation; Indian Himalayan region
Contamination of water is a serious environmental problem as it affects the human health and the biodiversity adversely in aquatic ecosystems. Contaminated water can lead to the serious health problems of the associated populations. The World Health Organization (WHO) reported the relevance of waterborne infectious microorganisms accounted for the global burden of disease as well as the deaths [1]. While the microorganisms are widely distributed in nature, their abundance and diversity may be used as indicators of water quality [2]. Bacteriological quality of drinking water is usually expressed in terms of the occurrence of particular species of bacteria. Fecal coliforms (FC) and fecal streptococci (FS) are widely used as bacterial indicators for assessment of fecal pollution with respect to water quality in fresh water sources [3,4]. Coliforms in general, and Escherchia coli in particular, are important among bacterial indicators that are used in water quality monitoring and assessment [5]. Importance of water resources with respect to biological and physico-chemical communities along with the seasonal variations under mountain ecosystems is increasingly receiving attention in recent years [6-8]. Conservation and sustainable use of freshwater resources has been considered of great importance on earth [9]. Despite of the known crucial role of the microbial communities in biogeochemical processes, studies on the subject line are limited. The Himalayan rivers make crucial source of water under mountain ecosystem and are known to have important place in Indian culture and tradition. Due to remoteness, the mountain locations usually remain neglected for their pollution assessment. The mountain streams generally contain few organisms at the source, but as they flow into lower areas especially those having large amounts of organic material, the number and types of organisms increase. Some are accidental contaminants while others are aquatic organisms. In countries like India, assessment of river water is essential with respect to the various kinds of anthropogenic activities, as the river water is used for domestic purposes including source of drinking water [10]. Polluted river water can contain a large variety of pathogenic microorganisms including bacteria, protozoa and viruses. Fecal pollution can be brought to rivers through non-point sources (surface runoff and soil leaching), the wild life animals and grazing livestock feces, and also the farmyard manure used in agricultural fields [11]. River Jataganga, located at a pilgrim town in district Almora of Uttarakhand under Indian Himalayan region (IHR), is a unique location surrounded by deodar (Cedrus deodara) forest and comprising a cluster of 124 stone temples. The place is observed for organizing a number of festivals round the year involving all kinds of anthropogenic activities. Besides, the location is also used for cremation of the bodies by the local people. The uniqueness of the site offers an opportunity for assessment of the anthropogenic pressure as reflected in terms of colonization of microbial communities. The aim of the present study is to assess the colonization of various groups of bacterial community namely total viable counts (TVC), total coliforms (TC), fecal coliforms (FC) and fecal streptococci (FS) along the physico-chemical status with respect to the influence of anthropogenic activities and the seasonal variation, in two consecutive years.
Material and Method
Study area
Jageshwar, a hub of religious and tourist activities, is situated at the confluence of three streams that converge to form river Jataganga, a tributary of river Saryu (29°39’N 79°35’; altitude- 1870 m amsl), in Almora district of Uttarakhand, India. Six locations (referred as J1-J6) selected in the Jageshwar area for the present study were: Site J1- the Dandeshwar temple, Site J2- approximately 200 meters away from the Jageshwar temple with lesser human activities, Site J3- Brahmkund where the pilgrims perform a variety of rituals, Site J4- the confluence of J3 and J5 with contaminated water due to water runoff from the confluence and a cremation site, Site J5- an outlet for the offerings used in the temple, and Site J6- away from the temple with lesser human activities.
Sample collection
The water samples were collected in sterile (autoclaved) containers (in triplicate) from the 6 study sites. The sampling was done in three seasons, i.e. summer, rainy and winter, in two consecutive years: Year 1: June (summer), August (rainy) and January (winter) and; Year 2: in similar manner as in case of Year 1.
Bacteriological analysis of water samples
Three standard methods were used for enumeration of bacteria in water samples.
(1) Standard plate count (SPC) method: One ml of appropriate dilution was inoculated on Tryptone yeast extract (TY) agar plates and results were recorded as colony forming units (CFU) following 48 h of incubation at 25° and 37°C.
(2) Determination of coliforms by Most probable number (MPN) method: For determination of coliforms, five test tubes containing 10 ml of double strength lactose broth and 10 test tubes containing single strength lactose broth with Durham’s tubes were taken. The water samples were inoculated in each lactose broth tubes i.e. 10 ml water sample into each five tubes containing 10 ml double strength lactose broth, 1 ml water sample into five tubes containing 5 ml single strength broth, and 0.1 ml water sample into each 5 tubes containing 5 ml single strength lactose broth. All the test tubes were incubated at 37°C for 48 h. Following incubation, all the tubes were observed for acid and gas production. The production of acid and gas indicated the presence of coliforms and thus test was considered positive.
Loop full culture from tubes showing positive results was inoculated in Eosin-methylene blue (EMB) agar and Endo agar, and inoculated at 37°C for 24 h. For further determination of fecal coliforms (FC) and fecal streptococci (FS), lactose fermenting and non fermenting colonies were isolated from MPN tubes, loop full of culture from tubes of MPN test were inoculated into Brilliant green bile and MUG-EC broth, respectively, and incubated at 44.5°C for 24 h.
(3) Membrane filter technique (MFT): For this method, 100 ml water sample was placed through thin sterile membrane filter (pore size 0.45 μm) that is kept in a special filter apparatus contained in a suction flask. The filter disc containing the ‘trapped’ microorganisms was transferred to a sterile Petri dish having an absorbent pad saturated with a selective liquid medium (FC and Endo broth). Number of colonies, developed following incubation at 37°C for 24 h, were recorded. Number of coliforms per 100 ml of water sample were calculated by the formula=colony count/volume of sample used × 100.
