Blepharoptosis as a Complication of Botulinum Toxin Injection: Understanding the Role of Neurovascular Variations
Received: 24-Jun-2023, Manuscript No. ijav-23-6554; Editor assigned: 27-Jun-2023, Pre QC No. ijav-23-6554; Accepted Date: Aug 31, 2023; Reviewed: 12-Jul-2023 QC No. ijav-23-6554; Revised: 28-Jul-2023, Manuscript No. ijav-23-6554; Published: 31-Aug-2023, DOI: 10.37532/1308-4038.16(8).290
Citation: Jameie SB. Blepharoptosis as a Complication of Botulinum Toxin Injection: Understanding the Role of Neurovascular Variations. Int J Anat Var. 2023;16(8):355-358.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Since the first approval of Botulinum Toxin A (BTX) for the treatment of eye muscle disorders in 1989, the use of BTX for cosmetic purposes has increased sharply. The procedure can lead to various complications. Fortunately, most of the complications of BTX cosmetic injection are generally mild and temporary. One of the more serious complications that can occur is upper eyelid ptosis. Although this type of ptosis is usually self-limiting and does not typically lead to the development of any secondary adverse effects, it is important to note that upper eyelid ptosis can be associated with impaired vision, diplopia, and hyperemia. In serious cases, series of treatments and procedures can be used to expedite recovery. Most of these complications can be avoided through the skill and knowledge, precise anatomical analysis of the individual patient, and thorough understanding of the product. The rate of ptosis due to cosmetic BTX injection can be as high as 6.5% if. However, even with appropriate care, there is still a small chance of ptosis that may be associated with anatomical and neurovascular variations. Provided that the necessary guidelines are followed, BTX injection remains a more effective and safer cosmetic procedure than its surgical counterpart.
Keywords
Botulinum toxin A; Ptosis, Blepharoptosis; Neurovascular anatomical variation
INTRODUCTION
Botulinum toxin (BTX) is an exotoxin produced by the bacterium Clostridium botulinum. The toxin itself was discovered in 1905 by Tchitchikine and was described as a neurotoxin [1]. Botulinum toxin has eight different serotypes (A, B, C alpha, C beta, D, E, F, and G) [2]. Out of these serotypes, only type A and B are approved for medical use. BTX-A is commonly used to control frown lines appearing predominantly in hyperactive superciliary and corrugator muscles. Dependent on the dosage, this effect would usually last around 3-6 months.
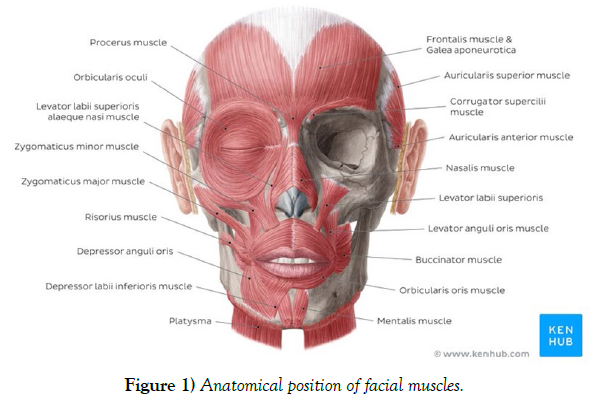
BTX is composed of two chains; the heavy chain binding to the nerve ending and a lighter chain having the role in toxifying [2]. This toxin mainly affects the striated muscles by inhibiting the release of neurotransmitter “Acetylcholine” at the neuromuscular junction. While having a high dosage of toxicity with a lethal dose of 10-9 g/kg body weight, it has been found that a pharmaceutically altered version of this toxin could be potentially used to temporarily disable a targeted muscle. This voluntary disability could be used for aesthetic or treatment usage. Although based on Allergan’s 2014 Annual report more than half of BTX injections had medical-related purposes, the cosmetic utilization has skyrocketed in the past decades as it is usually a lower risk alternative to surgeries for minor facial adjustments [7]. As of date, there are mainly four different FDA approved botulinum toxin products on the international market; Onabotulinum toxin A (Botox® (Allergan)), Abobotulinum toxin A (Dysport® (Speywood/Ipsen)), Incobotulinum toxin A (Xeomin), Prabotulinum toxin A (Jeuveau). The suggested conversion ratio for Botox to Dysport is 1:5 (100U in one vial of Botox vs 500U in one vial of Dysport product) [3]. The unit potency of Myobloc, a formulation of B subtype, is estimated to be 50-100 times lower than that of Botox. These products need to be maintained in the temperature of 2-8 °C. The normal dosage for the treatment of the glabellar lines is around 10-20U. The beneficial effects of these procedures usually last around 8 to 16 weeks. Injection sessions should be at least 3 months apart using the lowest effective dose to minimize the chances of any BTX antibody developing. Lower dosage would usually result in safer outcomes. Large dosage of drugs such as aminoglycoside could also have an inhibitory effect on the release of acetylcholine, which could be misinterpreted as a BTX injection complication. BTX is not subjected to any major drug interaction; however contradictions to neuromuscular drugs could be possible. Quinolones such as Chloroquine were also