Can amoxicillin clavulanate be used for treating MRSA?
2 Liaquat National Hospital, Karachi, Pakistan, Email: uzmasaad@gmail.com
Received: 27-Sep-2017 Accepted Date: Oct 26, 2017; Published: 01-Nov-2017
Citation: Jamil S, Saad U, Hafiz S. Can amoxicillin clavulanate be used for treating MRSA? J Pharmacol Res December-2017;1(1):21-23.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objective: To determine the frequency of beta lactamase producing Staphlococcus aureus and their sensitivity to Amoxicillin clavulanate in major cities of Pakistan. Setting: Various laboratories of the country with one as the central Laboratory. Materials and Methods: Seven hundred and ninety two consecutive clinical isolates of Staphylococcus aureus were collected from 8 laboratories all over Pakistan i.e. Karachi, Peshawar, Lahore, Sukkhur, Islamabad, Quetta, and Mirpur, Azad Kashmir. Antibiotic sensitivity was done by Kirby Bauer disc diffusion method and Beta lactamase production was identified by using Nitrocefin test. Results: Forty two percent of the isolates were found to be Methicillin resistant Staphylococcus aureus (MRSA) out of which 87.9% were positive for Beta lactamase production 52.1% of these Beta lactamase producing MRSA were sensitive to amoxicillin-clavulanate and the remaining (47.9%) were resistant. Conclusion: If beta lactamase producing Staphlococcus aureus are tested against beta-lactam antimicrobial agents in combination with clavulanic acid or sulbactam (Beta-lactamase inhibitors), they become susceptible to the Beta-lactam antimicrobial agents. This might have therapeutic and epidemiological implications in near future.
Keywords
Methicillin resistant Staphylococcus aureus; Vancomycin intermediate Staphylococcus aureus; Vancomycin resistant Staphylococcus aureus; Clinical laboratory standard institute; Penicillinase resistant penicillins; Minimum inhibitory concentration; Penicillin binding proteins; Center of disease control
Introduction
For a long time penicillin group of antibiotics have been the main stays for the management of variety of infections caused by the genus Staphylococcus. During the course of time, resistance to antibiotic was acquired by the genus and a proportion of organisms have become resistant to Methicillin and Cloxacillin. The incidence of methicillinresistant Staphylococcus aureus (MRSA) has gradually increased, with strains shown to cause up to 21% of skin infections and 59.6% of nosocomial pneumonia [1,2].
Methicillin resistant Staphylococcus aureus (MRSA) has gained much attention in the last decade, as MRSA is a major cause of hospital acquired infections. The preferred drugs against Staphylococcus aureus infections are the β-lactam antibiotics. S. aureus has developed resistance to the β- lactam antibiotics due to the production of chromosomal or plasmid mediated β-lactamases. This results in the limitation of the therapeutic options to a few antimicrobials, which are not only toxic and complicated to administer but also expensive [3,4]. As a consequence, patients have to be hospitalized for a longer duration, treatment costs are increased, and mortality also rises. This has a major impact on individual patients and institutions [5]. An additional concern of great significance is the emergence of vancomycin intermediate Staphylococcus aureus (VISA) and more recently vancomycin resistant S. aureus (VRSA) [6]. In addition to this, MRSA has become established outside the hospital environment and is now appearing in community populations. Thus, the number of effective exogenous antibiotics is declining; therefore concerted efforts are to be made to identify antimicrobial materials which could be used for the treatment of such pathogens. The research papers read in local forums in Pakistan claimed around 35% MRSA in Pakistan [7].
However all of these studies were done in an isolated setting where the overall picture of Beta lactamase producing MRSA in Pakistani population was not available hence a prospective study was planned. Regional laboratories in Pakistan were requested to participate in the study with the intention of generating local data regarding the prevalence of Beta lactamase producing MRSA in Pakistan and its sensitivity to Amoxicillin clavulanate.
Materials and Methods
A prospective laboratory based study was designed with eight laboratories participating from Karachi, Peshawar, Lahore, Sukkhur, Islamabad/ Rawalpindi, Quetta and Mirpur Azad Kashmir. These laboratories received clinical samples from various parts of the city and adjoining areas. These satellite laboratories were requested to collect all Staphylococcus aureus isolates received for testing and send them to the Central Laboratory for confirmation, beta lactamase production and their sensitivity to Amoxicillin clavulanate.
Clinical samples received and identified were screened for Staphylococcus aureus. All consecutive Staphylococcus aureus isolates based on cultural, morphological and biochemical characteristics were collected along with the clinical report form. The isolates were from Pus, blood, urine, aspirates and ear and eye swabs. These isolates were subcultured, checked for purity, rechecked and the identification of the isolates was confirmed in accordance with the standard protocol [8,9]. The antibiotic susceptibility pattern was determined by Kirby Bauer disc diffusion method according to CLSI guidelines [10], against Cefoxitin and Amoxicillin-clavulanate. The isolates found to be Methicillin Resistant and Amoxicillin clavulanate resistant were based on disc sensitivity producing a zone of inhibition <22 mm with 30 μg cefoxitin and <18 mm with 20/10 μg amoxicillin calvulanate respectively. Nitrocefin strip was used for beta lactamase production according to the standard protocol [10].