Preliminary characterization of pure bacterial isolates
The pure cultures from the above experiments were isolated following subculturing method. The number of cultures was narrowed on the basis of colony morphology and microscopic features. Each distinct culture was given a code number and preserved on agar slants at 4°C and in glycerol stocks at -20°C. The culture and growth characteristics of the pure bacterial isolates were studied on TY agar and Endo agar, following incubation at 25°C for 72 h. Observations on colony morphology (size, shape, elevation and margin) were recorded. For cell morphology, Gram staining was performed and the slides were viewed under an Image analyzer microscope (Nikon H550S).
Biochemical and physiological characterization of bacterial isolates
Biochemical tests, viz. utilization of carbon sources and production of extracellular and intracellular enzymes were performed following standard procedures. Catalase and oxidase activities were determined by formation of oxygen bubbles with 3% hydrogen peroxide solution, and by the oxidation of TMPD (tetramethyl-phenylenediamine dihydrochloride, provided in the form of discs), respectively. Hydrolysis of starch was performed by flooding Gram’s iodine, on colonies grown on starch agar (2% starch) and observing presence or absence of clearing around the colony. Lipase activity was determined by growing the isolates on tributyrin agar (1% tributyrin as substrate) and observing the absence or presence of a zone around the colony. The IMViC tests consisting of (a) Indole production, (b) Methyl- red, (c) Voges-Proskauer, and (d) Citrate utilization were performed. Utilization of carbon sources was determined by a change in the color of Andrade peptone water containing 0.1% Andrade indicator. For physiological growth characteristics, the isolates inoculated on TY agar plates were incubated at different temperatures (4, 9, 14, 21, 28, 35, 42 and 50 and 55°C) and pH levels (4-12, with an interval of 0.5 units) to determine minimum, optimum and maximum temperatures, and pH requirements. For determination of salt tolerance, the isolates were inoculated on TY agar with different salt concentrations (0.5 to 10.0%) following incubation at 28°C for 72 h.
Determination of physico-chemical factors
Various physico-chemical factors namely temperature, pH, total dissolved solids (TDS), conductivity, and dissolved oxygen (DO), biological oxygen demand (BOD) and chemical oxygen demand (COD) were performed in laboratory following standard titrimetric methods [3]. Temperature, electric conductivity and pH of water samples were recorded using thermometer, TDS-Scan and pH meter, respectively.
Statistics
Determination of physico-chemical parameters and enumeration of water microbes by SPC, MPN and MFT were conducted in triplicates. The value for each sample was calculated as the mean ± SD (standard deviation). Analysis of variance and significant difference among means were tested following Duncan’s Multiple Range Test (DMRT) using SPSS version 16.
Result and Discussion
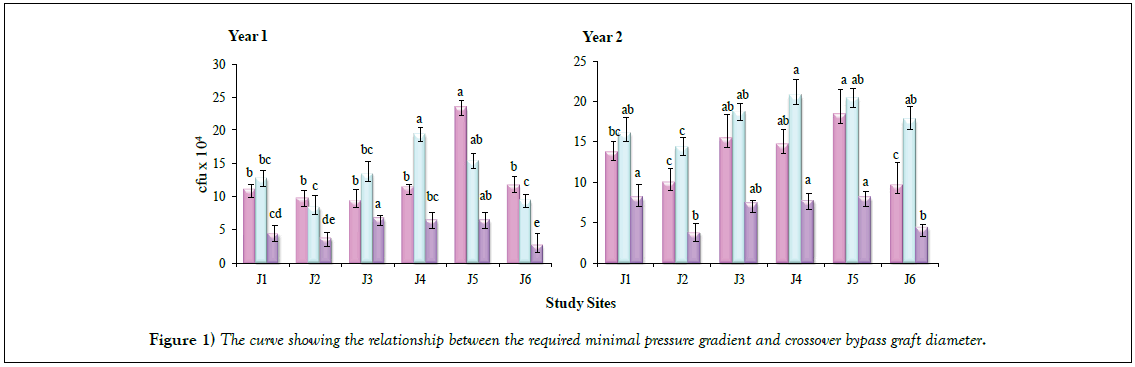
Total viable counts (TVC) recorded on various groups of bacteria under consideration, assessed for six sites in three seasons during two consecutive years, are presented in Figure 1. Maximum TVC were recorded in rainy season followed by summer and winter for all the sites in both the years, except in case of Site J4 and Site J5 where maximum counts were recorded in summer in Year 1. Site J4 gave maximum TVC while minimum counts were recorded for the Sites J2 and J6, in both the years. The results on TVC revealed significant variation (p<0.05) with respect to two consecutive years, in different seasons. Higher values of TVC at Site J3 can be attributed to the anthropogenic activities, including a variety of rituals, mainly during summer and rainy seasons when a large number of pilgrims and tourists visit the location. Similarly, Site J5 was likely to bear the colonization of larger bacterial populations due to the offerings, often consisting of large amount of nutritionally rich materials such as the variety of carbohydrates (mono to polysaccharides) that can be utilized by microbial populations resulting in luxurious growth.
Seasonal differences among the microbial community structure in sediments of freshwater streams with prevalence of proteobacteria and actinobacteria has been studied by Bucci et al. [12]. Sudden increase in microbial load in running water during rainy season has also been reported in earlier studies [5,13]. Higher TVC during rainy season, in comparison to summer and winter seasons, is indicative of the act of precipitation at the sources and the amount of microbial pollution. These results are in line with the earlier observations reported by Sood et al. [5] and Kumarasamy et al. [14]. High surface flows during rainy seasons resulting in increase in erosion and the transport of sediment carrying bacteria into rivers are also on record [15]. Influence of seasonal changes on colonization of biological indicators with respect to water quality and nutrient concentrations has been studied for Biaka river Catchment of Southern Poland [16].
