Can γH2AX assay be an endpoint in drug discovery?
Received: 19-Oct-2017 Accepted Date: Nov 20, 2017; Published: 25-Nov-2017
Citation: Yashavarddhan MH, Shukla SK, Sharma AK. Can γH2AX assay be an endpoint in drug discovery? Clin Pharmacol Toxicol Res. December-2017;1(1):9-12.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Biomarker development is essential in the drug discovery field for effectiveness, toxicity, and optimization of doses when a drug is translated during pre-clinical to clinical trials. The present review highlights the prospective application of the γH2AX assay for pharmacological and toxicological evaluation in drug development. γH2AX is a phosphorylated form of H2AX protein belongs to the family H2A and is a well-known biomarker for measurement of DNA double strand breaks (DSBs). During chemotherapy, cancer cells are targeted by drugs that induce DSBs thereby result in cell death. In such scenario, γH2AX could be an informative marker for the effectiveness of the chemotherapeutic drug as wells as its associated toxicity in surrounding normal cells. Moreover, the canonical calculation of dose conversion of a drug from pre-clinical animal models to clinical trials in humans or non-human primates may not be effective all the time. The γH2AX assay may be used as one of possible marker for such dose conversion for any drug capable to generate DSBs. Besides, it can also be used as an effective biomarker in radiation countermeasures and bio-dosimeter which has been substantiated in many research articles. Considering the importance of γH2AX in various fields, this biomarker could be helpful in safety and efficacy assessment of new drugs.
Keywords
γH2AX, Clinical pharmacology, toxicity
Introduction
Drug discovery is an excellent area of research which needs vast and latest updated knowledge for the development of any new drug for human use. So far, a tremendous progress has been made in formulating guidelines required during various phases of drug development. Pharmacological mechanism of action integrated with safety evaluation play a paramount importance for drug discovery. Enormous biological parameters are being evaluated for understanding the mechanism of action for an agent. A number of bioassays have been put to use for safety, efficacy and mechanistic aspects as per the guidelines of the various drug approving agencies operating across the world. These bioassays serve as an essential tool for analyzing the pharmacological mechanism of an agent. The search for a suitable biomarker is an ongoing process which requires a pragmatic approach based on various ways of experimentation.
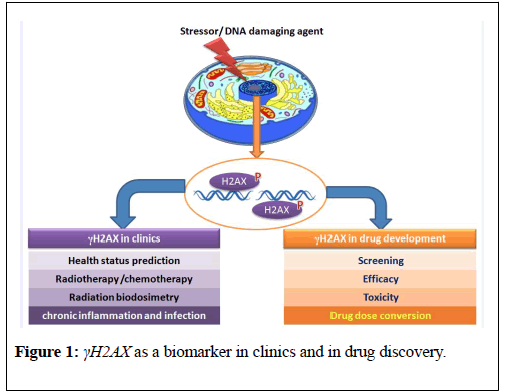
From past twenty years, since the discovery of γH2AX, this marker has been used vastly by various researches to understand the mechanistic aspects of DNA double strand breaks (DSBs) response pathway. However, the use of this protein in applied research is still needed to be explored.The present review potentiates application of γH2AX as a biomarker in clinics and in drug discovery field (Figure 1).
Figure 1: γH2AX as a biomarker in clinics and in drug discovery.
γH2AX Genesis
Any stress agent that generates DSB leads to an extensive response in the chromatin region flanking the break. A large number of protein species accumulates at DSB sites forming large nuclear aggregates [1]. Ser-139 phosphorylated form of H2AX (γH2AX) protein is a unique biomarker of DNA double strand break. In 1998, Rogakou et al., first time discovered this variant of histone protein in mammalian systems exposed to an ionizing radiation [2]. H2AX is a histone H2A variant which constitutes 2-25% of histone H2A [2,3] and is composed of a central globular domain, flanked by N-terminal and a unique COOH terminal prone to posttranslational modifications like acetylation, biotinylation, phosphorylation, methylation, and ubiquitination [2,4]. The proteins responsible for the phosphorylation of the H2AX protein are ataxia telangiectasia mutated (ATM), AT and Rad3-related protein (ATR), and DNA-dependent protein kinase (DNA-PK) [5]. However, Peng et al. in 2008, suggested its autophosphorylation ability [6].
Kinetics of γH2AX
The persistence of phosphorylated form of H2AX in the cellular system is debatable that it persisted for shorter or longer duration [7]. Previous reports convey the formation of γH2AX within a minute which generally persists up to 24-48 h [8-10]. However, recently many research articles have shown its persistence for days or even months in various model systems [7,11-13]. The short existence of this marker in cells can allow quick estimation, however, posing a great limitation for evaluation of DNA damage for longer time points. Moreover, the persistence of this protein for many days indicates the residual DNA damage that could be used as a marker for assessment of manifestation of prolonged damage.