reported to have an inhibitory reaction on BTX. Large dosage, long-term treatment, and short intervals between the injections could lead the body in developing antibodies resistant to BTX. This resistance was seen in 5-10% of the patients [4]. Additionally, lower protein load per doses since the year 1997 onward decreases the less likelihood of antibody development [5]. The effects of BTX on long-term immunologic pathology are not yet clear. It is not expected for BTX to be presented in measurable quantities in peripheral blood following proper injection procedure (Stern et al., 2018). Since Botox has been categorized as a “C” class drug by the FDA, utilization for cosmetic purposes is not yet approved during pregnancy. Cosmetic Botox injections are not recommended in children under the age of 18 (Stern et al., 2018). Although low risk, there are some complications that could potentially arise mainly due to the injection nature of this procedure. The most common side effects in BTX injection include bleeding, headache, bruising, swelling, and pain, which can be controlled by administering the lowest possible concentration of the product and the utilizationof thinner needles for the injection. Upper eyelid ptosis is one of the more common adverse effects that are mostly associated with poorly administration of Botox mainly to the glabellar complex [6]. The main cause of this complication is thought to be the diffusion of this toxin to the surrounding area and therefore affecting the Levator palpebral muscle. Although this type of ptosis doesn’t normally require any special treatment, it could be quite difficult for the patient to do day-to-day tasks. This adverse effect is even more critical in patients with jobs in which the patient is more publicly presented. This review article aims to thoroughly discuss the cause, prevention, and suggested treatments of the upper lid ptosis as an unfavorable outcome in cosmetic utilization of Botulinum Toxin (Figure 1).
PATHOLOGY
Blepharoptosis is a condition characterized by the drooping of the eyelid, which can potentially interfere with the patient’s vision. The severity of the condition can be categorized as mild (1-2 mm), intermediate (3-4 mm), or severe (>4 mm). Besides the aesthetic outcome, ptosis may cause pain, excessive secretion in the lacrimal glands, and hyperemia. Ptosis may worsen during the daytime. The condition can have neurogenic, myogenic, aponeurotic, traumatic, or mechanical causes, and it can occur isolated or in association with infections, hereditary causes, immunologic problems, and degenerations. Currently, the most common cause of acquired ptosis is the complication due to the injection of botulinum toxin (BTX), particularly when injected into the glabellar complex, as the toxin can diffuse to the surrounding area. Early symptoms of ptosis, such as the feeling of heaviness in the eyelid, can appear as early as 48 hours after the procedure and can take up to 2 weeks to fully develop. Ptosis is mainly unilateral but can also develop bilaterally, and in some cases, it can start on one side and spread to the opposite. Other similar complications, such as Pseudo Ptosis, can result from redundant skin, mainly observed in elderly patients. In a cross-sectional review study, out of 10,577 cases of complications due to Botulinum Toxin A injection, Ptosis was reported in 6.1%. Unlike other complications, blepharoptosis was not observed in any of the control groups in experimental trials. It was discovered that the administration of onabotulinumtoxinA [Botox®, Vistabel®] has a slightly greater chance of developing adverse effects such as pain, swelling, and ptosis. Diplopia was also reported in 3% of patients when treating muscle spasm with BTX. Based on the data from Carruthers et al., blepharoptosis was reported in 6.5%. It should be noted that the chance of re-occurring ptosis in repeated injection sessions tends to decrease. The rate of ptosis was 3%, 2.2%, and 0.8% after the first, second, and third treatment sessions. Based on a study conducted on 56 subjects, including 20 with repeated injection sessions every 3-6 months over a period of 1-3 years, there was no record of long-term blepharoptosis. Although the adverse effects of BTX on nerves are not entirely understood, a study with direct injection to the nerve in rats resulted in no reported damage. It is also noteworthy that ptosis could be voluntarily induced for corneal protection or eyelid fissure asymmetry correction. MRD1 (Margin Reflex Distance), which measures the distance between the upper lid margin and the corneal distance, is the most common protocol for