Brain metastasis from ovarian cancer
Received: 11-May-2023, Manuscript No. Pulcmr-23-6419; Editor assigned: 12-May-2023, Pre QC No. Pulcmr-23-6419 (PQ); Accepted Date: May 26, 2023; Reviewed: 20-May-2023 QC No. Pulcmr-23-6419 (Q); Revised: 24-May-2023, Manuscript No. Pulcmr-23-6419(R); Published: 28-May-2023, DOI: 10.37532/pulcmr-2023.6(2).68-71
Citation: Herrmann T, Jahanmohan JP, Molnar L, et al. Brain metastasis from ovarian cancer. J Cancer Metastasis Res. 2023; 6(2):68-74.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: Brain metastasis from ovarian cancer is a rare phenomenon and only a few studies have focused on it. The present trial aimed to evaluate the predictive factors involved in its development, and the outcomes for women with this kind of evolution, based on exploration of real-life data.
METHODS: This was a single-center retrospective study conducted on data of fifty women with brain metastasis from ovarian cancer treated from 2001 to 2021.
RESULTS: The presence of a high-grade serous carcinoma was a significant factor for the development of secondary brain lesions. Older age at metastatic diagnosis and platinum sensitivity were good predictive factors. Surgery of the primary lesion was also a significant factor for good prognosis. In multivariate analysis age at brain metastasis, more than 2 lesions and sensitivity to platinum salts were three significant prognostic factors contrary to the biomarker CA 125, the initial stage, and the number of previous lines of treatment. The prognosis with this dissemination remains poor, with a median overall survival of 14 months.
CONCLUSION: This study provided real-life data with clinical characterization and an evaluation of therapeutic strategies in case of encephalic involvement. Age at brain metastasis and the presence of more than 2 lesions were factors for poor prognosis, whereas sensitivity to platinum salts appeared to be a factor for good prognosis. This study should be extended with the use of larger databases and a more detailed molecular investigation to adapt the therapeutic management for this category of patients.
Key Words
Neoplasm metastasis; Brain; Ovarian cancers; Risk factors; Real-life
Introduction
Ovarian Cancer (OC) ranks as the seventh cause of cancer among women in developed countries [1]. It can affect women of any age, but OC incidence increases with age. The prognosis of this malignancy is poor with a five-year survival rate below 50% [2]. However, there is variability according to the histological type [3]. Epithelial tumors account for around 80% of OCs. Among these, several types have been identified [4]. High-grade Serous Carcinomas (HGSC) are the most frequent, reaching 70% of all OCs. This subtype has the poorest prognosis [3]. In 70% of cases, diagnosis is made in the advanced stages, with no symptoms, or symptoms that are not clinically suggestive, explaining the poor pronosis.
Cancer cells in ovarian carcinomas spread via several routes. Direct extension from the ovary is common and could explain the development of metastasis in the peritoneum. Transperitoneal dissemination is less frequent and leads to the occurrence of distant metastases (omentum, bowel, spleen, or pelvic organs for example) [5]. In 70% of cases, pelvic or para-aortic node involvement is found via lymphatic dissemination. More rarely, dissemination can occur via the hematogenous system, and affect sites such as the pleura, the liver or the lung [6]. In even rarer cases, other secondary locations have been reported, such as the skin, the heart, or encephalic metastases. Invasion of the Central Nervous System (CNS) is rare. It was first described by Mayer et al. in 1978 [7]. Since then, a few studies have been carried out and have suggested an increase in incidence in recent years. This increase could be explained by improved imaging, but also by better outcomes with the new treatments available for ovarian cancer, allowing metastatic dissemination to other sites. Brain metastases from OCs account for 0.49% to 6.1% according to the literature [8]. Neurological symptoms are variable: headaches, seizures, or neurological disturbances. Currently, there is no established reliable marker. The biomarker CA 125, used for peritoneal relapse, is not a predictive marker for the occurrence of brain metastases. Significant risk factors have been identified, such as distant spread at the time of encephalic metastases [9], multiple brain lesions, or a short interval between initial diagnosis and CNS involvement [10]. In contrast, low tumor grade, platinum sensitivity or good performance status have been established as favorable prognostic parameters [10]. The management of CNS metastases is based on radiotherapy. When possible, a tri-modal approach with surgery, radiotherapy, and chemotherapy is implemented, improving outcomes for patients [11]. Nevertheless, no treatment guidelines have been clearly established, and the prognosis remains poor once brain metastases have been diagnosed. Additional data is required to improve diagnosis and therapy.