γH2AX in clinics
γH2AX can play a major role in clinical settings viz-a-viz diagnosis and mortality/morbidity prediction of radiation-exposed individuals. Apart from this it has also wider applications in radiation bio-dosimetry assessment. Moreover, γH2AX can also help in the prediction for a type of radiation exposure and in treatment of chronic inflammation/infection.
γH2AX in diagnosis
At instances DSBs may leads to cancer however, paradoxically; this also helps in killing cancerous cells. γH2AX, which is generated upon DSBs generation, could play an important role in diagnosis and effectiveness of treatment in patients suffering from carcinoma [14]. Worldwide, the major accepted methods used for the treatment of cancer are radiotherapy and chemotherapy [15]. Even after the advancement in these treatments modalities for treatment of tumor, very limited success has been achieved in this direction. The probable reasons are ineffectiveness and associate toxicity with almost all the treatment protocols in addition to contributory factors responsible for tumor formation. Basically, these two treatment protocols kills the cancerous cells by generating the DSBs [16]. So, the biomarker, γH2AX, has been measured in various clinical cases involving the patient for evaluation of DNA damage making a strong reasoning to be used as a marker for determining efficacy of treatment protocol, dose standardization/optimization and associated toxicity in surrounding tissues [17-19]. Taking this into consideration γH2AX has been studied against variety of cancer types, including the adrenal renal cancer [20], head& neck cancer [21], cervix cancer [22], breast cancer [23], rectal cancer [24], lung cancer [25], and ovary cancers [26].
γH2AX in prediction of mortality/morbidity of patents
γH2AX could be a marker for prediction of mortality/ morbidity in patients undergoing clinical treatments for radiation or chemical or some chronic diseases. In a case study published by Gupta et al, 2013, they have reported the detection of γH2AX after a month in metal scrap workers accidentally exposed to Co60 radiation [11]. This indicated the persistence of γH2AX form for longer period. Moreover, every patient has shown variable level of γH2AX. The patient who exhibited a highly significant level γH2AX consequently died later which clearly indicate the role of this protein in predicting severity, in terms of overall health status, of an individual [11]. In another study published by Yashavarddhan et al., confirmed these observations in rabbit model system [7]. In this study, they have explained γH2AX marker in studying the severity of radiation exposure and morbidity status of animals. Besides extensive radiation related studies, there are few reports showing a presence of this biomarker in chronic obstructive pulmonary disease (COPD) and tumorigenesis [4,27,28]. Activation of the proto-oncogene in chronic inflammation leads the development of cancer [29,30]. Their activations affect the cell cycle checkpoints that lead to the production of reactive oxygen species which damage the DNA. This damage can be measured by γH2AX. COPD is one of the examples of chronic inflammation in which few research articles have shown the measurement of γH2AX [29]. Viral infections targeting the DNA may also be addressed by the use of this biomarker [30]. Considering all the available literature, γH2AX may be a potential biomarker for the assessment of mortality, morbidity, and health status of individuals exposed to genotoxic agents.
Radiation biodosimetry and prediction of type of radiation exposure
Extensive data are available for estimation of γH2AX in radiation biodosimetry. The linear response of this protein with radiation doses prompted various investigators to use it as a biomarker for radiation biodosimetry [31-33]. The gold standard marker for quantification of radiation damage is dicentric assay which is labor intensive and needs skills as well as longer time for delivery of the result [34]. However, the formation of γH2AX within a minute of the exposure and its measurement using foci measurement or through flow-cytometric techniques makes it a very useful biomarker for dose assessment. The quick estimation of dose in radiation-exposed patient could certainly help the clinician to decide the treatment regimen. The interlaboratory evaluations are undergoing to validate this marker for radiation dose assessment. Additionally, the γH2AX may also predict the type of radiation exposure such as acute or repeated. The findings have shown larger γH2AX foci size and longer persistence for repeated radiation exposure compared to acute exposure [7,11]. However, further validation of these reports by other investigators could support this view.
γH2AX in pharmaceutical development
Drug development is a tedious process that needs lot of time and efforts for the translation of a drug molecule from bench to market. Chemotherapeutic drugs have shown the DNA damage induction in cells which can be evidenced by γH2AX assay [35]. Olive and Banath in 2009 used this assay to measure the effectiveness of Cisplatin, which is a worldwide used chemotherapy drug, in cancerous cells [36]. The similar experimental approach was adopted by various researchers to measure the effectiveness of various chemotherapy drugs like gemcitabine, busulfan, and melphalan, against a different type of cancer [37]. Additionally, this maker has been recently used for evaluation of the radioprotective efficacy of radiation countermeasure agents which were in pre-clinical stage [7,10,38,39].