assessing the severity of ptosis. The normal value of MRD is ranging from 4 to 4.5 mm. Another method of identifying the severity of ptosis is LF (Levator Function), which is defined by the measurement of the elevation of the upper lid margin, with a normal amount being around 15mm. Due to its objective view, MRD1 is easier to use as it does not require any voluntary movement by the patient. Serious adverse outcomes of botulinum injection are more common in patients older than 45 years of age and weighing more than 130 lb. Upper eyelid ptosis does not increase the odds of developing any other serious outcome. In patients with neuromuscular diseases, injection of Botox should be at the lowest dosage and treated with extra caution. Based on two experimental trials conducted by Carruthers et al., it was determined that individuals under the age of 50 respond better to Botox treatment. However, there is a suggestion that the efficacy of a 20U Botox dosage seems to decrease with age. Patients older than the age of 75 are more susceptible to experiencing underlying neuromuscular conditions. Crist reports a 57-year-old woman with a history of Bell’s palsy who developed upper eyelid ptosis after Botox treatment for frown lines. In this particular case, the patient also had diminished depth perception. It was also determined that female patients had a higher response to the treatment than male patients. Patients with any type of muscle dystrophy should be excluded from the procedure. In most cases of lid ptosis, the Herring law applies. Ptosis is usually benign and does not necessarily require any treatment. The process of non-interfered healing can take from 2 to 4 weeks [8].
CAUSE
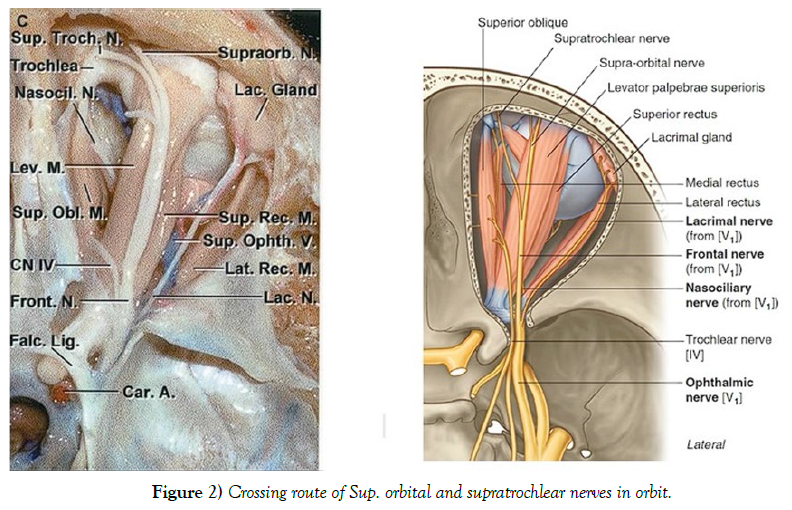
The main muscles targeted for injection with botulinum toxin (BoNT) are the Frontalis, Orbicularis Oris, Corrugator, Temporalis, and Procerus muscles. The primary cause of ptosis resulting from BoNT injection is believed to be the diffusion of the toxin into the surrounding area of the procedure, reaching two main muscles responsible for elevating the upper eyelid: the Levator Palpebrae Superioris (LPS) and Müller’s muscle. The LPS muscle is more susceptible to minute volumes of diffused toxin as it possesses a limited number of motor end plates. There are two possible theories surrounding the mechanism of this diffusion. Firstly, the toxin may reach the LPS muscle as it travels through the Orbital Septum. However, the mechanism of this effect and the ability of the toxin to travel through the fibrous tissue of the septum is not yet fully understood. Secondly, the toxin may be diffused through tributary branches of the supraophthalmic neurovascular structures, mostly traveling parallel to the LPS muscle, which is mainly caused by deep injections. Recently, the possibility of BoNT diffusing through neural networks has been raised since reciprocal inhibition between two agonist and antagonist muscles has been reported after peripheral injection. It has also been established that BoNT A and E could travel retrograde in the axons of neurons, possibly diffusing the toxin to adjacent areas. Since the supraophthalmic nerve travels superiorly to the LPS muscle, the potential retrograde movement and diffusion of the toxin entering the nerve could have a role in paralyzing the muscle. The diffusion of the toxin through the supraophthalmic vein is another possibility, but it is less likely since this vein travels underneath the superior rectus and LPS muscle, leaning towards higher chances of affecting both muscles. However, there are no notable complaints of interference in the function of the superior rectus muscle following a BoNT injection. The accuracy of these theories is still unclear due to the lack of studies on living patients and the unavoidable non-physiologic conditions present in a cadaver. These effects are more common in patients with absent or reduced Orbital Septum, mostly in elderly patients. A secondary ptosis often occurs in patients who already use the Frontalis muscle to uplift the upper eyelid (by about 1 mm). This effect causes significantly more frontal wrinkles, increasing the referral for an injection, which contradictorily leads to a mild ptosis due to the paralysis of the Frontalis muscle. Disability of the Frontalis muscle could also have a role in brow ptosis, which could be mistaken for an eyelid ptosis. This is more common in patients who normally use this muscle to lift the brows for better vision. There is no evidence suggesting that upper eyelid ptosis would cause any permanent degeneration or atrophy in the muscles of the patients involved (Figure 2).