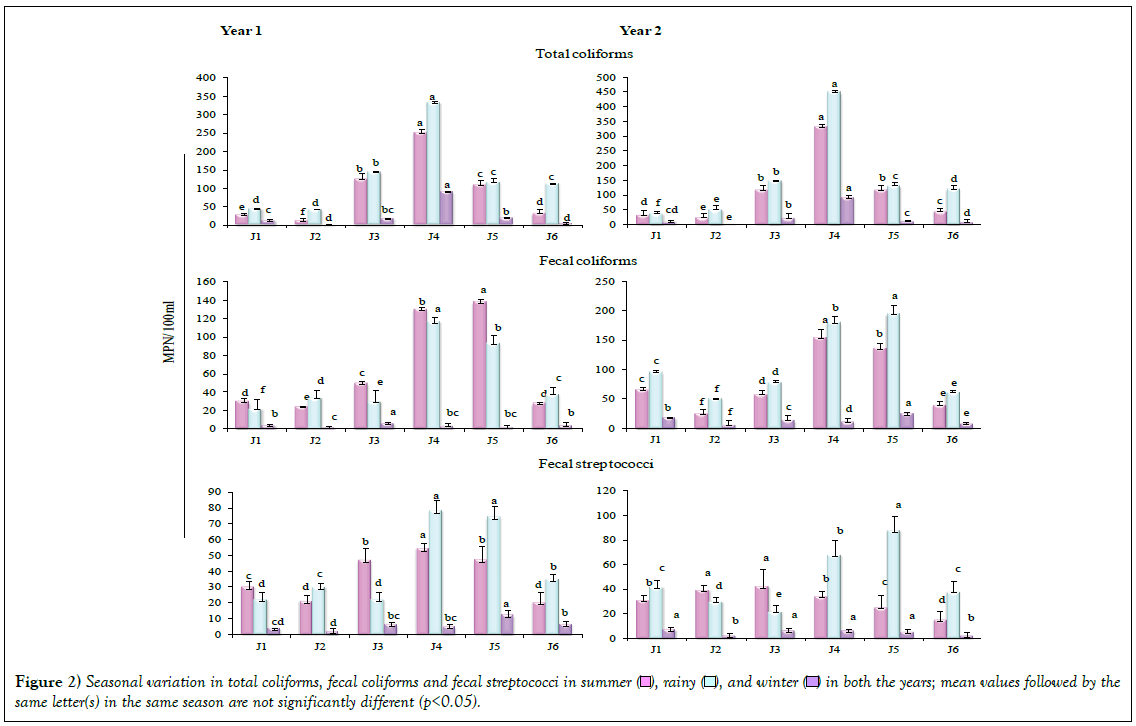
The populations of specific groups of bacteria, total coliforms (TC), fecal coliforms (FC) and fecal streptococci (FS) are presented in Figure 2. Across the studied six sites in three seasons a significant variation (p<0.05) was observed in TC, FC and FS, in both the years. TC were higher in rainy season in comparison to summer and winter; maximum coliforms colonized Site J4 in both the years. FC was higher at the four Sites (J1, J3, J4 and J5) in summer and at the Sites J2 and J6 in rainy season, in Year 1. In Year 2, TC was higher in rainy season in comparison to summer and winter, at all the sites. Maximum FC was recorded in Site J5. Values for FS were also recorded higher in rainy season in comparison to summer and winter in both the years, being maximum at Site J4. In the present study, FC were recorded at four sites (J1, J3, J4 and J5) in summer season, while in case of Site J2 and J6, FC represented larger populations in rainy season in year 1; FC were higher in rainy season in comparison to summer and winter, in both the years at all the sites.
Fecal microorganisms are mainly brought to aquatic environments through the discharge of domestic and industrial wastes [11]. Colonization by higher TC as well as the FC during monsoon season has been attributed to the act of precipitation at the source [17]. Higher FC/FS ratio in rainy season and much lower during winter season can rationally be attributed to the anthropogenic pressure during rainy season and, in addition, due to the runoff act from other water stretches. Water pollution due to the presence of fecal coliforms during rainy season arising due to the presence of animal dung carried by run-off to the rivers has been reported by Ajibade et al. [4]. Srivastava and Srivastava [18] also reported higher bacterial populations belonging to TC, FC, FS and FC/FS in monsoon season. Disposal of the domestic waste and the animal excreta pertaining to the livestock by the local people is likely to support the increase in TC and FC in river Jataganga. Factorial analysis revealed significant (p<0.05, p<0.01) effect of year, location and season, individually or in combination, on the bacterial counts (Table 1). Various environmetric methods, including the factor analysis, have been used to study the spatial variations of water quality variables and to determine the origin of pollution sources [19].
| Source of variation | DF | Bacterial counts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total viable counts | Total coliforms | Fecal coliforms | Fecal streptococci | ||||||
| MS | F value | MS | F value | MS | F value | MS | F value | ||
| Year | 1 | 184.08 | 37.58*** | 5590.08 | 926.0*** | 18200 | 4498*** | 14.82 | 2.55 |
| Locations | 5 | 112.5 | 22.97*** | 135840 | 22500*** | 21231 | 5247*** | 1918 | 330.36*** |
| Seasons | 2 | 927.37 | 189.33*** | 134392 | 22260*** | 59046.9 | 14590*** | 15521 | 2673*** |
| Year*Locations | 5 | 6.57 | 1.34 | 2954.59 | 489.41*** | 912.19 | 225.44*** | 202.5 | 34.88*** |
| Year* Seasons | 2 | 48.44 | 9.89*** | 1610.11 | 266.71*** | 5651.95 | 1397*** | 220.95 | 38.06*** |
| Locations* Seasons | 10 | 25.06 | 5.12*** | 15792.9 | 2616*** | 4929.08 | 1218*** | 1216.2 | 209.49*** |
| Year*Location*Seasons | 10 | 15.67 | 3.20** | 859.72 | 142.41*** | 489.78 | 121.04*** | 159.28 | 27.44*** |
Table 1: Analysis of variance with reference to the effect of year, location, season and their interaction with bacterial counts.
