Biochemical effects of effluent pollutants from coal mining activities on the freshwater snails, Helisoma duryi
Received: 09-Sep-2020 Accepted Date: Sep 25, 2020; Published: 02-Oct-2020
Citation: Change JB, Siwela AH, Basopo N. Biochemical effects of effluent pollutants from coal mining activities on the freshwater snails, Helisoma duryi. J Environ Chem Toxico. 2020;4 (3); 1-4.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
We assessed the biochemical effects of effluent pollutants from coal mining activities using the freshwater snail, Helisoma duryi, as a bioindicator species. Water samples were collected from 3 different sites in a coal mining area in Hwange. Physical parameters were measured on site and these were pH, total dissolved solids (TDS), temperature and conductivity. The water was analyzed for zinc, lead, copper and cadmium. Fresh water snails, in groups of 60, were exposed to sampled water diluted with tap water in the ratios 95:05 for sites 1 (coal processing plant) and 2 (underground acid rock drainage point) and 10:90 for site 3 (power production plant). The exposure duration was 28 days with snails collected at 7 day intervals to determine time dependent effects of effluent from mining activities. After the exposure duration, post mitochondrial fractions were prepared and used to determine catalase, superoxide dismutase and acetylcholinesterase activities. Very low acidic figures were observed in the water samples with site 2 having a pH of 2.13. High levels of TDS (1.21 ppt) and conductivity of 2.42 mS were observed at Site 1. High levels of zinc (540.0 mg/L ± 4.86 mg/L), copper (53.2 mg/L ± 0.16 mg/L), lead (9.6 mg/L ± 0.03 mg/L) and cadmium (4.3 mg/L ± 0.07 mg/L) were observed in the water samples. Time depended inhibitions of acetylcholinesterase and catalase were observed in snails exposed to water collected from Site 3 whilst superoxide dismutase activity showed a decrease over time. These results suggest that pollutants from the mining activities affect the reactive oxidative status of aquatic biota as indicated by the alterations of the studied enzymes in the freshwater snail H. duryi. There is therefore, need for constant monitoring of water bodies in coal mining areas to safeguard the health of aquatic life from adverse effects of effluent from coal mining activities.
Keywords
Coal mining; Catalase; Superoxide dismutase; Acetylcholinesterase; Heavy metals; Eeffluent; Bioindicator
Introduction
Extensive mining activities carried out by both skilled and unskilled miners in Zimbabwe adversely affect the environment. The problems associated with such activities are land degradation, deforestation and extensive water pollution with deleterious effects to both flora and fauna. The occurrence of metals in effluent water is not only associated with ground and surface water contamination, but is also responsible for the degradation of aquatic environments, leading to mortality and morbidity of animal species, as well as infection of human beings. Chemical waste management and disposal from coal mines should be aimed at reducing toxic heavy metals such as mercury, nickel, chromium, copper, lead, cadmium and arsenic [1].
In coal mining areas, the most common mineral containing reduced forms of sulfur is iron pyrite (FeS2) [2] Pyrite is the predominant species in coal and associated rocks and is the major acid-producer [3]. Acid mine drainage (AMD) is a major driving force responsible for heavy metal pollution. It is generated by the formation of sulphuric acid when sulphur-containing minerals such as pyrite undergo weathering in the environment [4]. Acid rock drainage causes metals trapped in geologic material in the mineral ores to be released into the environment thereby rendering them available for ecological uptake. Heavy metals are of high environmental concern because they cannot be removed from aquatic systems by self-purification but instead accumulate in the underlying sediments where they can enter food chains [5]. As a result, sediments are a sink as well as a source of heavy metal contaminants. They reflect the quality of particular surface water.
The aim of this study was to assess the impact of acid rock drainage in aquatic bodies in the vicinity of a coal mine by determining levels of selected metal residues and assessing the effect of the sampled water on freshwater snail Helisoma duryi.