In this study, we analyzed all cases of brain metastases among patients with OC at the Jean Perrin Center in Clermont-Ferrand. The primary objective was to obtain real-life data and characterize brain metastasis in OC. Secondly, the aim was to define risk factors for the development of brain metastases as well as factors for poor prognosis
Methods
Population
A retrospective single-center study was conducted at the Jean Perrin Center in Clermont-Ferrand among patients treated for ovarian cancer from 2001 to 2021. All patients were selected from the database using the following keywords: "ovarian cancer" and "brain involvement". Among the 2553 patients, we detected 50 patients with brain metastases, who were included in the study. Each patient was informed about the research by a non-opposition letter. They were free to oppose the use of their personal medical data. In compliance with French legislation, the database was notified to the CNIL (the French regulatory body for data privacy). Ethics approval for the study was obtained on 10th June 2022 (IRB 00013040).
Data collected
From the patients’ electronic medical records, the following patient medical data was collected: age at first diagnosis, personal and family history, symptoms revealing OC, histology, stage, surgery and margins, treatment used for primary cancer, date of CNS involvement, number of previous lines of treatment, use of bevacizumab or not, platinum sensitivity, number of lesions and localization, neurological symptoms at metastasis diagnosis, CA 125 at diagnosis and at brain metastases diagnosis, management of the brain metastases, and date of death or the latest follow-up assessment. There was no distinction between death caused by systemic ovarian cancer, death caused by the progression of brain metastases or nonspecific death on medical records.
Statistical Analysis
Statistical analyses were performed using R software (v. 4.1.0, RProject, GNU GPL). Hypothesis testing was two-sided, at a significance level of 5%. Categorical variables were expressed in absolute and relative frequencies, and continuous variables were expressed as means and Standard Deviation (SD), or medians and interquartile range (according to data distribution). The normality of the continuous variables was assessed using the Shapiro-Wilk test. Overall survival after brain metastases were defined as the time between diagnosis of brain metastases and death regardless of cause and patients who were alive at the time of the latest follow-up were statistically censored. Event-free survival was calculated as the time between the initial diagnosis of ovarian cancer and diagnosis of brain metastases. Kaplan-Meier’s method was used for survival rate estimation, and the comparison between survival curves used the logrank test. Cox regressions were used to assess the association between risk factors and survival time. Multivariate Cox models were fitted using a variable selection method based on a LASSO-type penalized regression including all factors with a p-value< 0.2 in the univariate analysis.
Results
Cohort characteristics
Fifty patients were included in the study among the 2553 ovarian cancer patients (2%). The cohort characteristics are shown in Table 1. The median age at OC diagnosis was 57.7 years (min 43.4; max 84.4). The main histological type was High-Grade Serous Ovarian Cancer (HGSOC) (76%). Stage III-IV accounted for 92% of the cases. CA 125 at OC diagnosis was 76 to 3215, with a median at 819 UI. At OC diagnosis, the first management was based on surgery and chemotherapy with neoadjuvant chemotherapy for 66% of the cohort. Initial chemotherapy included platinum salts in every case. Half of the cases received bevacizumab and 21 patients had received it before the onset of brain metastases. The median disease-free interval between primary diagnosis and CNS involvement was 34.5 months (IC 95%) with a median age at metastatic diagnosis of 61.8 (min 47.3; max 87.5). Patients had received between 0 and 9 treatment lines prior to the brain metastatic stage, including treatment with polyADP Ribose Polymerase Inhibitors (PARP-i). Platinum sensitivity was defined as time between the last platinum salt injection and disease relapse beyond 6 months. Thirty-seven patients responded to platinum salts, amounting to 77.1% of our cohort. The mutation status for BRCA was known only for about half of the patients because of late diagnosis or lack of material. Among the 26 cases where a genomic study could be performed, there were 9 patients with a somatic or constitutional BCRA mutation.