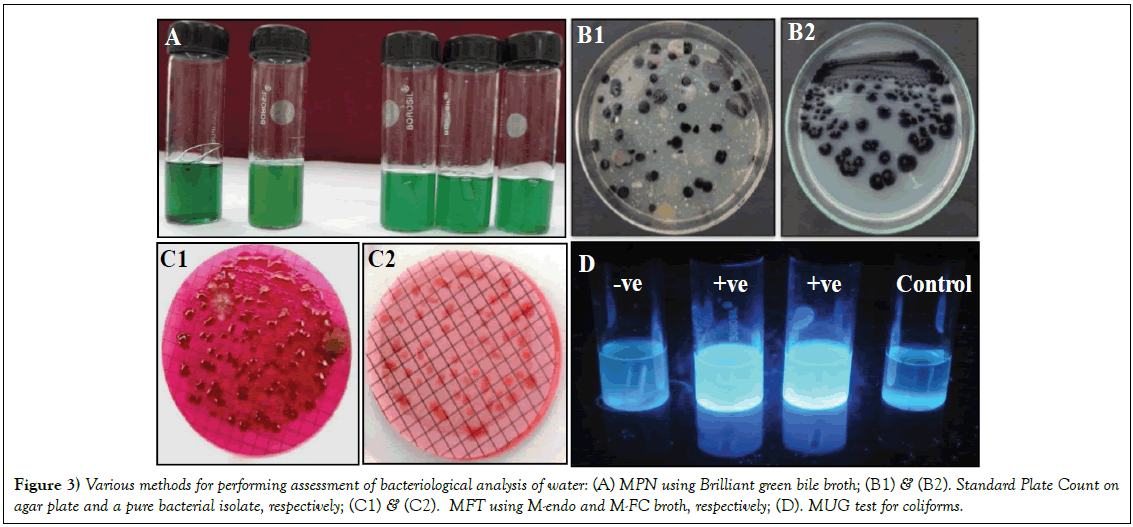
Amongst the methods (Figure 3), used in the present study, the MFT gave maximum bacterial counts in rainy season, followed by summer and winter, in both the years (data not shown). The specific tests for detection of coliforms in water samples are performed following MPN and MFT [20,21]. MFT is one of the common tests to measure the water quality with respect to the TC on the membrane. Presence of E. coli and other coliforms on the membrane indicates towards the fecal contamination of water bodies. MFT has advantage over MPN due to its fully quantitative estimation and allowing high volume of water to be accessed. However, the enzymatic methods are needed for further confirmation of the kind of contamination in MFT method. These enzymatic methods are generally based on the metabolic reactions of the bacteria where specific chromogenic/fluorogenic substrates are used to detect the specific enzyme activity. The associated biochemical properties are applicable in identifying the particular bacterial group. The MUG test associated to the MPN allows the growth of specific coliforms in the defined media substrates eliminating the other bacteria [22]. Varying permissible levels of the contaminants for assessment of water quality have been given by different agencies, such as, WHO, ICMR, APHA, ISI, BIS [23].
Figure 3: Various methods for performing assessment of bacteriological analysis of water: (A) MPN using Brilliant green bile broth; (B1) & (B2). Standard Plate Count on agar plate and a pure bacterial isolate, respectively; (C1) & (C2). MFT using M-endo and M-FC broth, respectively; (D). MUG test for coliforms.
A total of 110 isolates were obtained from the water samples. The pure cultures, based on their colony morphology and biochemical and physiological parameters, were found to be represented by 12 distinct groups of bacteria. Results on colony morphology, microscopic features, growth requirements (O2 requirement, temperature and pH range and salt tolerance) and IMViC tests of the representative bacteria are presented in Table 2. Based on these characters, the bacterial isolates mainly belonged to Enterobacteriaceae (Citrobacter, Escherichia, Hafnia, Klebsiella, Salmonella and Serratia) followed by Micrococcaceae (Micrococcus and Staphylococcus), Pseudomonadaceae (Pseudomonas) and Bacillaceae (Bacillus). The coliforms, generally referred to the genera of the family Enterobacteriaceae, and are considered as indicator bacteria. All the bacterial species could grow on TY agar, while only 8 species exhibited growth (Citrobacter, Enterobacter, Escherichia, Hafnia, Klebsiella, Psuedomonas, Salmonella and Serratia) on Endo agar. All the bacterial species were positive for catalase, except Streptobacillus and negative for oxidase, except Micrococcus. Bacillus and Serratia were positive for amylase while Bacillus, Serratia, Klebsiella and Streptobacillus were positive for lipase. Only Salmonella and Pseudomonas species were positive for production of H2S. All the species were positive for utilization of lactose except the species of Salmonella and Streptobacillus. Species of Serratia, Salmonella and Streptobacillus did not utilize sucrose and all the species were positive for mannitol, except Streptobacillus.