Materials and Methods
Sampling and physical parameter analysis
Water and sediment samples were collected in triplicate from three sites namely; Site 1 was effluent from coal processing plant, Site 2 was underground acid rock drainage point and Site 3 was effluent from a power production plant. Glass reagent bottles were used for water samples whilst polythene bags were used for sediment samples. Municipal water was used as the control. Temperature, pH, conductivity and total dissolved solids parameters were measured at each site.
Exposures
Snails were bred in cement aquaria outdoors as described by Naik and Hasler in 2002. They were collected and acclimatized to laboratory conditions for 24 hours before exposures. For the concentration screening studies, snails were exposed to different dilutions of water samples collected from the 3 different sites over 48 hours to determine the LC-50 values. The samples were diluted in the following ratios 90%, 75%, 50%, 25%, 10%, 7.5%, 5% and 2.5%. (Sample: tap water). Sixty snails (in triplicate) were exposed to sub-lethal levels of effluent, 5% concentration from Sites 1 and 2 and 90% concentration for Site 3. Ten snails were collected on day 1, 7, 14, 21 and 28 from each exposure tank [6-15].
Biochemical analysis
The snails were sacrificed, deshelled, weighed and homogenized with 5 volumes of 0.1 M potassium phosphate buffer (pH 7.4) and centrifuged at 10 000 × g for 10 minutes. The supernatant was stored at –80°C until required. Protein content in the post-mitochondrial fractions of the whole snail homogenates was determined using the method of Lowry et al. [17]. The post- mitochondrial fractions (PMF) were used to determine catalase, superoxide dismutase and acetylcholinesterase activities.
Superoxide dismutase
The activity of superoxide dismutase (SOD) in post-mitochondrial fractions was determined following the method of Sun et al. The reaction mixture contained 0.5 mL sample or standard copper, zinc superoxide dismutase (Cu, Zn-SOD) and 2.45 mL SOD Assay Reagent (SODAR) The SODAR contained 40 mL of 0.3 mM xanthine, 20 mL of 0.6 mM ethylenediaminetetraacetic acid (EDTA), 12 mL of 400 mM sodium carbonate, 6 mL of 0.1% w/v bovine serum albumin and 20 mL of 150 μM nitroblue tetrazolium. The working range for the Cu, Zn-SOD standard curve was 0-300 ng/mL. One enzyme unit of SOD was defined as the amount, which inhibited the nitroblue tetrazolium reaction by 50%. Cu, Zn-SOD activity in the samples will be calculated as units/mg protein by comparison to the standard curve.
Catalase
The activity of catalase (CAT) was determined spectrophotometrically according to the method of Clairborne in 1979. The reaction mixture consisted of 2950 μL of 19 mM hydrogen peroxide solution in 50 mM potassium phosphate buffer (pH 7.0) and 50 μL PMF (1 mg/mL protein). The blank had 3000 μL of buffer with no substrate. The mixture was incubated at 25°C the rate of decrease in absorption was monitored at a wavelength of 240 nm.
Acetylcholinesterase
The enzyme activity was measured using acetylthiocholine iodide as a substrate following the Ellman method that was modified for a plate reader by Kallander et al.
The reaction mixture contained 50 μL of 0.1 mg/mL PMF, 110 μL of 0.01 M Tris- hydrochloric acid buffer pH 8 and 50 μL of 0.4 mM 5, 5 dithio-bis-(2 nitro benzoic acid) DTNB. The mixture was incubated for 3 minutes before adding 30 μL of 0.5 mM acetylthiocholine iodide. The rate of production of a complex between thiocholine and DTNB was followed for 3 minutes at 412 nm.
Heavy metal analysis
Water
The water samples were filtered using a Whatman 40 micron filter paper and then kept in the refrigerator for analysis.
Statistical analysis
Data was reported as mean enzyme activity of specific antioxidant enzymes. Two-way ANOVA was used to assess statistical differences using the GraphPad prism 8 programs.