TABLE 1. Patient characteristics
| Characteristics | N | % | ||
|---|---|---|---|---|
| Median age at diagnosis (years) [range] | 57,7 (43,4-84,4) | - | ||
| Histology | - | - | ||
| High-grade serous carcinoma | 38 | 76 | ||
| Poorly differentiated non-mucinous | 1 | 2 | ||
| Clear cell adenocarcinoma | 3 | 6 | ||
| Mucinous adenocarcinoma | 1 | 2 | ||
| Endometrioid adenocarcinoma | 6 | 12 | ||
| Large-cell carcinoma | 1 | 2 | ||
| Initial stage | - | - | ||
| IV | 17 | 34 | ||
| IIIC | 28 | 56 | ||
| IIIA | 1 | 2 | ||
| IC | 3 | 6 | ||
| Unknown | 1 | 2 | ||
| Median CA 125 U/ml [range] | - | - | ||
| At diagnosis | 819 (424-1628) | - | ||
| At brain metastases | 95 (8-1710) | - | ||
| BRCA mutation | - | - | ||
| Yes | 9 | 18 | ||
| No | 17 | 34 | ||
| unknown | 24 | 48 | ||
| Associated breast cancer | - | - | ||
| Yes | 5 | 10 | ||
| No | 44 | 88 | ||
| Unknown | 1 | 2 | ||
| Neoadjuvant chemotherapy | - | - | ||
| Yes | 33 | 66 | ||
| No | 16 | 32 | ||
| Unknown | 1 | 2 | ||
| Surgery | - | - | ||
| Yes | 46 | 92 | ||
| No | 4 | 8 | ||
| Residual disease | - | - | ||
| R0 | 27 | 59 | ||
| R1 | 12 | 26 | ||
| R2 | 3 | 6 | ||
| Unknown | 4 | 9 | ||
| Adjuvant chemotherapy | - | - | ||
| yes | 50 | 100 | ||
| no | - | - | ||
| PARP inhibitor | - | - | ||
| yes | 16 | 32 | ||
| no | 34 | 68 | ||
| Previous lines of treatment before brain lesions | - | - | ||
| 0 | 2 | 4 | ||
| 1 | 23 | 46 | ||
| 2 | 6 | 12 | ||
| 3 | 5 | 10 | ||
| 4 | 6 | 12 | ||
| 5 | 3 | 6 | ||
| 6 | 2 | 4 | ||
| 7 | 1 | 2 | ||
| 9 | 1 | 2 | ||
| Unknown | 1 | 2 | ||
| Symptoms at CNS involvement | - | - | ||
| Headaches | 14 | 28 | ||
| Seizures | 11 | 22 | ||
| Sensory-motor deficit | 13 | 26 | ||
| Dysarthria | 5 | 10 | ||
| Vertigo | 10 | 20 | ||
| Memory issues | 3 | 6 | ||
| Unknown | 2 | 4 | ||
| Number of lesions | - | - | ||
| 1 | 17 | 34 | ||
| > 1 and ≤ 5 | 16 | 32 | ||
| > 5 | 16 | 32 | ||
| Carcinomatous meningitis | 1 | 2 | ||
| Histological confirmation | -- | - | ||
| Yes | 12 | 24 | ||
| No | 36 | 72 | ||
| Unknown | 2 | 4 | ||
| Treatment for brain metastasis | - | - | ||
| Neurosurgery | - | - | ||
| In combination | 9 | 18 | ||
| Alone | 9 | 18 | ||
| Radiotherapy | - | - | ||
| In combination: WBRT | 30 | 60 | ||
| In combination : stereotactic RT | 8 | 16 | ||
| Chemotherapy in combination | 35 | 70 | ||
| Monitoring | 3 | 6 | ||
| Unknown | 1 | 2 | ||
Seventeen patients had a single brain lesion (34%), 16 had between 2 and 5 brain lesions (32%), 16 had more than 5 brain lesions (32%) and only one patient had meningeal involvement (2%). Brain metastases were symptomatic in the majority of cases (96%) with headaches, seizures, sensory-motor deficit, dysarthria, vertigo, or memory issues. The association of neurological symptoms was frequent. Histological confirmation was provided in 24% of cases from biopsies or surgical removal. At brain metastases diagnosis, CA 125 was 8 to 1710, with a median at 95 UI.
