CD68 immunostaining of tubular epithelium is an excellent indicator of Calcineurin inhibitor toxicity in kidney transplant patient
2 King Abdulla University Hospital, Amman, Jordan, Email: farsak@gmail.com
3 Department of Nephrology, Istishari Hospital, Amman, Jordan, Email: nidal@gmail.com
Received: 03-Oct-2017 Accepted Date: Oct 19, 2017; Published: 26-Oct-2017
Citation: Abu Farsakh, HA, Abu Farsak HN, Badran N. CD68 immunostaining of Tubular epithelium is an excellent indicator of Calcineurin inhibitor toxicity in kidney transplant patient. J Histol Histopathol Res 2017;1(1):15-18.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
CONTEXT: Calcineurin inhibitor toxicity [CNI] is a major cause of posttransplant renal impairment. Its prompt recognition is essential in renal transplant management.
DESIGN: A retrospective study on 162 cases of kidney transplant core biopsies were reviewed over the last 4 years in our Nephropathology Department. The diagnosis of CNI was rendered in 54 cases [33%]; while rejection was rendered in 78 cases [48%]. In all cases, C4d and CD68 were performed by immunohistochemistry on formalin fixed, paraffin embedded tissue. The pattern of CD68 positivity was then analyzed into two patterns: histiocyte staining pattern and tubular epithelium granular pattern.
RESULTS: C4d was positive in 44 out of 162 cases [27%]. CD68 was positive in 133 cases out of 162 [83%]. The pattern of CD68 positivity was then analyzed into two patterns: histiocyte staining pattern in 82 out of 162 cases [51%], and tubular epithelium granular pattern in 53 out of 162 cases [33%]. In the remaining 27 cases [16%], both patterns are seen within the same case. 53 [out of 54 cases: 98%] with CNI showed tubular epithelium granular pattern at least focally. On the other hand, only one case with rejection out of 78 cases [1%] showed granular tubular epithelium pattern.
CONCLUSION: CD68 immunostaining in tubular granular pattern in biopsies from kidney transplant patient is an excellent marker for diagnosing CNI in kidney transplant patients.
Abbreviations
CNI: Calcineurin Inhibitor Toxicity; TMA: Thrombotic Microangiopathy
Calcineurin inhibitor [CNI], discovered in late 1970s, with cyclosporine followed by tacrolimus [1-3]. The nephrotoxicity of cyclosporine was reported in the first publications on the clinical use of cyclosporine in humans after renal transplantation, followed by other publications on its long-term use with irreversible renal functional deterioration [4,5].
CNI toxicity can be divided into acute and chronic CNI nephrotoxicity. The acute changes were reported in different structures: arteries, tubules and endothelial cells by causing acute arteriolopathy without histologic changes, tubular isometric vacuolization and thrombotic microangiopathy [6-12]. Chronic CNI nephrotoxicity, on the other hand, includes the following reported changes [9,10,12-16]: Interstitial fibrosis and tubular atrophy [typically striped], medial arteriolar hyalinosis, glomerular capsular fibrosis, global glomerulosclerosis, focal segmental glomerulosclerosis [FSGS], juxtaglomerular apparatus hyperplasia, and/or tubular microcalcifications. The Pathogenesis of CNI nephrotoxicity is attributed to at least the following means: Its vasoconstriction effect on the afferent arterioles, by an increase in endothelin and thromboxane and activation of the renin-angiotensin system as well as a reduction of vasodilator factors like prostacyclin, prostaglandin E2, and nitric oxide [5,17]. It also directly activates apoptosis genes and increase apoptosis in tubular and interstitial cells, thereby inducing tubular atrophy [5,18,19]. The third possible way is its direct toxic effect on the tubules [20].
CNI and rejections are two major important diagnostic dilemmas that face the nephrologist and the nephropathlogist in the post-transplant period. The diagnosis has to be timely and accurate. Unfortunately, the treatment of each category is in the opposite direction of the other. Any mistakes in this field can results in loss of the patient’s kidney and revert him to dialysis soon. The aim of this study is to diagnose changes of CNI by an objective tool, like immunohistochemistry and confirm them as soon as possible.