Results
Physico-chemical analysis of water samples
Results of physico-chemical analysis of water samples are shown in Table 1. Water collected from Site 1 (coal processing plant) and Site 2 (underground acid rock drainage point) had the higher values of conductivity and total dissolved solids whilst exhibiting low pH when compared to the values for water samples obtained from the control site.
| Point | pH | Temperature (°C) | Conductivity | Total Dissolved Solids |
|---|---|---|---|---|
| Site 1 | 3.00 | 24.6 | 2.42 mS | 1.21 ppt |
| Site 2 | 2.13 | 16.6 | 2.41 mS | 1.20 ppt |
| Site 3 | 5.77 | 22.4 | 253 µS | 128 ppm |
| Control | 5.84 | 23.7 | 64.9 µS | 32.4 ppm |
Table 1: Physico-chemical parameters of water samples.
The control and Site 3 (power production plant) samples had similar pH, their values lying in the range 5.7 to 5.9. There was however significant differences in the conductivity values of 64.9 μS and 253 μS between samples collected from the control site and Site 3 respectively whilst total dissolved solids values of 32.4 mg/L and 128 mg/L were observed between samples from Site 3 and those from the control site.
Heavy metal analysis
Results of heavy metal analysis for water are shown in Table 2.
| Variables | Sampling site (mg/kg) | ||
|---|---|---|---|
| Heavy metal | 1 | 2 | 3 |
| Lead (Pb) | 9.6 ± 0.03 | 7.1 ± 0.17 | 4.5 ± 0.05 |
| Cadmium (Cd) | 4.3 ± 0.07 | 3.9 ± 0.11 | 1.1 ± 0.05 |
| Copper (Cu) | 53.2 ± 0.16 | 33.5 ± 0.10 | 34.0 ± 0.34 |
| Zinc (Zn) | 525.0 ± 2.63 | 540.0 ± 4.86 | 31.3 ± 0.06 |
Table 2: Heavy metal analysis of water.
From 4.5 mg/kg to 9.6 mg/kg whilst copper concentrations were in the range of 33.5 mg/kg to 53.2 mg/kg. Cadmium concentrations were in range 1.1 mg/kg to 4.3 mg/kg and zinc concentrations were in the range 31.3 mg/ kg to 540.0 mg/kg were observed from the samples obtained from the three sampling sites.
Superoxide dismutase
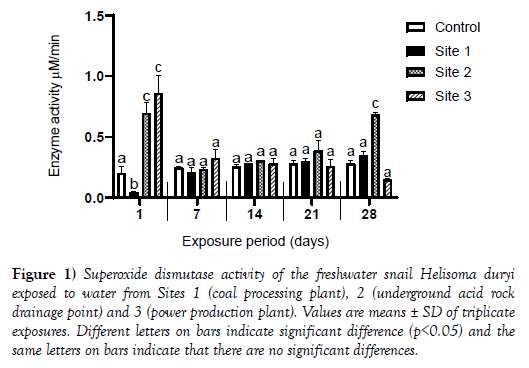
Results of superoxide dismutase activity are shown in Figure 1.
Figure 1: Superoxide dismutase activity of the freshwater snail Helisoma duryi exposed to water from Sites 1 (coal processing plant), 2 (underground acid rock drainage point) and 3 (power production plant). Values are means ± SD of triplicate exposures. Different letters on bars indicate significant difference (p<0.05) and the same letters on bars indicate that there are no significant differences.
There was a significant increase in SOD activity on days 1, 7, 14 and 21 in snails exposed to water from Site 1 when compared to enzyme activity in snails exposed to the control water. The enzyme activity of snails exposed to water from Site 1 dropped to levels of control values on day 28. A similar trend was observed for snails exposed to water from Site 2 with the exception of results on day 1 where statistically non-significant increase in SOD activity (p>0.05) was observed compared to SOD activity of snails exposed to the control water. There was a significant increase in enzyme activity for snails exposed to water from Site 3 on days 1 and 28 whilst a significant decrease (p<0.05) in enzyme activity was observed on days 7, 14 and 21 compared to SOD activity in snails exposed to control water. A statistically significant decrease (p<0.05) in enzyme activity was observed in snails exposed to Site 1 and 2 on (Figure 2).