Results
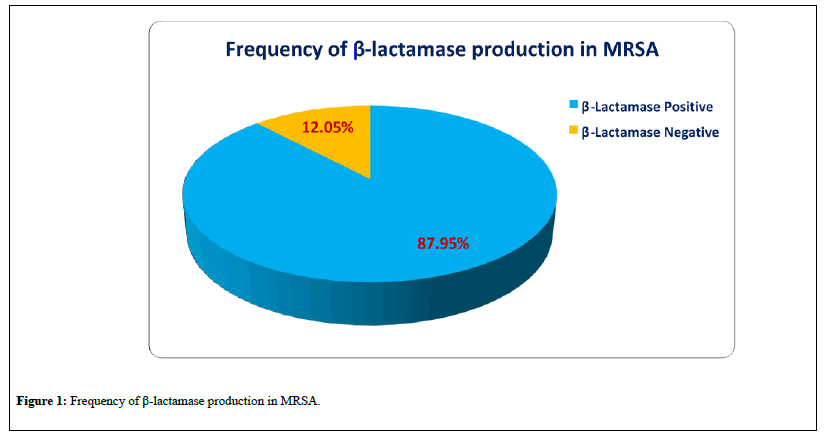
A total of 875 isolates of Staphylococcus aureus were received from the collaborating Laboratories, out of which 792 were viable. Methicillin resistant Staphylococcus aureus (MRSA) accounted for 42% (n=332) of the isolates as shown in Table 1, out of which 87.9% (n=292) were positive for Beta lactamase production Figure 1. Beta lactamase producing
| SL No. |
Laboratory No. |
Specimen | Total Staph. aureus |
MRSA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Inpatients | Outpatients | Total | No. | % | No. | % | |||
| 1 | Lab Karachi | 11 | 95 | 66 | 161 | 151 | 94 | 87 | 58 |
| 2 | Lab Karachi | 12 | 67 | 33 | 100 | 94 | 94 | 54 | 57 |
| 3 | Lab Lahore | 21 | 31 | 69 | 100 | 98 | 98 | 60 | 61 |
| 4 | Lab Islamabad | 32 | 51 | 41 | 92 | 85 | 92 | 39 | 46 |
| 5 | Lab Peshawar | 41 | 93 | 94 | 187 | 158 | 84 | 57 | 36 |
| 6 | Lab Quetta | 51 | 4 | 38 | 42 | 27 | 64 | 7 | 26 |
| 7 | Lab Azad Kashmir | 61 | 42 | 47 | 89 | 81 | 91 | 26 | 32 |
| 8 | Lab Sukkur | 71 | 37 | 67 | 104 | 98 | 94 | 2 | 2 |
| 9 | Total | 420 | 455 | 875 | 792 | 91 | 332 | 42 | |
Table 1: Age wise distribution of patients in two groups.
Discussion
In this study the prevalence of MRSA strains in various cities of Pakistan over 10 months period was found to be 42%.
MRSA were further categorized into:
1. Beta-lactamase producers (87.9%)
2. Non-Beta-lactamase producers (12.05%)
In non-Beta lactamase producing MRSA, altered PBP2a was considered as the sole cause of methicillin/cefoxitin resistance as depicted by amoxicillin-clavulanate resistance by disc method.
On the other hand, Beta lactamase producing MRSA were further categorized into two types,
1. Amoxicillin-clavulanate resistant strains
2. Amoxicillin-clavulanate sensitive strains
Current CLSI guidelines suggest that Oxacillin resistant Staphylococcus must be reported as cephems and other beta-lactams resistant. Therefore, another factor to be considered is the role of beta-lactamase inhibitors used in combination with other beta-lactam antimicrobial agents to treat infections caused by S. aureus with borderline susceptibility or resistance. Clavulanic acid in combination with amoxicillin is now commercially available. Since clavulanic acid and sulbactam inhibit staphylococcal betalactamase, theoretically these combinations could be used to treat MRSA infections.
There is limited information on the use of beta-lactamase inhibitors in combination with Beta-lactam antimicrobial agents for treating MRSA infections. Lacey has indicated that the role of these drugs is uncertain, [11] but the successful use of clavulanic acid and amoxicillin in treating staphylococcal soft tissue infections has been reported for animal models [12] and for children [13]. The important question on therapy for infections caused by these borderlines.
(Non-heteroresistant) S. aureus is whether they have to be treated with vancomycin, the drug that is recommended to treat methicillin-resistant S. aureus infections in Pakistan and in other parts of the world. Vancomycin is known for its potential toxicity and cost. We emphasize, however, that vancomycin is the drug of choice for inherently resistant S. aureus infections. These questions of therapy cannot be answered until appropriate clinical trials are conducted to compare the efficacy of different regimens of therapy.
Another important consideration is whether to stick to isolation precautions for hospitalized patients who are infected or colonized with these borderline, non-heteroresistant S. aureus.