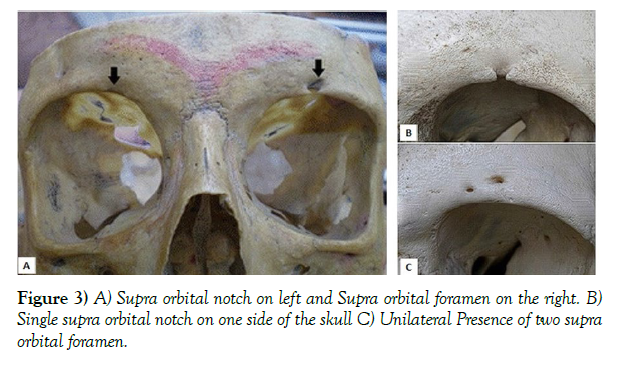
Neurovascular and anatomical foramina variations in different skull types could potentially play a role in the development of ptosis after botulinum toxin (BoNT) injection. The presence of a supraorbital foramen instead of a notch could be one such variation. Because the foramen is located at a higher distance to the supraorbital ridge than a notch, the supraorbital nerve and adjacent artery and veins could be more exposed at a superficial level, increasing the chances of blepharoptosis. Based on multiple studies done on different skulls with a variety of ethnicities, it was found that the chances of a foramen being present instead of a notch are estimated at around 25%. In most cases, the foramen was only present on one side of the skull, which could somewhat explain the slightly higher occurrence of unilateral ptosis with enough attention to the procedure. Based on a study done by Tomaszewska et al., it was discovered that Europeans in colder climates have a higher chance of this variation than their warmer counterparts. Another study on different variations in the foramina of 110 skulls (70 men and 40 women) showed that females had a higher number of supraorbital foramen instead of a notch. It should also be mentioned that the distance of this foramen or notch to the midline of the face could have an effect on the development of this complication as well. (Figure 3) (Table 1).
| Single Foramen (N, %) | Single Notch (N, %) | Double Exits (N, %) | Total | |
|---|---|---|---|---|
| Nanayakkara 2018 | 31(26.72) | 85(73.28) | N/A | 116 |
| Agthong 2005 | 90(44.33) | 102(50.25) | 11(5.42) | 203 |
| Saylam 2003 | 100(27.50) | 281(70.25) | 9(2.25) | 390 |
| Webster 1986 | 83(38.43) | 133(61.57) | N/A | 216 |
| Chung and Kim 199550 | 87(70) | 37(30) | N/A | 124 |
Table 1) Morphological aspect of supra orbital nerve exits based on different studies.