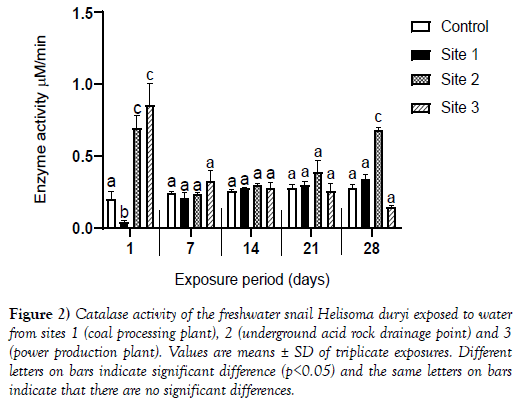
Figure 2: Catalase activity of the freshwater snail Helisoma duryi exposed to water from sites 1 (coal processing plant), 2 (underground acid rock drainage point) and 3 (power production plant). Values are means ± SD of triplicate exposures. Different letters on bars indicate significant difference (p<0.05) and the same letters on bars indicate that there are no significant differences.
Statistically significant increase of catalase activity (p<0.05) in snails exposed to water from Site 1 was observed throughout the exposure period compared to the basal enzyme activity in snails exposed to control water on Day 1. However, the activities of catalase for Day 1 exposures were significantly reduced when compared to activities of the enzyme in snails exposed to control water.
The activities of the enzyme in snails exposed to water from Site 1 remained the same as the enzyme activity values in snails exposed to control water from Day 7 to Day 28. There was a significant increase in enzyme activity on Day 1 which was followed by a twofold decrease in enzyme activity for snails exposed to water from sites 2 and 3 when compared to enzyme activity in snails exposed to control water on Days 7, 14, 21 and 28 save for the enzyme activity for snails exposed to Site 3 which resorted to Day 1 values.
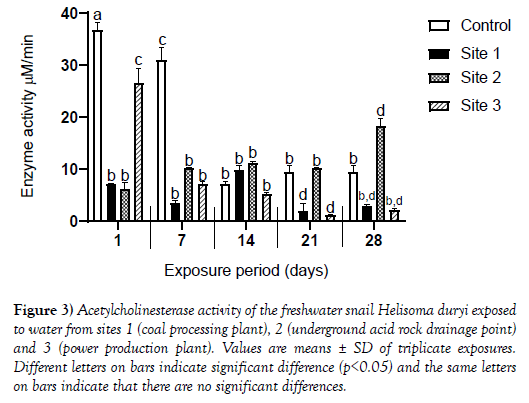
Acetylcholinesterase
There was a statistically significant decrease in acetylcholinesterase activity in snails exposed to water from Sites1 and 3 on day 1, 7, 21 and 28 whilst the activity of the enzyme on day 14 remained statistically the same when compared enzyme activity in snails exposed to control water. On day 28, a statistically significant increase in activity of the enzyme in snails exposed to water from Site 2 compared to enzyme activity in snails exposed to control water was observed.
Discussion
The measurement of TDS integrates all anions and cations and some ions are substantially more toxic than other ions. Different aquatic organisms vary in their sensitivity to TDS toxicity depending on different life stages, for example most fish species are sensitive to TDS during fertilization [6-10]. Acid rock drainage produces sulphuric acid and elevated concentrations of ions. The acid facilitates the dissolution of other minerals causing a high load of dissolved solids. This is probably the reason for the observed low pH and high TDS at Sites 1 and 2 (Table 1). Electrical conductivity gives an indication of the amount of total dissolved solids in water hence high TDS values correlate with high conductivity in water (Yilmaz and Koc, 2014). According to World Health Organization (WHO) guidelines, freshwater that has TDS values below 1000 ppm is good enough for both drinking and irrigation. Our results show that Site 3 and control (Table 1) satisfy this requirement. However, water from both the control site and Site 3 has a pH lower than the World Health Organization recommended pH value of 6.5 or higher for drinking water (WHO, 2010).