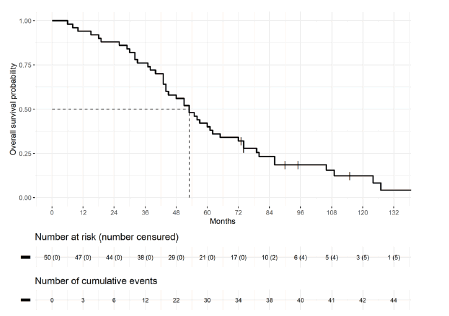
Several treatment options were implemented for CNS metastases: radiotherapy alone in 9 cases (18%), simple monitoring in 3 cases (6%), and combined therapies for 76%. Combined therapies included surgery plus radiotherapy, surgery plus radiotherapy and chemotherapy, or radiotherapy plus chemotherapy. Sixteen patients received PARP-i as part of their treatment. All BRCA-mutated patients received olaparib, corresponding to 9 patients in the cohort. Niraparib was the other prescribed PARP-i. Depending on the brain lesions, patients were treated with Whole Brain Radiotherapy (WBRT) or stereotactic radiotherapy, with 40 patients and 8 patients, respectively. Median overall survival from diagnosis of the primary ovarian cancer was 53 months [(IC 95%) 44-72]. At the end of analyses, five patients were still alive (Figures 1 and 2).
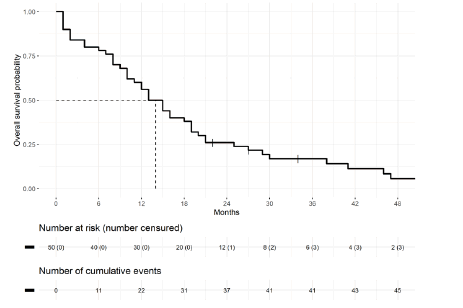
Figure 2: Overall survival from the diagnosis of brain metastases in ovarian cancer
BRCA: Breast Cancer Gene, CA 125: Cancer Antigen 125, CNS: Central Nervous System, PARP: Poly (ADP-ribose) Polymerase, RT: Radiotherapy, WBRT: Whole Brain Radiotherapy
Predictive factors for CNS involvement
In univariate analysis, the statistically significant predictive factors highlighted were platinum sensitivity (p<0.001) and age at brain metastasis diagnosis (p=0.018). The biomarker CA 125 and the use of bevacizumab were not statistically significant factors.
In addition, in this univariate analysis, factors for primary lesion surgery, the histology of High-Grade Serous Carcinomas (HGSC), and the initial stage of the disease had a p-value <0.2 and were therefore considered for the multivariate analysis.
In the multivariate Cox regression, the presence of an HGSC was a significant factor for the development of secondary brain lesions with HR=3.7 IC 95% [1.37-9.88] (p=0.009). Older age at metastatic diagnosis and platinum sensitivity were factors for good prognosis with p<0.001 (HR=0.93; IC 95% [0.89-0.96] and HR = 0.1 IC 95% [0.03-0.28], respectively). Surgery of the primary lesion was also a significantly good prognostic factor (HR=0.23; IC 95% [0.06-0.85] p = 0.027) in this model (Table 2).
TABLE 2. Prognostic factors for central nervous system involvement in ovarian cancer from multivariate analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI of HR | p-value | HR | 95% CI of HR | p-value |
| HGSC | 0.5 | 0.24– 0.99 | 0.062 | 0.56 | 0.24 – 1.23 | 0.16 |
| Age at brain metastasis | 1.045 | 1.009 – 1.082 | 0.014 | 1.045 | 1.004 – 1.088 | 0.032 |
| diagnosis | ||||||
| CA 125 at brain metastases diagnosis | 1.001 | 1 – 1.002 | 0.021 | 1.001 | 1 – 1.002 | 0.114 |
| Number of previous lines of | 1.209 | 1.03 – 1.42 | 0.023 | 1.19 | 0.97 – 1.46 | 0.09 |
| Treatment | ||||||
| More than 2 brain lesions | 1.72 | 0.89 – 3.3 | 0.09 | 2.39 | 1.1 – 5.2 | 0.28 |
| Platinum sensitivity | 0.31 | 0.15 – 0.65 | 0.004 | 0.26 | 0.11 -0.58 | 0.001 |
The median overall survival once the brain metastatic diagnosis was made was 14 months. In the multivariate model, three parameters were significant prognostic factors for death from brain metastases from OC: age at brain metastasis diagnosis (HR=1.045; IC 95 [1.004- 1.088] p=0.032), more than 2 brain lesions (HR=2.39; IC 95 [1.1-5.2] p=0.028) and sensitivity to platinum salts (HR=0.26; IC 95 [0.11- 0.58] p=0.001). The biomarker CA 125, the initial stage, and the number of previous lines of treatment were not statistically significant for outcomes with encephalic involvement from OC (Table 3).