γH2AX in toxicity studies
Toxicity measurement of a drug is a major parameter for its development. An ideal drug should be nontoxic or limited undesirable effect. OECD guidelines for toxicity show various standard assays for evaluation of toxicity of any compound. Genotoxicity studies generally involve micronuclei, comet, and chromosomal assays. Researchers have also used γH2AX assay for evaluation of toxicity [40]. The method for measurement of γH2AX requires minimum time and very quick, so it may be used as supportive parameters for estimation of genotoxicity.
γH2AX in drug dose conversion
From last few decades dose conversion from lower animals to higher animals applies the classical conversion formula [41] which may not be effective for many reasons. There is need to develop reliable assays which can support in dose conversion. Considering the available literature the γH2AX assay could be helpful in deciding such dose conversion particularly for the drug inducing DNA DSBs.
Conclusion
The entire above summary about γH2AX clearly shows its relevance in a different domain of drug discovery along with its usefulness in clinics. The characteristic formation of this protein in the cells exposed to any stress and resulted in DSBs has made it a marker for evaluation of toxicity, efficacy and mechanism study. Commendable publication in last few decades indicates its immense potential as a biomarker in radiation biodosimetry. Henceforth, the researcher should take advantage of this marker in various fields of clinical therapeutics, diagnosis and in drug discovery for efficient and quick evaluation.
Acknowledgements
The authors are thankful to Director, Institute of Nuclear Medicine & Allied Sciences (INMAS), Delhi, India, for providing support in writing this mini review.
Conflict of Interest
We declare that we have no conflict of interest.
REFERENCES
- Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25(5):409-33.
- Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858-68.
- Tanaka T, Kurose A, Huang X, et al. ATM activation and histone H2AX phosphorylation as indicators of DNA damage by DNA topoisomerase I inhibitor topotecan and during apoptosis. Cell Prolif. 2006;39(1):49-60.
- Dickey JS, Redon CE, Nakamura AJ, et al. H2AX: functional roles and potential applications. Chromosoma. 2009;118(6):683-92.
- Redon CE, Dickey JS, Bonner WM, et al. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res. 2009;43(8):1171-8.
- Peng L, Wang S, Yin S, et al. Autophosphorylation of H2AX in a cell-specific frozen dependent way. Cryobiology. 2008;57(2):175-7.
- Yashavarddhan MH, Shukla SK, Srivastava NN,et al. γH2AX formation kinetics in PBMCs of rabbits exposed to acute and fractionated radiation and attenuation of focus frequency through preadministration of a combination of podophyllotoxin and rutin hydrate. Environ Mol Mutagen. 2016;57(6):455-68.
- Horn S, Barnard S, Rothkamm K. Gamma-H2AX-based dose estimation for whole and partial body radiation exposure. PloS One. 2011;6(9):e25113.
- Kuo LJ, Yang LX. γ-H2AX-a novel biomarker for DNA double-strand breaks. In vivo. 2008;22(3):305-9.
- Yashavarddhan MH, Shukla SK, Chaudhary P, et al. Targeting DNA Repair through Podophyllotoxin and Rutin Formulation in Hematopoietic Radioprotection: An in Silico, in Vitro, and in Vivo Study. Front. Pharmacol. 2017;8:750.
- Gupta ML, Srivastava NN, Dutta S, et al. Blood biomarkers in metal scrap workers accidentally exposed to ionizing radiation: A case study. Hum Exp Toxicol. 2013;32(12):1311-22.
- Bhogal N, Kaspler P, Jalali F, et al. Late residual γ-H2AX foci in murine skin are dose responsive and predict radiosensitivity in vivo. Radiat Res. 2010;173(1):1-9.
- Ahmed EA, Agay D, Schrock G, et al. Persistent DNA damage after high dose in vivo gamma exposure of minipig skin. PLoS One. 2012;7(6):e39521.
- Bonner WM, Redon CE, Dickey JS, et al. γH2AX and cancer. Nat Rev Cancer. 2008;8(12):957-67.
- Sattar S, Alibhai SM, Fitch M, et al. Chemotherapy and radiation treatment decision-making experiences of older adults with cancer: A qualitative study. J Geriatric Oncol. 2017;S1879-4068(17):30143-1.
- Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105(4):370-88.
- Willers H, Gheorghiu L, Liu Q, et al. DNA damage response assessments in human tumor samples provide functional biomarkers of radiosensitivity. Seminars Radiat oncol. 2015;25(4):237-50.