There are several immunohistochemical markers that are useful for rejections, C4d, CD8 and CD68 [21, 22]. So far, there is no objective stain that can diagnose reliably CNI toxicity. Trials to detect CNI toxicity was attempted by Meehan et al. by performing immunohistochemistry to CD61, a platelets marker [23]. CD61 stained arterioles in cases with CNI toxicity, especially in cases with thrombotic Microangiopathy [TMA] [23]. But, in their study, it was not helpful when no TMA is present. CD68 immunohistochemical marker of histiocytes. It is useful in confirming rejection by staining the histiocytes in renal biopsies [21,22]. We noticed that CD68 stains tubular epithelium cells in cases with CNI toxicity, but it does not stain them in cases of rejection. This observation made us to retrospectively study all cases of renal transplant biopsies that were submitted to our nephropathology department, by using CD68 to sort cases that were positive in the tubular epithelium vs. those that are positive in the histiocytes.
Design
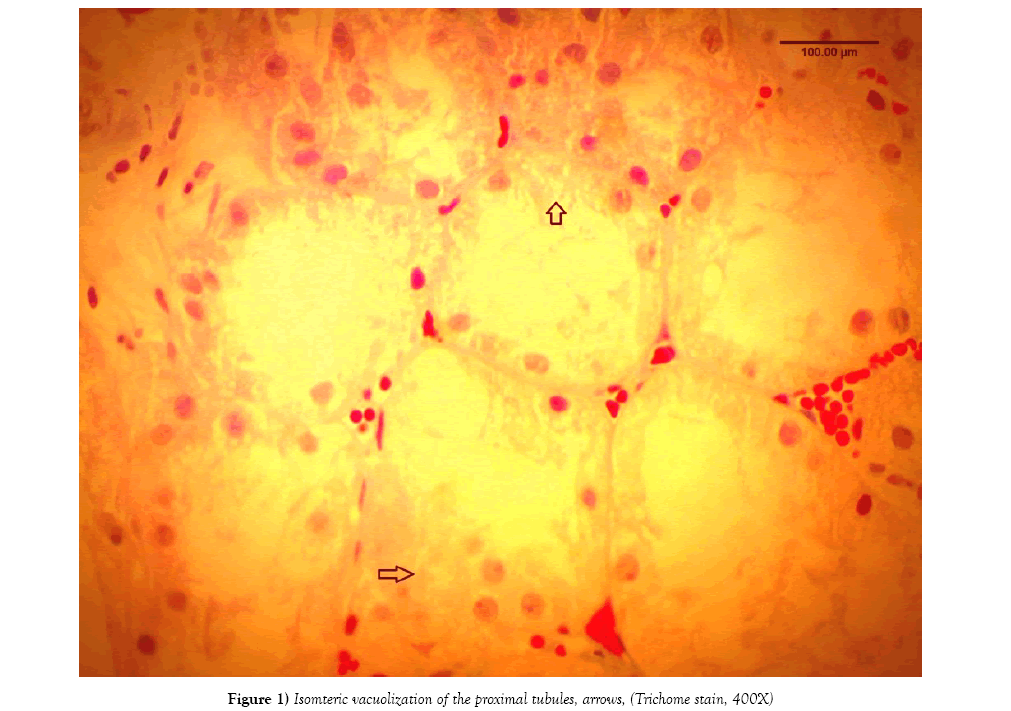
A retrospective study on 162 cases of kidney transplant core biopsies were reviewed over the last 4 years in our Nephropathology Department. The diagnosis of CNI nephrotoxicity was rendered in 54 cases [33%] based on morphological changes reported in several papers [4-10] (Figure 1) while rejection was rendered in 78 cases [48%]. In all cases, C4d and CD68 were performed by immunohistochemistry on formalin fixed paraffin embedded tissue. The immunostainings were performed via link-label chromogen detection system. C4d is [Biomedica, rabbit polyclonal, dilution 1:30] and CD68 [bioGenex KPI clone, Ready to use], The pattern of CD68 positivity was then analyzed into two patterns: histiocyte staining pattern and tubular epithelium granular pattern.
