Case report: the clinical significance of an azygous anterior cerebral artery
Scott Jennings1* and Ali Haider2
1Surgical Registrar, The Canberra Hospital, Garran ACT 2605, Australia
2Resident Medical Officer, The Canberra Hospital, Garran ACT 2605, Australia
- *Corresponding Author:
- Dr. Scott Jennings, BSc MBBS (Hons)
Surgical Registrar, The Canberra Hospital, Garran ACT 2605, Australia
Tel: +61 458438864
E-mail: scott.jennings@act.gov.au
Date of Received: July 19th, 2011
Date of Accepted: September 22nd, 2011
Published Online: December 5th, 2011
© Int J Anat Var (IJAV). 2011; 4: 185–187.
[ft_below_content] =>Keywords
anterior cerebral artery, azygous, variation
Introduction
The circle of Willis was originally described in 1664 [1]. The circle of Willis is a structure receiving arterial blood supply from the internal carotid and basilar arteries. The circle allows for the equalization of blood flow between cerebral hemispheres allowing for anastomotic circulation [2,3]. The circle of Willis then divides into 3 paired vascular divisions: anterior, middle and posterior [2]. The paired anterior cerebral arteries arise from the internal carotid arteries, connected by the anterior communicating artery to complete the anterior portion of the circle. These arteries then supply the medial surface of each cerebral hemisphere, superior to the corpus callosum extending posteriorly as far as the parieto-occipital sulcus [2]. Comparatively, animals with a less developed prefrontal cortex, such as fish, reptiles and amphibians do not posses an anterior communicating artery, with two anterior cerebral arteries laying in parallel. Snakes, tortoises and crocodiles occasionally exhibit a unified anterior cerebral artery, the midline vessel known as the azygous anterior cerebral artery (AACA) [4]. Lower primates also show considerable variation with no anterior communicating artery, whereas a gorilla’s anatomy best resembles that of humans [4,5]. The AACA in humans is rare with an incidence between 0.1-1.1% [3,6].
The clinical significance of an AACA is the alteration of arterial hemodynamics of the frontal lobe and the increased incidence of malformations like agenesis of the corpus callosum, hydranencephaly, saccular aneurysms and arterio-venous malformations [3]. Aneurysms have been reported in one study at an incidence of 41% of identified AACA cases and at an incidence of between 13-71% [3,7]. This exceedingly rare anatomical variation has very important implications for routine inpatient care and treatment of potential neurological and neurosurgical conditions.
Case Report
A 67-year-old Caucasian male, presented to the Emergency Department with an apparent cerebrovascular event. He appeared conscious, but not speaking or moving, suffering a grand mal seizure after arrival. A decision to intubate was made, but spontaneous-breathing returned. Physical examination showed no response to painful stimuli. Pupils were mid-range and not responding to light. The remainder of the physical examination was unremarkable as were routine bloods and an ECG.
Urgent head-CT showed nil obvious acute or chronic intracranial pathology. Discussion with the closet referral center suggested reperfusion, however as consciousness began returning at this point, it was felt thrombolysis not appropriate. The patient was then admitted to the high-dependency unit with continued seizures requiring phenytoin. The following day, the patient’s limbs began moving and he attempted communicating. A lumbar puncture was normal, as was follow-up cranial CT. Prophylactic heparin was not prescribed due to concern of an occult cerebral hemorrhage not apparent on the previous CT scans (compression stockings placed). Intravenous ceftriaxone and acyclovir were commenced, covering potential bacterial and viral pathogenesis. Collateral history from a friend had revealed the patient had been “unwell” for the previous 6 weeks.