TABLE 3. Evaluation of the prognostic factors for overall survival after diagnosis of brain metastases in ovarian cancer in multivariate analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI of HR | p-value | HR | 95% CI of HR | p-value |
| HGSC | 0.5 | 0.24 – 0.99 | 0.062 | 0.56 | 0.24 – 1.23 | 0.16 |
| Age at brain metastasis | 1.045 | 1.009 – 1.082 | 0.014 | 1.045 | 1.004 – 1.088 | 0.032 |
| diagnosis | ||||||
| CA 125 at brain metastases diagnosis | 1.001 | 1 – 1.002 | 0.021 | 1.001 | 1 – 1.002 | 0.114 |
| Number of previous lines of | 1.209 | 1.03 – 1.42 | 0.023 | 1.19 | 0.97 – 1.46 | 0.09 |
| treatment | ||||||
| More than 2 brain lesions | 1.72 | 0.89 – 3.3 | 0.09 | 2.39 | 1.1 – 5.2 | 0.28 |
| Platinum sensitivity | 0.31 | 0.15 – 0.65 | 0.004 | 0.26 | 0.11 -0.58 | 0.001 |
Discussion
This retrospective single-center trial evaluated the characteristics of brain metastases in ovarian cancer patients diagnosed from 2001 to 2021. Brain metastasis in ovarian cancer is rare, accounting for less than 6% of the cases [8]. On average, patients were 61.8 years old at the time of brain involvement with a median free interval from primary diagnosis of 34.5 months. These observations were consistent with other published data. Indeed, the development of brain metastases varies according to studies from 11 months to 46 months [12–14]. As expected, metastases were symptomatic for a large majority of the patients, with headaches, seizures, sensory-motor deficit, dysarthria, vertigo, or memory issues. These symptoms are suggestive of brain involvement. Thomakos et al. highlighted the fact that the diagnosis of metastases was confirmed in 90% of cases when these neurological symptoms appeared [5]. These symptoms can be confused with Paraneoplastic Neurological Syndrome (PNS). In ovarian cancer, PNS is most often characterized by a sub-acute cerebellar degeneration and other neurological symptoms [15]. PNS can be a telltale sign of neoplasia occurring a few months or even years after diagnosis of ovarian primary cancer [16, 17].
According to the literature, PNS is rare in cases of HGSC. Unlike brain metastases diagnosed by imaging, PNS can be highlighted by the presence of onconeuronal antibodies such as anti-Yo,anti-cdr2- like, or anti-NMDAR antibodies [18–20]. The treatment remains the same but the prognosis is different with a poorer prognosis in case of proven cerebral metastases. In this series, 34% of the patients had a single metastatic site, which was concordant with other research [14, 21, 22]. This parameter appeared to be a factor for good prognosis in the multivariate analysis. After CNS involvement, prognosis remained poor with a median overall survival of 14 months. Although outcomes were generally poor after metastatic diagnosis, overall patient survival was longer in our study than that reported in the literature [12, 13, 21]. The patients were mostly treated in a multimodal approach combining surgery, radiotherapy and systemic treatments. The increase in available therapies as well as the improvement of treatments used could explain in part this increase in survival [23]. Indeed, 8 patients were eligible for stereotactic radiotherapy in this trial. This technique is defined as a “high precision” irradiation technique. It delivers high doses (4 to 25 Gray) in a limited number of fractions (usually 1 to 5) with a high dose gradient [24]. For several decades, the combination of Whole Brain Radiation Therapy (WBRT) and a stereotactic approach has provided improvements in overall survival for patients with brain metastases from solid cancers [23,25]. Due to its neurotoxicity, WBRT has been increasingly avoided in the therapeutic strategy, especially for cases with fewer than 5 brain lesions [25,26]. This localized irradiation technique enables new irradiations to be performed in case of recurrence, either by new stereotaxis or by WBRT [27-29]. Thus, local control is improved for a longer period of time.