Until more data are gathered, one has to confine to the conservative approach which is to consider the isolates of these patients as methicillinresistant S. aureus and to implement the isolation precautions as advocated by the Centers for Disease Control guidelines.
Until many of the therapeutic and epidemiological questions are answered, the most conservative approach is to treat the isolates as intrinsically resistant methicillin-resistant S. aureus and not be concerned about the mechanisms.
If a different decision is to be made, it will be based on indivisual discretion and it should be made because of a high incidence of the beta lactamase- mediated oxacillin-resistant strains. This decision can therefore be taken by the appropriate hospital personnel, e.g., microbiologist, infectious disease physician, and epidemiologist. If laboratory workers wish to determine whether a borderline culture is heteroresistant, they could do an MIC (minimum inhibitory concentration) test that includes a beta-lactamase inhibitor in combination with the beta-lactam antimicrobial agent, they could also do a disk diffusion test with amoxicillin-clavulanic acid [14], or they could use the 24-h agar screen test [15].
The terms methicillin resistant, intrinsically resistant, occult resistant [16] and heteroresistant are used interchangeably to refer to those strains with chromosomally mediated resistance not only to methicillin but also to oxacillin, nafcillin, cloxacillin, flucloxacillin, cephalosporins, and probably all beta-lactams. This resistance is related to altered or lowaffinity targets, since the PBPs (penicillin binding proteins) appear to be involved [17]. Acquired-resistant S. aureus would be those strains that are resistant to a PRP because of the beta-lactamase they produce which is probably always acquired and plasmid mediated. If S. aureus isolate is found to be resistant to oxacillin, for example, it could be further tested as described above and further designated as either intrinsically or acquiredresistant S. aureus. If these acquired resistant S. aureus are tested against these beta-lactam antimicrobial agents in combination with clavulanic acid or sulbactam (Beta-lactamase inhibitors), they become susceptible to the Beta-lactam antimicrobial agents. Additional studies are needed to determine the therapeutic and epidemiological implications of these findings. Several new antibiotics showing promising activity may be used against these multidrug-resistant bacteria. However, as the history of bacterial resistance has taught us, it will be a matter of time until these organisms adopt mechanisms of resistance to these new drugs. The key then lies in preventive measures. Surgeons and physicians must adhere to the precautionary guidelines recently set forth by the CDC and HICPAC: chief among these guidelines being the elimination of inappropriate antibiotic usage, especially inappropriate vancomycin use.
REFERENCES
- Pechere JC. Current and future management of infections due to methicillin resistant staphylococci infections: the role of quinupristin/dalfopnstin. J Antimicrob Chemother. 1999;44:11-8.
- Shopsin B, Mathema B, Martinez J, et al. Prevalence of methicillin-resistant and methicillin susceptible Staphylococcus aurcus in the community. J Infect Dis. 2000;182:359-62.
- Perry CN, Jarris B. Linezolid: a review of its use in the management of serious gram-positive infections Drugs. 2001;61:525-51.
- Kim T. Oh PI, Simor AE. The economic impact of methicillin-resistant Staphylococcus aureus in Canadian hospitals. Infect Control Hosp Epidemiol. 2001;22:99-104.
- Cosgrove SE, Qi Y, Kaye KS, et al. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay and hospital charges. Infect Control Hosp Epidemiol. 2005;26:166-74.
- Apple aum PC. Reduced glycopeptide susceptibility in methicillin- resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents. 2007;30:398-408.
- Hafiz S, Shabuddin ANH, Biennial conference of Pakistan Society for Microbiology at Bhurban, Pakistan, Karachi:MRSA, 1997.
- Clinical Microbiology Procedures Handbook. Washington D.C: American Society for Microbiology. NCCLS: Performance Standards for Antimicrobial Susceptibility Testing. 1998;18:100-08.
- NCCLS: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria Grow Aerobically; Approved Standard- 5th Ed. 2000.
- Baker CN, Huang MB, Tenover FC. Optimizing testing of Methicillin resistant Staphylococcus spp. Diag Microbiol Infect Dis. 1994;19:167-70.
- Lacey RW. Treatment of staphylococcal infections. J Antimicrob Chemother. 1983;11:3-6.
- Brook I, Coolbaugh JC, Walker RI. Antibiotic and clavulanic acid treatment of subcutaneous abscesses caused by Bacteroides fragilis alone or in combination with aerobic bacteria. J Infect. 1983;148:156-9.
- Fleisher GR, Wilmott CM, Campos JM. Amoxicillin combined with clavulanic acid for the treatment of soft tissue infections in children. Antimicrob Agents Chemother. 1983;24:679-81.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobic disc susceptibility test, 3rd ed. Approved standard M2-A3.1984.
- Thornsberry C, McDougal LK. Successful use of broth microdilution in susceptibility tests for methicillin resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983;18:1084-91.
- Hindler JA, Inderlied CB. Effect of the source of Mueller-Hinton agar and resistance frequency on the detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21:205-10.
- Hartman BJ, Tomasz A. Low-affinity penicillinbinding protein associated with, ß-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513-16.