| S. No. | Colony morphology | Microscopic features | Growth parameters | IMViC tests | Tentative identification |
|---|---|---|---|---|---|
| 1 | Off white, entire, irregular colony with 3-5 mm dia | Gram +ve, bacilli, diplobacilli, palisade or short chains with subterminal spore, motile | FA,temp 4-45°C, pH 4–11, salt tolerance 9% | Indole –ve, MR +ve, VP–ve citrate –ve, | Bacillus sp. |
| 2 | Dark pink, circular, entire colony with 1-2mm dia | Gram -ve, long rod shaped, nonmotile, nonspore forming | FA, temp 4-45°C, pH 2-9, salt tolerance 9% | Indole -ve, MR –ve, VP +ve, citrate +ve | Citrobacter sp. |
| 3 | Pink, circular, entire colony with 1-2mm dia | Gram -ve, rod shaped, motile, nonspore forming | FA, temp 4-45°C, pH 4-10, salt tolerance 7% | Indole –ve, MR –ve, VP +ve, citrate +ve | Enterobacter sp. |
| 4 | Pink with metallic sheen, circular, entire colony with 1-2mm dia | Gram -ve, rod shaped, motile, nonspore forming | FA, temp 4-45°C, pH 4-11, salt tolerance 7% | Indole +ve, MR +ve, VP–ve, citrate –ve | Escherichia coli |
| 5 | Pink, circular, entire colony with 1-2mm dia | Gram -ve, thick rod shaped, motile, nonspore forming | FA, temp 4-45°C, pH 4-11, salt tolerance 9% | Indole –ve, MR–ve, VP +ve, citrate +ve | Hafnia sp. |
| 6 | Pink, circular, entire colony with 1-2mm dia | Gram -ve. rod shaped, nonmotile, nonspore forming | FA, temp 4-45°C, pH 2-9, salt tolerance 9% | Indole +ve, MR +ve, VP -ve, citrate +ve | Klebsiella sp. |
| 7 | White, irregular, circular colony with 3-5mm dia | Gram +ve, cocco-bacilli, motile, non-spore forming | Temp 4-45°C, pH 4-11, salt tolerance 9% | Indole –ve, MR –ve, VP –ve, citrate –ve | Micrococcus sp. |
| 8 | Pink, entire, circular colony with 2mm dia | Gram -ve, rod shaped | FA, temp range 4-45°C, pH range 4-11, salt tolerance 9% | Indole –ve, MR +ve, VP –ve, citrate +ve | Psuedomonas sp. |
| 9 | Pink, circular, entire colony with 1-2mm dia | Gram-ve, rods, motile, nonspore forming | FA, temp 4-45°C, pH 2-9, salt tolerance 9% | Indole –ve, MR +ve, VP +ve, citrate +ve | Salmonella sp. |
| 10 | Red, entire, irregular colony with 1-2mm dia | Gram -ve, very small rods, motile, nonspore forming | FA, temp 4-45°C, pH 2-9, salt tolerance 9% | Indole –ve, MR –ve, VP +ve, citrate +ve | Serratia sp. |
| 11 | Off white, entire, circular colony with 1-2mm dia | Gram +ve, cocci/ diplo cocci, nonmotile, nonspore forming | FA, temp 9-55°C, pH 5-9, salt tolerance 5% | Indole –ve, MR –ve, VP +ve, citrate +ve | Staphylococcus sp. |
| 12 | White, circular, irregular colony with 1-2mm dia | Gram –ve, rod long, wavy chains or filaments, nonmotile, nonspore forming | FA, temp 4-45°C, pH 2-9, salt tolerance 9% | Indole -ve, MR –ve, VP –ve, citrate –ve | Streptobacillus sp. |
Table 2: Characteristics and tentative identification of the bacterial isolates.
Generally, coliforms are found to dominate among bacterial contaminants and known to cause various diseases in animals and humans. The bacterial isolates mainly belonged to the family Enterobacteriaceae that is known to consist several pathogenic bacteria. The bacteria colonize the intestinal parts of animals including humans. Escherichia and Salmonella are considered as food and water-borne pathogens whereas species of Citrobacter, Klebsiella and Serratia are known for their opportunistic behaviour [24]. Hafnia is reported from various ecological samples, however, it is not a common human pathogen and often associated to the gastroenteritis [25]. Pathogenic Staphylococcus species are associated with inflammation and suppuration. Micrococcus species although are not considered as pathogens, although they find human body as a favorable habitat to colonize.