Metals occur naturally in the environment but due to anthropogenic activities their levels in ecosystems increase to levels that cause adverse effects to other organisms. Metals like lead are introduced in aquatic ecosystems as effluent from mining and industrial activities. Literature shows that metals induce oxidative stress in cells of living organisms and these results from an imbalance between the generation and elimination of reactive oxygen species. In the current study Sites 1 (coal processing plant) 2 (underground acid rock drainage) and 3 (power production plant) were polluted with lead at 9.6 ± 0.03, 7.1 ± 0.17 and 4.5 ± 0.05 respectively. In comparison to the Zimbabwe Environmental Management Agency Regulations of 2007 which defined the hazardous levels of zinc in water at 15 mg/l (S.I. 6 of 2007), sites 1 and 2 were heavily polluted with zinc with concentrations ranging from 525 mg/kg to 540 mg/kg (Table 2). The contributing factor to the high zinc concentrations is probably the fact that effluent from both sites is from plants that utilize coal [7]. Metals like lead probably cause production of reactive oxygen species (ROS) which, if not eliminated effectively, attacks macromolecules in living organisms [10-15]. The actual mechanism of elimination of ROS like the superoxide anion radical is generally by the dismutation activity of SOD producing hydrogen peroxide. The observed activation of SOD in snails in our study suggests that the detoxification of the pollutants by the snail’s defense system produces superoxide anion radicals as byproducts which are detoxified by the activity of SOD. Induction of CAT is a protective response by the snail’s system to combat the oxidative stress effects of hydrogen peroxide, a product of the dismutation of superoxide anion radical by SOD. These results are consistent with our earlier findings [15-20] and findings of [21-23] who also observed elevated CAT activities in snails exposed to metals such as lead, cadmium, iron and copper.
The first line of defense among antioxidant enzymes is superoxide dismutase and its increase in activity in our study suggests the consistent attempt by the snail’s defense system to counteract the toxic effects of superoxide radicals generated during normal metabolism associated with presence of metals in the snail’s body. General increases in the activities of superoxide dismutase were observed in snails exposed to Sites 2 (underground acid rock drainage point) and 3 (power production plants a response to the heavy metal pollution (Figure 1).
Significant inhibition of AChE in snails exposed to Site 3 was observed and was attributed to the high lead and zinc concentration. According to Calabrese and Baldwin [24,25], metals like aluminium have been found to have hormetic effects on AChE where inhibition can be a result of compensatory mechanisms following a disruption in homeostasis. Activation of AChE observed in Figure 3 for exposed snails can also possibly be a result of hormesis due to low concentrations of cadmium in the initial days of exposure to effluent. Hormesis a phenomenon characterized as a dose response with stimulation of AChE at low doses and inhibition at high doses. Inhibition of catalase observed for snails exposed to Site 3 is in agreement with a decrease in catalase activity that was observed in Lymnaea natalensis exposed to sediment and water contaminated by lead.
Figure 3: Acetylcholinesterase activity of the freshwater snail Helisoma duryi exposed to water from sites 1 (coal processing plant), 2 (underground acid rock drainage point) and 3 (power production plant). Values are means ± SD of triplicate exposures. Different letters on bars indicate significant difference (p<0.05) and the same letters on bars indicate that there are no significant differences.
Acknowledgements
This work was supported by funds from International Science Program, Uppsala University Sweden. Support from the Ecotoxicology Research Group and the Department of Applied Biology and Biochemistry of the National University of Science and Technology Zimbabwe was much appreciated.
Conflict of Interest
No conflict of interest.
REFERENCES
- Adler R, Rascher J. A strategy for the management of acid mine drainage from gold mines in Gauteng. Report. No. CSIR/NRE/PW/ER/2007/0053/C. CSIR, Pretoria; 2007.