Results
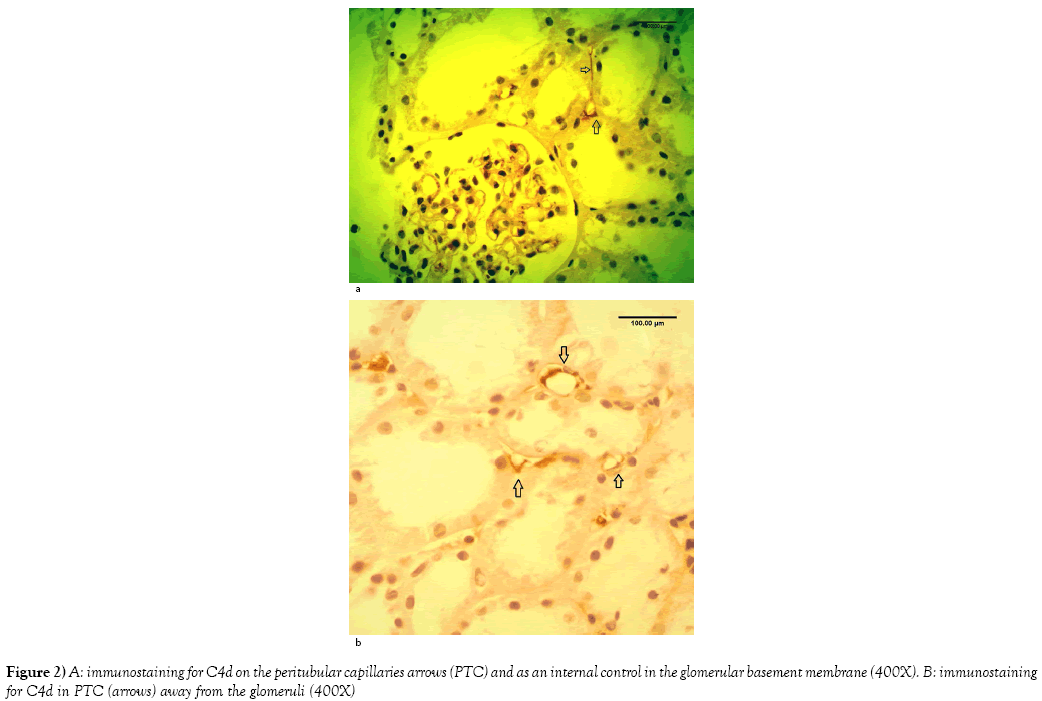
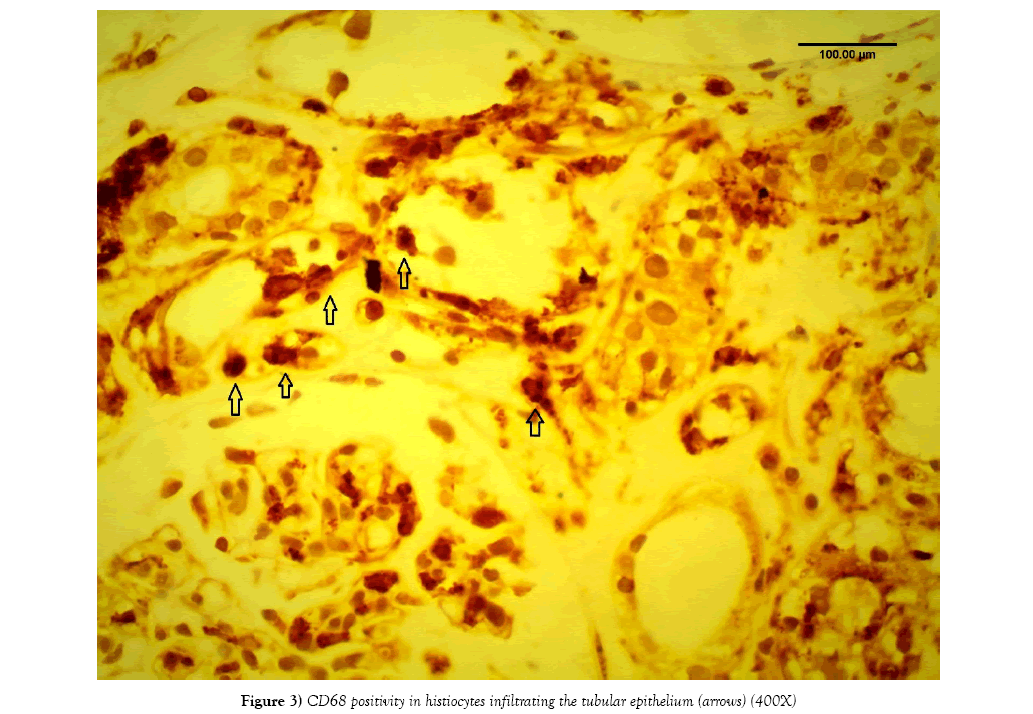
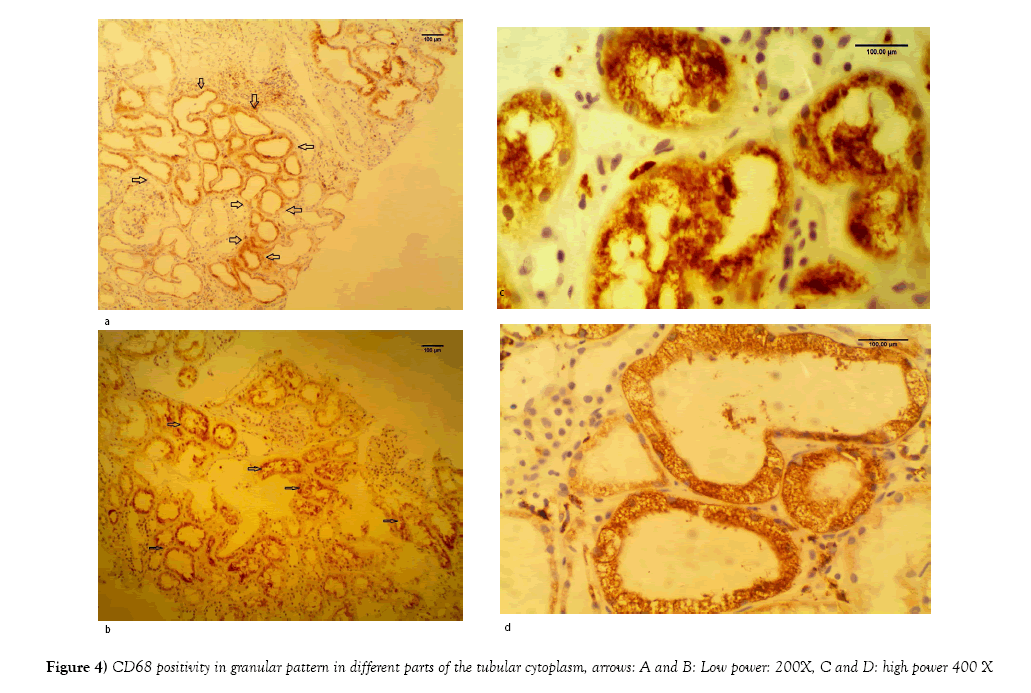
Rejection diagnosis [acute and/or chronic] was rendered in 78 out of 162 cases [48%]. C4d was positive only in 44 cases [27%] (Figures 2A and 2B). On the other hand, CD68 immunostainings was positive in 133 cases out of 162 [83%]. The pattern of CD68 positivity was then analyzed into two patterns: histiocyte-staining pattern in 82 out of 162 cases [51%] (Figure 3), and tubular epithelium granular pattern in 53 out of 162 cases [33%]. In the remaining 27 cases [16%], both patterns were expressed. [53 out of 54 cases: 98%] with CNI showed tubular epithelium granular pattern at least focally (Figures 4A-4D). On the other hand, only one case with rejection out of 78 cases (1%) showed granular tubular epithelium pattern.