Species of Bacillus and Pseuodomonas are generally known for their various biotechnological and environmental applications; some species from the respective genera are also known to be pathogenic. Based on the specificities of the study location, colonization of species of Bacillus and Pseudomonas can be attributed to the surrounding vegetation including number of conifers such as Pine, Cedrus and Taxus. Rhizosphere of these tree species are colonized by these bacterial species that are also known for their plant growth promoting and biocontrol abilities [26-28].
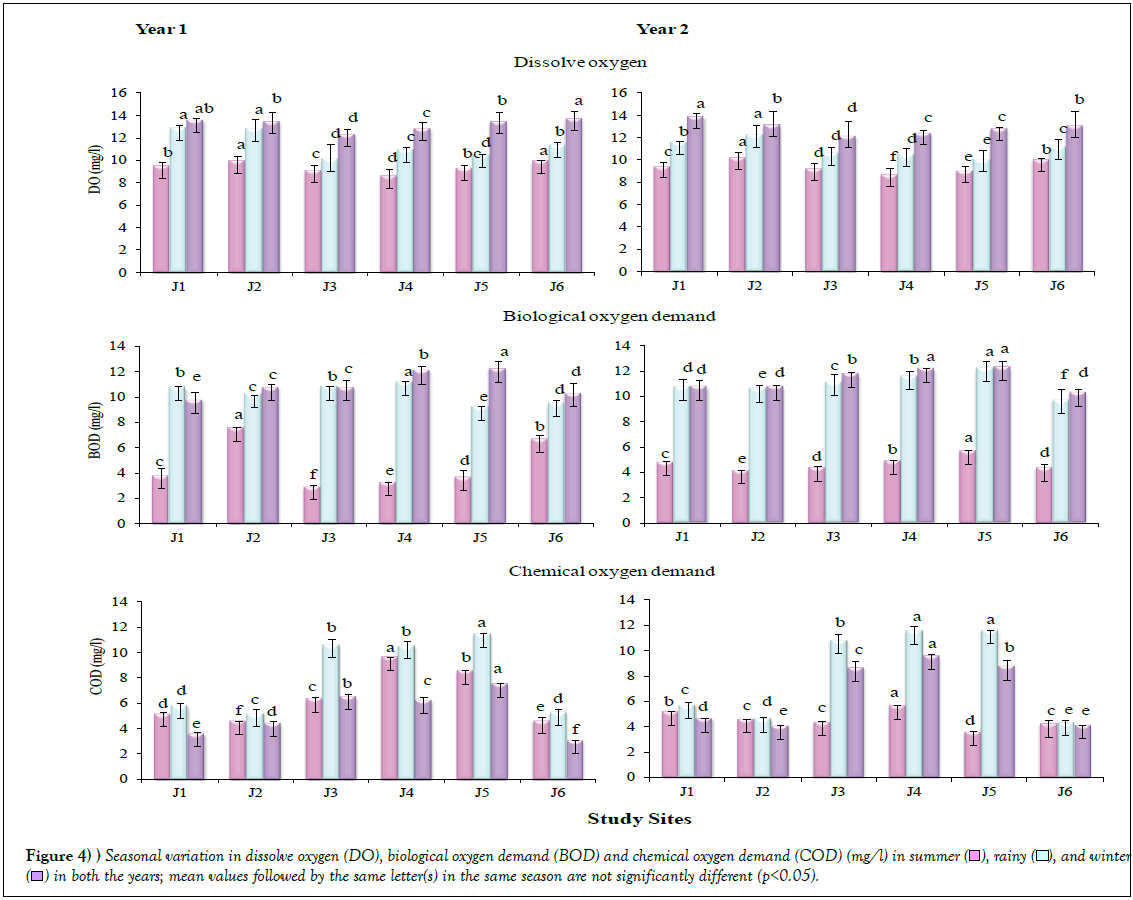
Physico-chemical analyses showed significant differences (p<0.05) across the seasons, in both the years (Table 3; Figure 4). The DO values were recorded to be highest in winter, followed by rainy and summer seasons, in both the years. Generally, it varied between 12 to 14 mg/l in winter, 10 to 13 mg/l in rainy and 8 to 10 mg/l in summer. However, all the values obtained were within the permissible limit [29]. The values for BOD were also found higher in winter, followed by rainy and summer seasons in both the years, on similar lines as in case of the findings on DO. The BOD values were recorded higher at the Sites J4 and J5. The values for COD were recorded higher in rainy season, followed by summer and winter, in both the years. Maximum COD was recorded at Site 5, in both the years.
| Study sites | Summer | Rainy | Winter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp | pH | TDS | Temp | pH | TDS | Temp | pH | TDS | |
| Year 1 | |||||||||
| J1 | 17.3 ± 0.03b | 7.34 ± 0.03b | 26.67 ± 0.05c | 10.4 ± 0.05e | 7.51 ± 0.04d | 27.56 ± 0.52d | 4.0 ± 0.02c | 7.3 ± 0.03a | 23.2 ± 1.00c |
| J2 | 16.5 ± 0.26c | 7.37 ± 0.08b | 26.21 ± 0.04d | 10.2 ± 0.05d | 7.59 ± 0.08d | 28.42 ± 0.71d | 4.1 ± 0.02c | 7.34 ± 0.01a | 23.56 ± 0.52c |
| J3 | 18.3 ± 0.05a | 7.61 ± 0.05a | 27.67 ± 0.06b | 11.3 ± 0.05c | 7.8 ± 0.02c | 30.21 ± 0.051c | 4.1 ± 0.03bc | 7.48 ± 0.04a | 25.63 ± 0.35b |
| J4 | 18.2 ± 0.18a | 7.57 ± 0.01a | 40.67 ± 0.04a | 11.5 ± 0.05b | 8.21 ± 0.04b | 38.56 ± 1.03b | 4.1 ± 0.08ab | 7.5 ± 0.02a | 25.83 ± 0.35b |
| J5 | 18.1 ± 0.05a | 7.53 ± 0.01a | 40.54 ± 0.02a | 11.6 ± 0.05ab | 8.42 ± 0.02a | 40.21 ± 1.42a | 4.3 ± 0.05a | 7.48 ± 0.01a | 28.56 ± 0.40a |
| J6 | 17.6 ± 0.13b | 7.41 ± 0.03b | 24.23 ± 0.01e | 11.6 ± 0.08a | 8.01 ± 0.10b | 29.23 ± 0.61cd | 4.2 ± 0.08a | 7.4 ± 0.01a | 24.63 ± 0.50bc |
| Year 2 | |||||||||
| J1 | 18.1 ± 0.12bc | 7.45 ± 0.03d | 28.50 ± 0.05b | 10.5 ± 0.05cd | 8.21 ± 0.04c | 26.21 ± 0.52f | 4.14 ± 0.02e | 7.22 ± 0.02d | 24.12 ± 1.80f |
| J2 | 17.5 ± 0.21d | 7.57 ± 0.08c | 27.21 ± 0.04d | 10.1 ± 0.05d | 7.91 ± 0.08d | 26.82 ± 0.71e | 4.25 ± 0.02b | 7.24 ± 0.03d | 25.06 ± 0.82e |
| J3 | 18.8 ± 0.15b | 7.81 ± 0.05b | 27.87 ± 0.06c | 10.8 ± 0.05bc | 7.87 ± 0.02d | 28.51 ± 0.051c | 4.21 ± 0.03c | 7.52 ± 0.01a | 26.23 ± 0.85d |
| J4 | 18.5 ± 0.81ab | 7.87 ± 0.01ab | 41.17 ± 0.04a | 11.2 ± 0.05ab | 8.54 ± 0.04a | 29.26 ± 1.03b | 4.18 ± 0.08d | 7.46 ± 0.05c | 26.3 ± 0.95c |
| J5 | 18.9 ± 0.51a | 7.93 ± 0.01a | 41.14 ± 0.02a | 11.4 ± 0.05a | 8.51 ± 0.02a | 38.21 ± 1.42a | 4.28 ± 0.05a | 7.51 ± 0.01ab | 28.96 ± 0.70b |
| J6 | 17.8 ± 0.13cd | 7.81 ± 0.03b | 23.53 ± 0.01e | 11.3 ± 0.08a | 8.41 ± 0.10b | 27.23 ± 0.61d | 4.19 ± 0.08cd | 7.48 ± 0.01bc | 29.63 ± 0.80a |
Table 3: Seasonal variation in physiological parameters for two consecutive years.