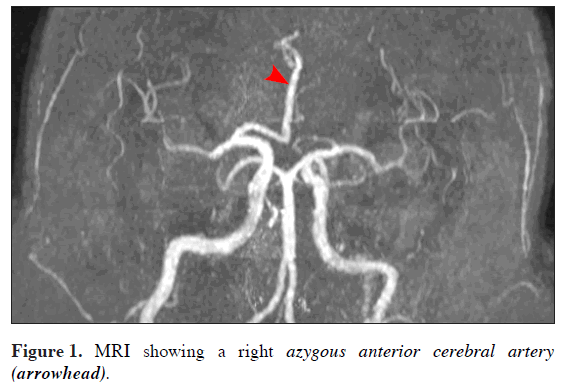
Although continued seizure activity, the clinical picture began improving. An MRI revealed multiple small foci of abnormal high signal, involving both the gray and white matter within anterior and middle cerebral territories (bilaterally). These multiple peripheral foci were thought to represent small infarcts. These infarcts were in different vascular territories, indicating possible multiple small emboli. The MRI also showed a single large caliber anterior cerebral artery (no other vascular variants identified) (Figure 1). Lacking a definitive diagnosis and in the context of improving clinical picture it was felt a follow-up MRI/MRA appropriate.
The patient continued to improve and on the last ward round he was less confused (able to laugh and smile). However, whilst being bathed he became unresponsive and fell from bed, progressing to full cardiac arrest. The medical emergency team responded and resuscitation commenced. It was noted the right calf appeared swollen during resuscitation and blood was noted in the endotracheal tube. A clinical diagnosis of massive pulmonary embolus (PE) was made and thrombolysis administered. Resuscitative efforts continued for 75 minutes, ending when unrecoverable electromechanical dissociation supervened. Subsequent autopsy revealed the cause of death to be massive PE.
Discussion
The paramount issues in this case surrounded diagnosis, reperfusion, risk of occult issues and deep vein thrombosis prophylaxis. Firstly, the diagnosis of the patient’s presenting complaint was difficult. The presentation would have initially suggested a catastrophic cerebral event, but cranial CT ruled out hemorrhage. Cranial CT remains the gold standard in the acute setting, helping rule out hemorrhagic insult and then allowing for consideration of reperfusion therapy when the diagnosis of infarction is certain. However, as high as 60% of acute cranial CTs are normal in the first few hours following ischemic-infarction [8].
The next consideration was occult hemorrhage continuing in the brainstem and what was the onset-time if an infarction was the diagnosis. Had the time prior to presentation exceeded 3 or even 6 hours? [9–11]. If so, the potential for creating further insult from reperfusion-injury was significant [12]. However, at this time the clinical picture improved, with a degree of consciousness returning and seizure activity being controlled. A second cranial CT continued to show nil intracranial pathology and more detailed imaging sort.
An MRI scan showed the above-mentioned findings and a right AACA (Figure 1). The formation of this AACA was best classified as bihemispheric with most of the major hemispheric vessels presumed to arise from this right-sided vessel [4]. However, it did not posses the left portion of the anterior communicating artery which would normally complete the circle of Willis [2]. The next consideration was the size of the AACA, it was enlarged, but was this a normal anatomical variant (supplying both hemispheres) or was it potentially aneurysmal? Chemical DVT prophylaxis was decided not appropriate at this juncture as the incidence of aneurysms (occult) is high and the artery is already of large caliber and surrounded by potential areas of infarct [3,7]. Another consideration was had there only been a transient pathology of this AACA causing the decreased conscious state and ongoing seizure activity?
However, the patient’s condition continued to improve until a fatal PE occurred, presumed from the right calf, which was noted to be swollen during resuscitation efforts. Thrombolytic therapy was given, but not successful and unfortunately death occurred before neurosurgical and neurology advice could be sought regarding further management.
Conclusion
In conclusion, this case highlights the therapeutic dilemma faced by clinicians in cases such as this. The treatment options had the potential to cause more damage than already sustained and how the risk of implementing prophylactic treatment could increase the consequences of a potentially devastating bleed from an AACA.