Discussion and Conclusion
CD68 is an immunostaining of protein called: macrosialin. The gene for macrosialin protein encodes a 110-kD transmembrane glycoprotein that is highly expressed by human monocytes and tissue macrophages. It is a member of the lysosomal/endosomal-associated membrane glycoprotein [LAMP] family [24,25]. The protein primarily localizes to lysosomes and endosomes with a smaller fraction circulating to the cell surface [24,25]. It is a type I integral membrane protein with a heavily glycosylated extracellular domain and binds to tissue- and organ-specific lectins or selectins (24,25]. The protein is also a member of the scavenger receptor family. Scavenger receptors typically function to clear cellular debris, promote phagocytosis, and mediate the recruitment and activation of macrophages. Alternative splicing results in multiple transcripts encoding different isoforms [24,25]. Tubular epithelium has a prominent endoplasmic reticulum that upon tubular toxic effect become more prominent. Calcineurin inhibitors exert toxic effect on tubules by enlargement of the endoplasmic reticulum and increased lysosomes [26]. Histologically, this effect manifests as isometric vacuolization of the tubular cytoplasm, Isometric tubular vacuolization could be the consequence of relative ischemia caused by afferent arteriolar vasoconstriction, or direct toxic tubulopathy [27,28]. The other toxic changes in the tubules, is inclusion bodies in the tubular epithelium cytoplasm. Ultrastructurally, these inclusion bodies represent giant mitochondria and autolysomes [29,30]. Another toxic effect has been attributed to inhibition of the cell cycle through cyclosporine-induced accumulation of p53 [31-33].
The authors propose that Macrosialin protein becomes more prominent in the tubular cytoplasm as a reaction to toxic effect of CNI. Thus, it becomes easily stainable by CD68. This may detect subclinical cases of CNI toxicity effect on the tubular cytoplasm and help in the early management of this problem. Our study proves that by performing a relatively a cheap immunohistochemical marker [CD68], nephropathologists can sort out cases of rejection [by strong staining of histiocytes] vs. CNI toxicity [by staining of tubular epithelium]. The cases that show mixed pattern of staining, the nephropathologists has to use, in addition, the clinical status, the other histological criteria for rejection or CNI toxicity and the amount of histiocytes that are stained. In our opinion, the wisely interpretation of CD68 immunostainings is very helpful in cases of renal transplant management. We advocate the use of this marker routinely in all cases of renal transplant.
Acknowledgement
This research was presented in part at Collage of American Pathologists CAP17
REFERENCES
- Calne RY, White DJ, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978;2: 1323-27.
- Starzl TE, Todo S, Fung J, et al. FK 506 for liver, kidney, and pancreas transplantation. Lancet 1989;2:1000–4.
- Nankivell BJ, Chow H, Philip J, et al. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: Comparison of cyclosporine and tacrolimus eras. Transplantation 2016;100: 1723–31.
- Myers BD, Ross J, Newton L, et al. Cyclosporine-associated chronic nephropathy. N Engl J Med 1984;311:699–705.
- Norma AB, Gerardo G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. American Journal of Physiology - Renal Physiology 2007;293(1):F2-F9.
- Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: New insight and preventive strategies. Curr Opin Crit Care 2001;7:384–89.
- Homan WP, Fabre JW, Williams KA, et al. Studies on the immunosuppressive properties of cyclosporine a in rats receiving renal allografts. Transplantation 1980;29:361–66.