- Bourton EC, Plowman PN, Smith D, et al. Prolonged expression of the γ‐H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer. 2011;129(12):2928-34.
- Yashavarddhan MH, Shukla SK, Sharma AK, et al. Response of Normal cells Following multiple radiation exposure under radiotherapy Setting. Def Life Sci J. 2017;2(3):335-42.
- Wasco MJ, Pu RT. Utility of antiphosphorylated H2AX antibody (γ-H2AX) in diagnosing metastatic renal cell carcinoma. Appl Immunohistochem Mol Morphol. 2008;16(4):349-56.
- Sak A, Grehl S, Erichsen P, et al. Gamma-H2AX foci formation in peripheral blood lymphocytes of tumor patients after local radiotherapy to different sites of the body: dependence on the dose-distribution, irradiated site and time from start of treatment. Int J Radiat boil. 2007;83(10):639-52.
- Sunada S, Hirakawa H, Fujimori A, et al. Oxygen Enhancement Ratio in Radiation-Induced Initial DSBs by an Optimized Flow Cytometry-based Gamma-H2AX Analysis in A549 Human Cancer Cells. Radiat Res. 2017;188(5):591-4.
- Yang SX, Polley EC, Nguyen D. Association of γH2AX at Diagnosis with Chemotherapy Outcome in Patients with Breast Cancer. Theranostics. 2017;7(4):945-51.
- Avkshtol V, Arora S, Lesh RW, et al. Peripheral Blood Lymphocyte Gamma-H2AX as a Predictor for Treatment Response in Rectal Cancer Patients. Int J Radiat Oncol Biol Phys. 2017;99(2):E576-7.
- Sunada S, Hirakawa H, Fujimori A, et al. Oxygen Enhancement Ratio in Radiation-Induced Initial DSBs by an Optimized Flow Cytometry-based Gamma-H2AX Analysis in A549 Human Cancer Cells. Radiat Res. 2017;188(5):591-4.
- Sedelnikova OA, Bonner WM. γ-H2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell cycle. 2006;5(24):2909-13.
- Redon CE, Weyemi U, Parekh PR, et al. γ-H2AX and other histone post-translational modifications in the clinic. Biochimic Biophys Acta. 2012;1819(7):743-56.
- Srivastava N, Gochhait S, de Boer P, et al. Role of H2AX in DNA damage response and human cancers. Mut Res. 2009;681(2-3):180-8.
- Pastukh VM, Zhang L, Ruchko MV, et al. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis. 2011;6:209-17.
- Kryston TB, Georgiev AB, Pissis P, et al. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711(1-2):193-201.
- Garty G, Chen Y, Turner H, et al. The RABiT: a rapid automated biodosimetry tool for radiological triage. II. Technological developments. Int J Radiat Biol. 2011;87(8):776-90.
- Redon CE, Nakamura AJ, Gouliaeva K, et al. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PloS One. 2010;5(11):e15544.
- Rothkamm K, Barnard S, Ainsbury EA, et al. Manual versus automated γ-H2AX foci analysis across five European laboratories: can this assay be used for rapid biodosimetry in a large scale radiation accident?. Mutat Res. 2013;756(1-2):170-3.
- Romm H, Wilkins RC, Coleman CN, et al. Biological dosimetry by the triage dicentric chromosome assay: potential implications for treatment of acute radiation syndrome in radiological mass casualties. Radiat Res. 2011;175(3):397-404.
- Redon CE, Nakamura AJ, Zhang YW, et al. Histone γH2AX and poly (ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16(18):4532-42.
- Olive PL, Banáth JP. Kinetics of H2AX phosphorylation after exposure to cisplatin. Cytometry B Clin Cytom. 2009;76(2):79-90.
- Nieto Y, Valdez BC, Thall PF, et al. Vorinostat combined with high-dose gemcitabine, busulfan, and melphalan with autologous stem cell transplantation in patients with refractory lymphomas. Biol Blood Marrow Transplant. 2015;21(11):1914-20.
- Srivastava NN, Shukla SK, Yashavarddhan MH, et al. Modification of radiation‐induced DNA double strand break repair pathways by chemicals extracted from Podophyllum hexandrum: An in vitro study in human blood leukocytes. Environ Mol Mutagen. 2014;55(5):436-48.
- Kashino G, Liu Y, Suzuki M, et al. An alternative mechanism for radioprotection by dimethyl sulfoxide; possible facilitation of DNA double-strand break repair. J Radiat Res. 2010;51(6):733-740.
- Bourton EC, Plowman PN, Smith D, et al. Prolonged expression of the γ‐H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer. 2011;129(12):2928-34.
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659-61.