The DO parameter is crucial for survival of aquatic organisms. The DO content in various stretches, in the present study, maintained similar trend for a particular season, irrespective to the site of sample collection. High value of DO, recorded in the winter season, can be attributed to the low temperature environment, in agreement with the APHA standards. However, the highest values may be attributed to the higher organic load. The contamination of water by biological, physico-chemical, organic and inorganic pollutants has been reported for resulting in the higher BOD values [30]. The total dissolved solids are considered as an indicator of the degree of dissolved substances. TDS in the entire study site was recorded within the minimum prescribed limits [3].
The pH values for all the sites were found to be on alkaline side in the rainy and summer seasons, while it was almost neutral in winter season, in both the years. Generally, the values for pH varied between 7.3 to 8.2 in summer, and 7.5 to 8.5 in rainy and 7.3 to 7.6 in winter season. On the similar lines, the values for temperature at the study location varied between 17.2 to 18.8°C in summer, 10.2 to 11.6°C in rainy and 4.03 to 5.01°C in winter. This was also reflected in the growth of pure bacterial cultures that could grow at low temperature as well (Table 2). Factorial analysis revealed that all the variables (effect of year, locations and seasons) individually and their interaction significantly (P<0.05) affected the physico-chemical parameters (Table 4). The pH of the water under study was found to be permissible, within the WHO standards (6.5-8.5). The pH of a water body is known as one of the important factors in determination of water quality as it affects other chemical reactions such as solubility and metal toxicity [31].
| Source of variation | Physico-chemical parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | DO | BOD | COD | Temp | pH | TDS | |||||||
| MS | F value | MS | F value | MS | F value | MS | F value | MS | F value | MS | F value | ||
| Year | 1 | 1.15 | 66.83*** | 3.84 | 1771*** | 1.2 | 101.80*** | 0.67 | 20.57*** | 0.8 | 118.52*** | 32.52 | 138.98*** |
| Locations | 5 | 6.72 | 390.93*** | 2.29 | 1055*** | 81.7 | 6971*** | 2.54 | 77.77*** | 0.46 | 67.75*** | 453.41 | 1938*** |
| Seasons | 2 | 119.45 | 6948*** | 463.1 | 214100*** | 71.25 | 6081*** | 1717.92 | 52580*** | 4.17 | 619.52*** | 358.59 | 1532*** |
| Year*Locations | 5 | 0.2 | 11.86*** | 4.7 | 2162*** | 1.36 | 115.90*** | 0.14 | 4.20** | 0.02 | 2.53** | 18.52 | 79.13*** |
| Year* Seasons | 2 | 0.57 | 33.35*** | 0.97 | 447.70*** | 24.01 | 2050*** | 1.43 | 43.67*** | 0.38 | 55.75*** | 105.26 | 449.79*** |
| Locations* Seasons | 10 | 1.13 | 65.96*** | 4.4 | 2031*** | 9.52 | 812.50*** | 0.78 | 23.81*** | 0.13 | 18.88*** | 87.34 | 373.23*** |
| Year*Location*Seasons | 10 | 0.31 | 17.83*** | 2.75 | 1269*** | 4.34 | 370.70*** | 0.04 | 1.31NS | 0.06 | 8.35*** | 14.42 | 61.60*** |
Table 4: Analysis of variance with reference to the effect of year, location, season and their interaction with physico-chemical parameters.
In summary, the variation in TVC, in the present study, appeared mainly influenced by the peculiarities of the study sites and also by seasonal variation. Selection of the procedure in determination of the bacteriological water quality may differ with the objectives of the study. Presence of coliforms is considered as one of the most important biological indicators in assessment and monitoring of water quality. The genus or species level identification indicated towards the specificities of the study sites. Overall, the dominance of Enterobacteriaceae, including biological indicators and pathogens with respect to water-borne diseases, indicated the high anthropogenic pressures at the respective sites. Frequent recording on bacteriophages on agar plates in all these experiments was an important observation that needs attention in future studies from such sites.
Conclusion
Diversity of microorganisms in natural freshwater systems plays a key role in determination of the quality of water. Detailed knowledge on microorganisms with respect to their functional aspects in freshwater habitats are an essential prerequisite for the sustainable management of freshwater resources. The aim of this study was to evaluate the impact of anthropogenic activities in various seasons on the colonization of various groups of microorganisms, mainly bacteria, as they are useful in assessment and monitoring of pollution status of water sources. The river Jataganga, presented a unique site due to its location, anthoropogenic activities and the surrounding vegetation. The results on colonization of specific groups of bacteria under influence of a range of biotic and abiotic factors can be used for taking preventive measures while drawing policies on cleanliness of the water bodies including rivers. Observations on bacteriophages on agar plates, that were used for enumeration of bacteria, indicated the presence of a self-purification system in the water bodies. However, due to the impact of extensive anthropogenic activities, the water bodies are likely to lose their self-purification capacity to a large extent. Biomonitoring of water pollution level at the tourist sites, that are periodically under the influence of anthropogenic activities, should be an important component at policy level. The consortium of bacterial populations representing biological indicators, causative organisms (pathogens) of waterborne diseases and plant growth promoting species, extends opportunity for microbe-microbe interaction studies in the water bodies under mountain ecosystem. Long term monitoring of the rivers under anthropogenic pressure will certainly support the planning of the environment related programmes at local levels.
Acknowledgements
Director, G.B. Pant National Institute of Himalayan Environment and Sustainable Development, Almora for extending the facilities and Ministry of Environment, Forest & Climate Change, Govt. of India, for financial support.
Conflict of Interest
The authors declare no competing interests.
REFERENCES
- WHO (World Health Organization). Mortality and Burden of Disease from Water and Sanitation. World Health Organization, Geneva, Switzerland. 2004; Available at:
- Okpokwasili GC, Akujobi TC. Bacteriological indicators of tropical water quality. Environ Toxicol. 1996;11:77-81.