- Homan WP, French ME, Millard P, et al. Studies on the effects of cyclosporin A upon renal allograft rejection in the dog. Surgery 1980;88:168–73.
- Mathis A, Egloff G, Ghin GL. Calcineurin inhibitor sparing strategies in renal transplantation, part one: Late sparing strategies. World J Transplant 2014;4(2):57–80.
- Naesens M, KuypersD, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009;4:481–508.
- Neeraja K, Suja Na, Sheryl S, et al. Semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. CJASN 2007;2(1):135-42.
- Maarten N, Dirk RJK, Minnie S. Calcineurin inhibitor nephrotoxicity. CJASN 2009;4(2):481-508.
- Randhawa PS, Shapiro R, Jordan ML, et al. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506: Clinical significance and comparison with cyclosporine. Am J Surg Pathol 1993;17:60–68.
- Ryffel B. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol 1998;49:356–63.
- Morozumi K, Takeda A, Uchida K, et al. Cyclosporine nephrotoxicity: how does it affect renal allograft function and transplant morphology. Transpl Proc 2004;36:S251–S56.
- Norma AB, Gerardo G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. American Journal of Physiology - Renal Physiology 2007;293(1):F2-F9.
- Dieperink H, Starklint H, Leyssac PP, et al. Glomerulotubular function in cyclosporine-treated rats: A lithium clearance, occlusion time/transit time and micropuncture study. Proc Eur Dial Transplant Assoc Eur Ren Assoc 1985;21:853–59.
- Shihab FS, Andoh TF, Tanner AM, et al. Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int 1999;56:2147–59.
- Thomas SE, Andoh TF, Pichler RH, et al. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int 1998;53:897–908.
- Heering P, Grabensee B. Influence of ciclosporin A on renal tubular function after kidney transplantation. Nephron 1991;59:66–70.
- Kulkarni PUM, Prayaga ADU. Dakshina Murthy K1 Renal allograft pathology with C4d immunostaining in patients with graft dysfunction. Indian J Nephrol 2011;21(4):239–44.
- Troxell M, Lanciault C. Practical applications in immunohistochemistry evaluation of rejection and infection in organ transplantation. Arch Pathol Lab Med 2016;140:910–925.
- Meehan SM, Baliga R, Poduval R, et al. Platelet CD61 expression in vascular calcineurin inhibitor toxicity of renal allografts. Hum Pathol. 2008;39(4):550-6.
- Fukuda Minoru. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking" (PDF). J Biol Chem 1991;266(32):21327-30.
- Dimitry AC, Murray CK, Veronika M, et al. CD68/macrosialin: Not just a histochemical marker, Laboratory Investigation 2016;97:4-13.
- Pallet N, Rabant M, Xu-DYC, et al. Response of human renal tubular cells to cyclosporine and sirolimus: A toxicogenomic study. Toxicol Appl Pharmacol 2008;229:184–96.
- Mihatsch MJ, Ryffel B, Hermle M, et al. Morphology of cyclosporine nephrotoxicity in the rat. Clin Nephrol 1986;25:S2–S8.
- Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. American Journal of Transplantation 2011;11:2123–31.
- Kim JY, Suh KS. Light microscopic and electron microscopic features of cyclosporine nephrotoxicity in rats. J Korean Med Sci 1995;10:352–9.
- Jamil RA, Mohamed HS, Samir GM. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol 2013;191(12):5785-91.
- Healy E, Dempsey M, Lally C, et al. Apoptosis and necrosis: Mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int 1998;54:1955–66.
- Lally C, Healy E, Ryan MP. Cyclosporine A-induced cell cycle arrest and cell death in renal epithelial cells. Kidney Int 1999;56: 1254–57.
- Paul J, Christian K, Sonia A, et al. Cyclosporine A induces senescence in renal tubular epithelial cells. American Journal of Physiology - Renal Physiology 2007;293(3):831-38.