In addition, 16 patients received chemotherapy plus PARP-i, such as olaparib or niraparib. The PARP-i molecules have now been approved for the management of ovarian cancer: olaparib, niraparib and rucaparib [30]. There is no evidence that these molecules can cross the blood-brain barrier. Nevertheless, case reports have shown that metastatic brain disease can be contained with the use of olaparib and niraparib [31, 32]. Moreover, in cases of breast cancer with BRCA mutations, adjuvant olaparib has shown a decrease in metastatic development, including in the CNS [33].
Among the 50 patients included in this study, histological evidence of the ovarian origin was available for [12]. Obtaining a cohort of this type is of interest because of its rarity. To our knowledge, very little is known about the genomics for brain involvement in ovarian cancer. In a retrospective trial, Balendran et al. analyzed 8 pairs of tumors with a cancer-specific enrichment kit covering 94 genes [34]. The authors highlighted a high proportion of variant alterations distributed over 22 genes, but each sequenced tumor presented a single molecular profile. As expected, the most frequent alterations involved homologous recombination and the TP53 gene.
Combinations of gene alterations have been identified in several cases such as TP53 and BRC A1; TP53 and ATM or TP53 and BRCA2. In contrast, there were more variants affecting BRCA1. This is concordant with Koul et al.â??s work and further suggests the value of anti-PARP drugs in the management of this metastatic evolution [35]. One of the limitations of this work was the small number of cases and the heterogeneity of the population, related to the long evaluation period. Indeed, the cases were collected over a period of more than 20 years when treatments available in ovarian cancer were undergoing a complete revolution, in particular with the advent of anti-PARP. Because of the small number of patients specifically treated with PARP inhibitors here, no relationship could be established. This particular point would be particularly interesting to evaluate, using larger databases. Because of this long collection period, a large number of data could not be collected or were not found (resection margin, BRCA status, therapeutic modalities). The histological confirmation of the ovarian origin is one of the strengths of this study. Further molecular analyses should be conducted using the histological material from this cohort. We plan to use a large sequencing panel and compare molecular profiles between the two sites. In addition to the potential genomic alterations highlighted, functional analyses will be conducted. These molecular analyses are needed to identify dissemination pathways and to optimize treatment in the era of personalized and targeted medicine. Currently, systematic brain monitoring is not recommended since it remains a rare dissemination. Nevertheless, the identification of predictive factors could enable practices for well-selected patients to be changed.Conclusion
This study provided real-life data with a clinical characterization and an evaluation of therapeutic strategies in case of CNS involvement. The prognosis with this dissemination remains poor with a median OS of 14 months. Age at the onset of brain metastases and the presence of more than 2 lesions were factors for poor prognosis, whereas sensitivity to platinum salts appeared to be a factor for good prognosis
Conflict of Interest
We have no conflict of interest to disclose.
Data sharing agreement
The data presented in this study are available on request.
References
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(4):284-96.
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer biology & medicine. 2017 Feb;14(1):9.
- Gockley A, Melamed A, Bregar AJ, et al. Outcomes of women with high-grade and low-grade advanced-stage serous epithelial ovarian cancer. Obstetrics Gynecology. 2017;129(3):439.
- Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. American J Surgical Path. 2004;28(4):496-504.
- Thomakos N, Diakosavvas M, Machairiotis N, et al. Rare distant metastatic disease of ovarian and peritoneal carcinomatosis: A review of the literature. Cancers. 2019 Jul 24;11(8):1044.
- Pradeep S, Kim SW, Wu SY, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer cell. 2014;26(1):77-91.
- Mayer RJ, Berkowitz RS, Griffiths CT. Central nervous system involvement by ovarian carcinoma. A complication of prolonged survival with metastatic disease. Cancer. 1978 Feb;41(2):776-83.
- Borella F, Bertero L, Morrone A, et al. Brain metastases from ovarian cancer: current evidence in diagnosis, treatment, and prognosis. Cancers. 2020 Aug 4;12(8):2156.
- Wohl A, Kimchi G, Korach J, et al. Brain metastases from ovarian carcinoma: An evaluation of prognostic factors and treatment. Neurology India. 2019 Nov 1;67(6):1431.
- Kim TJ, Song S, Kim CK, et al. Prognostic factors associated with brain metastases from epithelial ovarian carcinoma. Int J Gynecologic Cancer. 2007;17(6).