PREVENTION
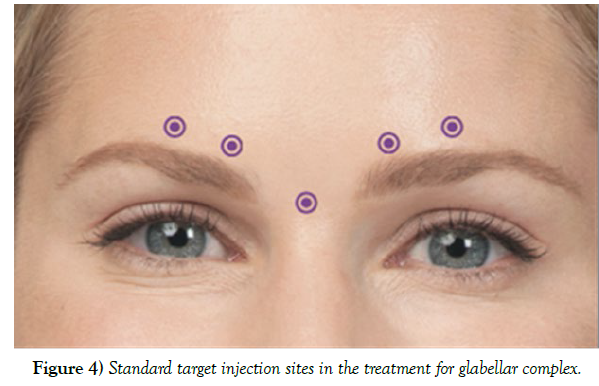
The percentage reported by the FDA for the occurrence of upper lid ptosis due to botulinum toxin (BoNT) injection performed on 264 patients was 5.4%. However, this number was achieved mostly by inexperienced injectors and using proper techniques can reduce this number to less than 1%. Based on a review article by Cavallini et al. on 35 other articles with over 8000 patients, the percentage of patients developing ptosis was estimated at 2.5%. For minimizing the risk of developing ptosis, a full medical history (including any pre-existing conditions) and photographs must be taken before the procedure. Patients’ allergies to BoNT and other products used to manufacture the injection (such as egg proteins or human albumin) should be taken into consideration as they may cause serious complications or even fatality. In case of such a reaction, further injection should be discontinued, and immediate medical attention should be sought. Based on published data from open studies, Dysport® (6.6%) has a higher percentage of associated ptosis than Botox® (1.4%). Proper assessment of the complete anatomy of the patient’s face plays a key role in reducing the chances of any potential problems arising post-procedure. BoNT injection should be avoided in patients with severe dry eyes or patients with previous multiple eyelid surgery as it could develop further complications. It is also important that patients fully understand the procedure to understand the risks of any potential complications and the elimination of any unachievable expectations. As discussed before, any effect of the frontalis muscle in lifting the upper lid could increase the chance of lid and brow ptosis [9]. The most common factors affecting the chances of ptosis developing during the procedure include muscle mass of the area injected, injection volume, speed of injection, needle gauge, and, most importantly, the usage of standard technique and the experience of the injector. Since this type of ptosis mainly occurs while treating the glabellar complex, it is important that the needle be entered pointing superiorly and from a safe distance from the brow, which is around 1cm. Injection should be placed superiorly and laterally to the orbital rim. Lid ptosis could develop from a misplaced lateral injection on the nasal bridge. Also, the mid-pupillary line should not be crossed at any given time as it could cause brow ptosis resulting from frontal muscle disability. The denervation radius of each injection is usually about 2.5-3 cm. Injection should be subdermal as it has a lesser chance of diffusion without any effect on the beneficial outcome. Avoiding deep injection is crucial due to absorption of toxin through the supraorbital vein and its branches passing adjacent to the levator palpebral muscle. Selecting the lowest volume and applying pressure using the non-injecting hand is also effective in reducing the chance of the toxin diffusing. Inexperienced injectors are advised to avoid treating the horizontal and frown lines at the same time. However, for an experienced user, it is recommended to treat the two areas at once for a more effective outcome. In cases where the injection sites need to be optimized, pre-orbital injection sites are recommended to be outside the “Orbital Zone” for minimizing the risk of developing complications (Figure 4).
Figure 4) Standard target injection sites in the treatment for glabellar complex.
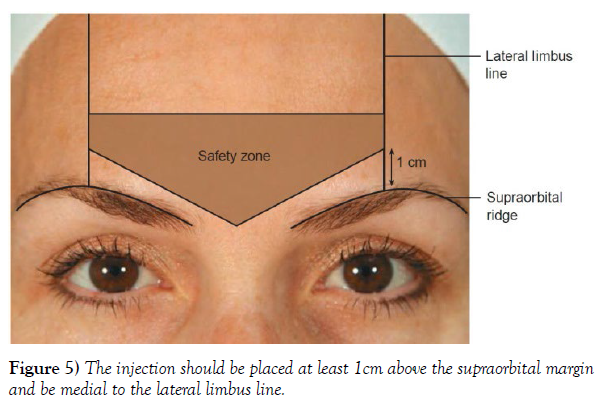
In a different injection technique demonstrated by Ghalamkarpour et al., injection above the medial brow head is replaced with the injection at the superior middle aspect of the corrugator muscle, minimizing the risk of the toxin diffusing to the lid-lifting muscles. The new technique was a better option for inexperienced injectors and patients with a history of upper lid ptosis. However, the utilization of this method is questionable since the initial success rate of the injection appears to be lower than the standard technique. Additionally, the low number of subjects in the study could have interfered with the results, since the percentage of patients developing blepharoptosis (BP) with the standard technique is already rather low. Another plausible technique to counter the development of BP caused by diffusion would be the utilization of a potentially new alternative pathway for BoNT proposed by Bomba-Warczak et al., which could eliminate the possibility of the diffusion of the toxin through the supraorbital nerve. Lastly, EMG-guided techniques can help improve the accuracy of the injections further, thereby minimizing the chances of complications, although this technique is not considered necessary by most authorities. Patients should be informed to maintain an upright position and avoid manipulating the area of injection for at least 4 hours after the procedure. Avoiding warm environments such as saunas and sunbaths is also recommended, as they could help develop certain issues. However, repeating certain facial expressions ten times an hour for 4 hours is recommended, as it can boost the entrance of the toxin to the nerve junctions of the injected areas (Figure 5).