This case also highlights the need for early specialist consultation and access to high-level diagnostic imaging. It is hoped the sharing of this very rare anatomical variant will alert fellow clinicians to the significance of a patient with an AACA, bearing in consideration the alteration of arterial hemodynamics of the frontal lobe and the increased incidence of malformations like agenesis of the corpus callosum, hydranencephaly, saccular aneurysms and arterio-venous malformations. An awareness of the potential of these malformations will allow more informed decisions regarding the management of patients presenting with an AACA in the acute context or as an incidental finding.
References
- Wolpert SM. The Circle of Willis. AJNR Am J Neuroradiol. 1997; 18: 1033–1034.
- McMinn RMH, ed. Last’s Anatomy: Regional and Applied. 9th Ed., Hong Kong, Churchill Livingstone. 2003: 599–601.
- Huh JS, Park SK, Shin JJ, Kim TH. Saccular aneurysm of the azygos anterior cerebral artery: three case reports. J Korean Neurosurg Soc. 2007; 42: 342–345.
- LeMay M, Gooding CA. The clinical significance of the azygous anterior cerebral artery. Am J Roentgenol Radium Ther Nucl Med. 1966; 98: 602–610.
- Watts JW. A comparative study of the anterior cerebral artery and the circle of Willis in primates. J Anat. 1934; 68: 534–550.
- Anterior Cerebral Artery. http://neuroangio.org/AnteriorCerebralArtery.aspx (accessed May 2011).
- Milenkovic Z, Puzic M, Vasovic L, Djuric S, Vucetic R, Dragan Stojanov. The azygos anterior cerebral artery aneurysms confirmed at operation. Facta Universitatis, Series: Medicine and Biology. 1997; 4: 40–43.
- Acute Cerebral Infarction. http://brighamrad.harvard.edu/Cases/bwh/hcache/93/full.html (accessed May 2011).
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359: 1317–1329.
- Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, Hommel M. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke. 1999; 30: 1326–1332.
- Larrue V, von Kummer R R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001; 32: 438–441.
- Elzawahry H, Hernandez-Frau P, Behrouz R. Reperfusion Injury in Stroke. http://emedicine.medscape.com/article/1162437-overview (accessed May 2011).
Scott Jennings1* and Ali Haider2
1Surgical Registrar, The Canberra Hospital, Garran ACT 2605, Australia
2Resident Medical Officer, The Canberra Hospital, Garran ACT 2605, Australia
- *Corresponding Author:
- Dr. Scott Jennings, BSc MBBS (Hons)
Surgical Registrar, The Canberra Hospital, Garran ACT 2605, Australia
Tel: +61 458438864
E-mail: scott.jennings@act.gov.au
Date of Received: July 19th, 2011
Date of Accepted: September 22nd, 2011
Published Online: December 5th, 2011
© Int J Anat Var (IJAV). 2011; 4: 185–187.
Abstract
This case reports the presentation of a 67-year-old Caucasian male who presented to the Emergency Department unresponsive after suffering what appeared to be an intracerebral bleed. A bleed was not confirmed at CT and the patient started to regain consciousness and the clinical picture improved over the next few days. A follow-up inpatient MRI revealed a single anterior cerebral artery, known as an azygous anterior cerebral artery. The presence of this rare anatomical variant and its potential associated anomalies proved a major consideration in the administration of routine deep vein thrombosis (DVT) prophylaxis. Administration of routine DVT prophylaxis had the potential to further exacerbate injury. Unfortunately the patient suffered a fatal pulmonary embolus before a definitive treatment plan could be arranged. This case highlights the need for early involvement of specialist advice, access to advanced imaging modalities and hopes to raise the awareness of this rare anatomical variant and give consideration to its potential serious complications with fellow clinicians.