- Cormio, G.; Loizzi, V.; Falagario, M. Changes in the Management and Outcome of Central Nervous System Involvement from Ovarian Cancer since 1994. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2011;114 (2);133– 136.
- Dauplat J, Nieberg RK, Hacker NF. Central nervous system metastases in epithelial ovarian carcinoma. Cancer. 1987;60(10):2559-62.
- Ratner, E.; Bala, M.; Louie-Gao, et al. Increased Risk of Brain Metastases in Ovarian Cancer Patients with BRCA Mutations. Gynecol. Oncol. 2019, 153 (3), 568–573.
- Marchetti C, Ferrandina G, Cormio G, et al. Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecologic oncology. 2016 Dec 1;143(3):532-8.
- Zaborowski MP, Spaczynski M, Nowak-Markwitz E, et al. Paraneoplastic neurological syndromes associated with ovarian tumors. J Cancer Res Clinical Onc. 2015:99-108.
- Rojas I, Graus F, Keime-Guibert F, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55(5):713-715.
- Liapi, A.; Sarivalasis, A. Paraneoplastic Cerebellar Ataxia Can Affect Prognosis in High-Grade Serous Ovarian Cancer: A Case Report. Case Rep. Oncol. 2020, 13 (2), 1006–1012.
- Anderson NE, Rosenblum MK, Posner JB. Paraneoplastic cerebellar degeneration: clinicalâ?ÂÃÂ?ÂÂimmunological correlations. Annals Neuro: Official J Amer Neuro Assoc Child Neurology Soc. 1988;24(4):559-567.
- Eichler TW, Totland C, Haugen M, et al. CDR2L antibodies: a new player in paraneoplastic cerebellar degeneration. PloS One. 2013;8(6):e66002.
- Florance, N. R.; Davis, R. L.; Lam, C.; et al. Anti-N-Methyl-D-Aspartate Receptor (NMDAR) Encephalitis in Children and Adolescents. Ann. Neurol. 2009, 66 (1), 11–18.
- Cohen ZR, Suki D, Weinberg JS, et al. Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neuro-oncology. 2004;66:313-25. [GoogleScholar]
[CrossRef]
- D’Andrea G, Roperto R, Dinia L, Caroli E, Salvati M, Ferrante L. Solitary cerebral metastases from ovarian epithelial carcinoma: 11 cases. Neurosurgical Review. 2005;28:120-3.
- Piura E, Piura B. Brain metastases from ovarian carcinoma. International Scholarly Research Notices. 2011.
- Dhermain F, Reyns N, Colin P, et al. Radiothérapie en conditions stéréotaxiques des métastases cérébrales. Cancer/Radiothérapie. 2015;19(1):25-29.
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. Jama. 2016;316(4):401-409.
- Minniti G, Scaringi C, Paolini S, et al. Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neuro-oncology. 2016;126:91-97.
- Jablonska PA, Tejero DS, Gonzalez AC, et al. Repeated stereotactic radiosurgery for recurrent brain metastases: An effective strategy to control intracranial oligometastatic disease. Critical Reviews in Oncology/Hematology. 2020 1;153:103028.
- Chidambaram, S, Pannullo, S. C, Schwartz, et al. Reirradiation of Recurrent Brain Metastases: Where Do We Stand? World Neurosurg. 2019;125;156–163.
- Konstantinopoulos PA, Lheureux S, Moore KN. PARP inhibitors for ovarian cancer: current indications, future combinations, and novel assets in development to target DNA damage repair. Amer Soc Clinical Onco Edu Book. 2020;40:e116-131.
- Gray S, Khor XY, Yiannakis D. Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Reports CP. 2019;12(8):e230738.
- Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nature reviews Clinical oncology. 2011;8(5):302-6.
- Tutt AN, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1-or BRCA2-mutated breast cancer. New Eng J Med. 2021: 2394-2405.
- Balendran S, Liebmann-Reindl S, Berghoff AS, et al. Next-generation sequencing-based genomic profiling of brain metastases of primary ovarian cancer identifies high number of BRCA-mutations. J Neuro-oncology. 2017;133:469-76.
- Koul A, Loman N, Malander S, et al. Two BRCA1-positive epithelial ovarian tumors with metastases to the central nervous system: a case report. Gynecologic Oncology. 2001;80(3):399-402.