TREATMENT
Although this type of ptosis is self-limiting and would normally disappear in 2-4 weeks without any special medical intervention, the facial asymmetry could be quite unappealing to the patient. In some cases, such as actors and politicians in which the facial presentation is vital, prescription of external medicine could be justified for a swifter recovery. Iopidine 0.5% (apraclonidine; Alcon Labs, Fort Worth, TX) eye drops with a dosage of 1 to 2 drops three times a day is the main medication prescribed in the treatment of ptosis. Other Alpha-adrenergic agonist ophthalmic drops such as Neosynephrine hydrochloride 2.5% can also be practical when used on the affected side. These agents, for the most part, cause the contraction of the smooth Müller muscle, which helps lift the upper eyelid by approximately 1-2 mm. This amount is often enough to achieve a relatively symmetrical face until the effects of ptosis wear off. Another treatment could be the first antitoxin for botulism (BAT; Emergent BioSolutions Canada Inc., Winnipeg, MB, Canada), which became commercially available in the United States in 2013, although it is not routinely used in clinical practice. Antidotes are mainly used in cases of accidental poisoning or an overdose, which are extremely rare in the case of cosmetic procedures due to lowvolume injections. In a study done in 1997, human immune globulin was extracted and used on 33 patients experiencing ptosis due to BTX injection. The study showed promising results, with the percentage of ptosis reducing to 11% compared to 37% for the control group without any repercussion on the intended beneficial effect of the original cosmetic procedure. This method was also used in one case of wrong muscle injection in the treatment for strabismus. The injection was done one hour after the initial injection, and it successfully prevented the paralysis of the muscle. Another helpful approach while treating with the aforementioned medications is the lowtreatment of the levator antagonist muscle (orbicularis oculi), which could provide additional aid in reducing the appearance of ptosis. Utilizing the treatments listed could significantly reduce the duration of this condition, even to just a few days [10].
CONCLUSION
Ptosis is one of the more common complications due to cosmetic Botox administration. The main cause of this occurrence is thought to be the diffusion of the toxin to the surrounding area, disrupting the muscles involved in lifting of the upper lid. The side effect of BTX A injection leading to blepharoptosis due to this diffusion could potentially be controlled by special care during and after the procedure. In cases of blepharoptosis, with enough attention to the procedure, it would seem that the possibility of neurovascular and anatomical variations in different individuals could have a role to play. The complication usually takes 3-7 days to develop and patients need to be closely monitored.
Currently, there is no officially recommended treatment for this condition, but there are some medications that can speed up the process of recovery or help restore facial symmetry until the patient recovers from the effect. The main way to prevent this complication is to utilize the standard guidelines and be fully aware of the procedure.
REFERENCES
- Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A: a focus on cosmetic procedures. Am J Clin Dermatol. 2005; 6(3):141-150.
- Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001; 20(2):109-120.
- Eleopra R, Tugnoli V, Quatrale R, Rossetto O et al. Different types of botulinum toxin in humans. Mov Disord. 2004; 199(8):53-S59.
- Vartanian AJ, Dayan SH. Complications of botulinum toxin a use in facial rejuvenation. Facial Plast Surg Clin North Am. 2005; 13(1):1-10.
- Odergren T, Hjaltason H, Kaakkola S. A double blind, randomised, parallel group study to investigate the dose equivalence of Dysport and Botox in the treatment of cervical dystonia. J Neurol Neurosurg Psychiatry. 1998; 64(1):6-12.
- Ranoux D, Gury C, Fondarai J, Mas JL et al. Respective potencies of Botox and Dysport: a double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2002;72(4):459-462.
- Carruthers A. Botulinum toxin type A: history and current cosmetic use in the upper face. Dis Mon. 2002; 48 (5): 299-322
- Frampton, JE, Easthope SE. Botulinum toxin A (Botox Cosmetic): a review of its use in the treatment of glabellar frown lines. American journal of clinical dermatology.2003; 4(10):709–725.
- Wang YC, Burr DH, Korthals GJ, et al. Acute toxicity of aminoglycosides antibiotics as an aid to detecting botulism. Appl Environ Microbiol. 1984; 48:951– 5.
- Lange DJ, Rubin M, Greene PE, et al. Distant effects of locally injected botulinum toxin: a double-blind study of single fiber EMG changes. Muscle Nerve. 1991; 14:672– 5.
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref
Indexed at , Google Scholar , Cross ref