- Zipper CE, Schoenholtz S, Soucek D, et al. Total dissolved solids in appalachian coalfield streams: Current Research Approaches.
- Skousen JG, Sexstone A, Ziemkiewicz PF. Acid mine drainage control and treatment. Reclam of drast disturb land. 2000;41:131-68.
- Gaikwad RW, Sapkal VS, Sapkal RS. Acid mine drainage: A water pollution issue in mining industry. Int J Adv. 2011;2(4):257-62.
- Dlamini CL, Fadiran AO, Thwala JM. A study of environmental assessment of acid mine drainage in Ngwenya, Swaziland. J Environ. 2013;2013.
- Weber-Scannell PK, Duffy LK. Effects of total dissolved solids on aquatic organism: a review of literature and recommendation for salmonid species. Am J Environ Sci. 2007.
- Wolkers-Dorfer C. Water management at abandoned flooded underground mines: fundamentals, tracer tests, modelling, water treatment. Springer Sci Rev. 2008.
- Calabrese EJ, Baldwin LA. Inorganics and hormesis. Crit Rev Toxicol. 2003;33(3-4):215-304.
- Siwela AH, Nyathi CB, Naik YS. A comparison of metal levels and antioxidant enzymes in freshwater snails, Lymnaea natalensis, exposed to sediment and water collected from Wright Dam and Lower Mguza Dam, Bulawayo, Zimbabwe. Ecotoxicol Environ Saf. 2010;73(7):1728-32.
- El-Shenawy NS, Mohammadden A, Al-Fahmie ZH. Using the enzymatic and non-enzymatic antioxidant defense system of the land snail Eobania vermiculata as biomarkers of terrestrial heavy metal pollution. Ecotoxicol Environ Saf. 2012;84:347-54.
- Basopo, N, Ngabaza. Toxicological effects of chlorpyrifos and lead on the aquatic snail Helisoma duryi. Adv in Biolog Chem. 2015;5(1): 225-233.
- Livingstone DR, Lips F, Martinez PG, et al. Antioxidant enzymes in the digestive gland of the common mussel, Mytilus edulis. Mar Biolog. 1992;112(2):265-76.
- Mangizvo R. Illegal dumping of solid waste in the alleys in the central business district of Gweru, Zimbabwe. J Sust Develop in Africa. 2010;12(2):110-23.
- Clifford SM, Parker TJ. The evolution of the Martian hydrosphere: Implications for the fate of a primordial ocean and the current state of the northern plains. Icarus. 2001;154(1):40-79.
- Tobin A. OHD processing of coal waste materials. Southern Illinois University at Carbondale; 2016.
- Kallander DB, Fisher SW, Lydy MJ. Recovery following pulsed exposure to organophosphorus and carbamate insecticides in the midge, Chironomus riparius. Arch Environ. 1997;33(1):29-33.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. Int J Biol Chem. 1951;193:265-75.
- Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int. 2011;2011.
- Naik YS, Hasler JA. An economical method for the maintenance of schistosome life-cycles using outdoor facilities. Discov Innov. 2002;14(3):221-4.
- Praveena SM, Radojevic M, Abdullah MH, et al. Application of sediment quality guidelines in the assessment of mangrove surface sediment in Mengkabong lagoon, Sabah, Malaysia. J. Environ Health Sci Eng. 2008;5(1):35-42.
- Shahidullah SM, Hakim MA, Alam MS, et al. Assessment of groundwater quality in a selected area of Bangladesh. Pak J Biol Sci 2000.
- Sun YI, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497-500.
- Tagwira F, Piha M, Mugwira L. Zinc distribution in zimbabwean soils and its relationship with other soil factors. Commun Soil Sci Plan. 1993;24(9-10):841-61.
- World Health Organization. Hardness in drinking-water: background document for development of WHO guidelines for drinking-water quality. World Health Organization; 2010.
- Yilmaz E, Koç C. Physically and chemically evaluation for the water quality criteria in a farm on Akcay. J Water Resource Prot. 2014;2014.