-Keywords
anterior cerebral artery, azygous, variation
Introduction
The circle of Willis was originally described in 1664 [1]. The circle of Willis is a structure receiving arterial blood supply from the internal carotid and basilar arteries. The circle allows for the equalization of blood flow between cerebral hemispheres allowing for anastomotic circulation [2,3]. The circle of Willis then divides into 3 paired vascular divisions: anterior, middle and posterior [2]. The paired anterior cerebral arteries arise from the internal carotid arteries, connected by the anterior communicating artery to complete the anterior portion of the circle. These arteries then supply the medial surface of each cerebral hemisphere, superior to the corpus callosum extending posteriorly as far as the parieto-occipital sulcus [2]. Comparatively, animals with a less developed prefrontal cortex, such as fish, reptiles and amphibians do not posses an anterior communicating artery, with two anterior cerebral arteries laying in parallel. Snakes, tortoises and crocodiles occasionally exhibit a unified anterior cerebral artery, the midline vessel known as the azygous anterior cerebral artery (AACA) [4]. Lower primates also show considerable variation with no anterior communicating artery, whereas a gorilla’s anatomy best resembles that of humans [4,5]. The AACA in humans is rare with an incidence between 0.1-1.1% [3,6].
The clinical significance of an AACA is the alteration of arterial hemodynamics of the frontal lobe and the increased incidence of malformations like agenesis of the corpus callosum, hydranencephaly, saccular aneurysms and arterio-venous malformations [3]. Aneurysms have been reported in one study at an incidence of 41% of identified AACA cases and at an incidence of between 13-71% [3,7]. This exceedingly rare anatomical variation has very important implications for routine inpatient care and treatment of potential neurological and neurosurgical conditions.
Case Report
A 67-year-old Caucasian male, presented to the Emergency Department with an apparent cerebrovascular event. He appeared conscious, but not speaking or moving, suffering a grand mal seizure after arrival. A decision to intubate was made, but spontaneous-breathing returned. Physical examination showed no response to painful stimuli. Pupils were mid-range and not responding to light. The remainder of the physical examination was unremarkable as were routine bloods and an ECG.
Urgent head-CT showed nil obvious acute or chronic intracranial pathology. Discussion with the closet referral center suggested reperfusion, however as consciousness began returning at this point, it was felt thrombolysis not appropriate. The patient was then admitted to the high-dependency unit with continued seizures requiring phenytoin. The following day, the patient’s limbs began moving and he attempted communicating. A lumbar puncture was normal, as was follow-up cranial CT. Prophylactic heparin was not prescribed due to concern of an occult cerebral hemorrhage not apparent on the previous CT scans (compression stockings placed). Intravenous ceftriaxone and acyclovir were commenced, covering potential bacterial and viral pathogenesis. Collateral history from a friend had revealed the patient had been “unwell” for the previous 6 weeks.
Although continued seizure activity, the clinical picture began improving. An MRI revealed multiple small foci of abnormal high signal, involving both the gray and white matter within anterior and middle cerebral territories (bilaterally). These multiple peripheral foci were thought to represent small infarcts. These infarcts were in different vascular territories, indicating possible multiple small emboli. The MRI also showed a single large caliber anterior cerebral artery (no other vascular variants identified) (Figure 1). Lacking a definitive diagnosis and in the context of improving clinical picture it was felt a follow-up MRI/MRA appropriate.
The patient continued to improve and on the last ward round he was less confused (able to laugh and smile). However, whilst being bathed he became unresponsive and fell from bed, progressing to full cardiac arrest. The medical emergency team responded and resuscitation commenced. It was noted the right calf appeared swollen during resuscitation and blood was noted in the endotracheal tube. A clinical diagnosis of massive pulmonary embolus (PE) was made and thrombolysis administered. Resuscitative efforts continued for 75 minutes, ending when unrecoverable electromechanical dissociation supervened. Subsequent autopsy revealed the cause of death to be massive PE.
Discussion
The paramount issues in this case surrounded diagnosis, reperfusion, risk of occult issues and deep vein thrombosis prophylaxis. Firstly, the diagnosis of the patient’s presenting complaint was difficult. The presentation would have initially suggested a catastrophic cerebral event, but cranial CT ruled out hemorrhage. Cranial CT remains the gold standard in the acute setting, helping rule out hemorrhagic insult and then allowing for consideration of reperfusion therapy when the diagnosis of infarction is certain. However, as high as 60% of acute cranial CTs are normal in the first few hours following ischemic-infarction [8].