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Waste Water. 20th ed. 1998; Washington DC.
- Ajibade WA, Ayodele IA, Agbede SA. Microbiological characteristics of waters in the major rivers in Kainji Lake National Park. Afr J Environ Sci Technol. 2008; 2(8):208-16.
- Sood A, Singh KD, Pandey P, et al. Assessment of bacterial indicators and physicochemical parameters to investigate pollution status of Gangetic river system of Uttarakhand (India). Ecol Indicators. 2008;8:709-17.
- Ahmed M, Victor R, Jashoul M, et al. Utilization of low quality water of mountain reservoirs: a case study from Al Jabal Al Akhdar, Oman. J Mt Sci. 2016;13:1423-30.
- Ouyang LL, Pan YD, Huang CM, et al. Water quality assessment of benthic diatom communities for water quality in the subalpine karstic lakes ofJiuzhaigou, a world heritage site in China. J Mt Sci. 2016;13(9):1632-44
- Li XY, Ding YJ, Han TD, et al. Seasonal variations of organic carbon and nitrogen in the upper basins of Yangtze and Yellow Rivers. J Mt Sci. 2017;14(8).
- Hahn MW. The microbial diversity of inland waters. Curr Opinion Biotechnol. 2006;217:256-61.
- Suthar S, Sharma J, Chabukdhara M, et al. Water quality assessment of river Hindon at Ghaziabad, India: impact of industrial and urban waste water. Environ Monit Assess. 2012; 165(1-4):103-12.
- Lika (Çekani) M, Nelaj E, Gjoni V. Evaluation of Microbiological Water Situation from 2005 to 2008 in Shkumbin River, Albania. Ohrid. 2010; Republic of Macedonia. 25.
- Bucci JP, Szempruch AJ, Caldwell JM, et al. Seasonal Changes in Microbial Community Structure in Freshwater Stream Sediment in a North Carolina River Basin. Diversity. 2014;6:18-32.
- Kistemann T, Claben T, Koch C, et al. Microbial load of drinking water reservoir tributaries during extreme rainfall and runoff. Appl Environ Microbiol. 2002;68:2188-97.
- Kumarasamy P, Vignesh S, James RA, et al. Enumeration and identification of pathogenic pollution indicators in Cauvery River, South India. Res J Microbiol. 2009;4(12):540-49.
- Strauch AM. Seasonal variability in fecal bacteria of semiarid rivers in the Serengeti National Park, Tanzania. Marine Freshwater Res. 2011;62:1191-1200.
- Lenart-Boroń A, Wolanin AA, Jelonkiewicz L, et al. Factors and mechanisms affecting seasonal changes in the prevalence of microbiological indicators of water quality and nutrient concentrations in waters of the Białka River Catchment, Southern Poland. Water Air Soil Poll. 2016;227:302.
- Borade S, Dhawde R, Maloo A, et al. Occurrence and seasonal variation in distribution of fecal indicator bacteria in Tapi Estuary along the west coast of India. Ind J Mar Sci. 2014;43(3):340-47.
- Srivastava A, Srivastava S. Assessment of physico-chemical properties and sewage pollution indicator bacteria in surface water of river Gomti in Uttar Pradesh. Int J Environ Sci. 2011;2 (1):325-36.
- Juahir H, Zain SM, Yusoff MK, et al. Spatial water quality assessment of Langat River Basin (Malaysia) using environmetric techniques. Environ Monit Assess. 2011;173:625-41.
- Tambekar DH, Hirulkar NB, Gulhane SR, et al. Evaluation of hydrogen sulphide test for detection of fecal coliform contamination in drinking water from various sources. Afr J Biotechnol. 2007;6(6):713-17.
- Nikaeen M, Pejhan A, Jalali M. Rapid monitoring of indicator coliforms in drinking water by an enzymatic assay. Iran J Environ Health, Sci Eng. 2009;6(1):7-10.
- Rompre A, Servaism P, Baudartm J, et al. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods. 2002;49: 31-34.
- Kumar M, Puri A. A review of permissible limits of drinking water. Ind J Occup Environ Med. 2012;61(1):40-44.
- Baylis C, Uyttendaele M, Joosten H, et al. The Enterobacteriaceae and their Significance to the Food Industry. ILSI Europe Report Series, Belgium, 2011.
- Janda JM, Abbott SL. The genus Hafnia: from Soup to Nuts. ClinMicrobiol Rev. 2006;19(1):12-8.
- Trivedi P, Pandey A. Recovery of plant growth promoting rhizobacteria from sodium alginate beads after three years following storage at 4°C. J IndMicrobiolBiotechnol. 2008;35:205-9.
- Malviya MK, Sharma A, Pandey A, et al. Bacillus subtilis NRRL B-30408: A potential inoculant for crops grown under rainfed conditions in the mountains. J Soil Sci Plant Nutr. 2012;12(4):811-24.
- Trivedi P, Pandey A, Palni LMS. Bacterial inoculants for field applications under Mountain Ecosystem: Present initiatives and future prospects. In: Maheshwari DK, editor. Bacteria in Agrobiology: Plant Probiotics, Springer, 2012; pp.15-44.
- BIS (Bureau of Indian Standards). Specification for drinking water. Ist revision; 1991; IS:10500. New Delhi.
- Padmanabha B, Belagali SL. Comparative study on the water quality index of 4 lakes in the Mysore city. Ind Environ Polln. 2005;25(10):873-6.
- Agbaire PO, Obi CG. Seasonal variations of some physico-chemical properties of river Ethiope water in Abraka, Nigeria. J ApplSci Environ Manage. 2009;13(1):55-7.




 ), rainy (
), rainy ( ), and winter (
), and winter ( ) in both the years; mean values followed by the same letter(s) in the same season are not significantly different (p<0.05).
) in both the years; mean values followed by the same letter(s) in the same season are not significantly different (p<0.05).