The next consideration was occult hemorrhage continuing in the brainstem and what was the onset-time if an infarction was the diagnosis. Had the time prior to presentation exceeded 3 or even 6 hours? [9–11]. If so, the potential for creating further insult from reperfusion-injury was significant [12]. However, at this time the clinical picture improved, with a degree of consciousness returning and seizure activity being controlled. A second cranial CT continued to show nil intracranial pathology and more detailed imaging sort.
An MRI scan showed the above-mentioned findings and a right AACA (Figure 1). The formation of this AACA was best classified as bihemispheric with most of the major hemispheric vessels presumed to arise from this right-sided vessel [4]. However, it did not posses the left portion of the anterior communicating artery which would normally complete the circle of Willis [2]. The next consideration was the size of the AACA, it was enlarged, but was this a normal anatomical variant (supplying both hemispheres) or was it potentially aneurysmal? Chemical DVT prophylaxis was decided not appropriate at this juncture as the incidence of aneurysms (occult) is high and the artery is already of large caliber and surrounded by potential areas of infarct [3,7]. Another consideration was had there only been a transient pathology of this AACA causing the decreased conscious state and ongoing seizure activity?
However, the patient’s condition continued to improve until a fatal PE occurred, presumed from the right calf, which was noted to be swollen during resuscitation efforts. Thrombolytic therapy was given, but not successful and unfortunately death occurred before neurosurgical and neurology advice could be sought regarding further management.
Conclusion
In conclusion, this case highlights the therapeutic dilemma faced by clinicians in cases such as this. The treatment options had the potential to cause more damage than already sustained and how the risk of implementing prophylactic treatment could increase the consequences of a potentially devastating bleed from an AACA.
This case also highlights the need for early specialist consultation and access to high-level diagnostic imaging. It is hoped the sharing of this very rare anatomical variant will alert fellow clinicians to the significance of a patient with an AACA, bearing in consideration the alteration of arterial hemodynamics of the frontal lobe and the increased incidence of malformations like agenesis of the corpus callosum, hydranencephaly, saccular aneurysms and arterio-venous malformations. An awareness of the potential of these malformations will allow more informed decisions regarding the management of patients presenting with an AACA in the acute context or as an incidental finding.
References
- Wolpert SM. The Circle of Willis. AJNR Am J Neuroradiol. 1997; 18: 1033–1034.
- McMinn RMH, ed. Last’s Anatomy: Regional and Applied. 9th Ed., Hong Kong, Churchill Livingstone. 2003: 599–601.
- Huh JS, Park SK, Shin JJ, Kim TH. Saccular aneurysm of the azygos anterior cerebral artery: three case reports. J Korean Neurosurg Soc. 2007; 42: 342–345.
- LeMay M, Gooding CA. The clinical significance of the azygous anterior cerebral artery. Am J Roentgenol Radium Ther Nucl Med. 1966; 98: 602–610.
- Watts JW. A comparative study of the anterior cerebral artery and the circle of Willis in primates. J Anat. 1934; 68: 534–550.
- Anterior Cerebral Artery. http://neuroangio.org/AnteriorCerebralArtery.aspx (accessed May 2011).
- Milenkovic Z, Puzic M, Vasovic L, Djuric S, Vucetic R, Dragan Stojanov. The azygos anterior cerebral artery aneurysms confirmed at operation. Facta Universitatis, Series: Medicine and Biology. 1997; 4: 40–43.
- Acute Cerebral Infarction. http://brighamrad.harvard.edu/Cases/bwh/hcache/93/full.html (accessed May 2011).
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359: 1317–1329.
- Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, Hommel M. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke. 1999; 30: 1326–1332.
- Larrue V, von Kummer R R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001; 32: 438–441.
- Elzawahry H, Hernandez-Frau P, Behrouz R. Reperfusion Injury in Stroke. http://emedicine.medscape.com/article/1162437-overview (accessed May 2011).