Circumaortic left renal vein ipsilateral to an adrenal incidentaloma: A case report
Received: 07-Oct-2017 Accepted Date: Oct 14, 2017; Published: 22-Oct-2017
Citation: Thomson F. Circumaortic left renal vein ipsilateral to an adrenal incidentaloma: A case report. Int J Anat Var. 2017;10(4): 85-87.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
During a routine dissection of a cadaver, a circumaortic left renal vein was found ipsilateral to an enlarged adrenal gland. There were two branches draining into the inferior vena-cava, the superior branch entered at the L1 level, and was smaller than the inferior branch which entered at L3. These variations can present medical and surgical issues, and are relatively rare findings, with a meta-analysis plus five other studies putting the incidence of circumaortic renal veins at 2.6%.
Keywords
Adrenal; Renal vein; Anatomy variation; Circumaortic; Incidentaloma
Introduction
Circumaortic renal veins and adrenal incidentalomas are anatomical variations that can be the source of several pathologies. Through advances in non-invasive imaging, and a rise in surgeries on both the kidneys and adrenals, these variations are being increasingly discovered. This case report describes the discovery of both variations in the same patient during a routine dissection. Both variations are rare, and this study also combines a meta-analysis of venous abnormalities with five further studies to suggest the incidence for circumaortic renal veins is 2.6%.
Case Report
Materials and methods
A standard dissection was performed of an 84-year old male cadaver who had died of primary lung cancer. When the abdomen was dissected out, two renal veins were discovered supplying the left kidney, one travelling anterior and the other posterior to the aorta. The dissection was then extended to remove the peri-renal fat, and to mobilise both the kidney and the adrenal gland of the left side.
Results
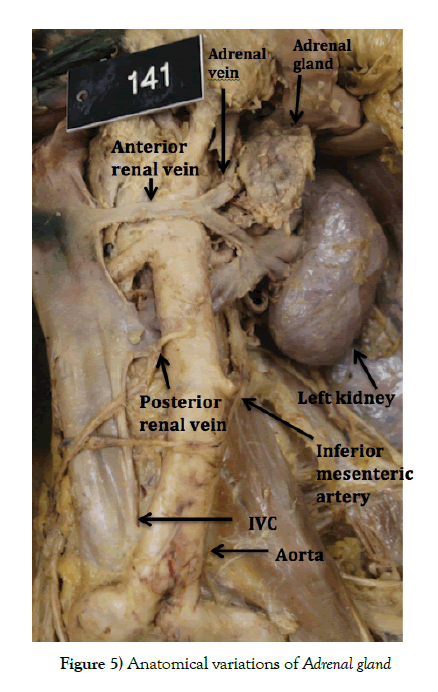
Of the two left renal veins, the superior most was smaller (9 mm diameter) and crossed anterior to the aorta and just below the superior mesenteric artery at approximately the L1 level. The inferior vessel was larger (14 mm diameter) and crossed posterior to the aorta at approximately the L2-3 level between the renal and inferior mesenteric artery. The kidney was otherwise normal, however the adrenal gland was found to be enlarged, measuring 80 mm in height and 30 mm in width. The inferior adrenal vein was also enlarged at 9 mm diameter, formed from two tributaries which merged before draining into the antero-superior renal vein. Both the right kidney and the right adrenal gland had no further anatomical variations of note.
Discussion
Pathogenesis
Adrenal incidentalomas are enlargements of the adrenal gland, which often produce no symptoms. The use of non-invasive 3D imaging has led to an increased discovery of them, and they are described radiologically as any adrenal tumour greater than 1 cm, discovered when adrenal disease was not the indication for imaging. Approximately 4% of abdominal CT scans have identified an adrenal incidentaloma, and around 15% of these are then found on further investigation to be sub-clinically hyperfunctioning, with the majority linked to catecholamine excess [1,2].
There is theoretically no limit to their size, with one being discovered measuring 35 x 25 x 11 cm in size. [3] In general, the larger the adrenal gland, the greater the chance of it producing symptoms, with one study suggesting over 45% of adrenal tumours more than 4 cm in size will be malignant [4], although this can be made more specific when other factors are considered, including specific imaging features [5] and various blood or urinary markers [6].
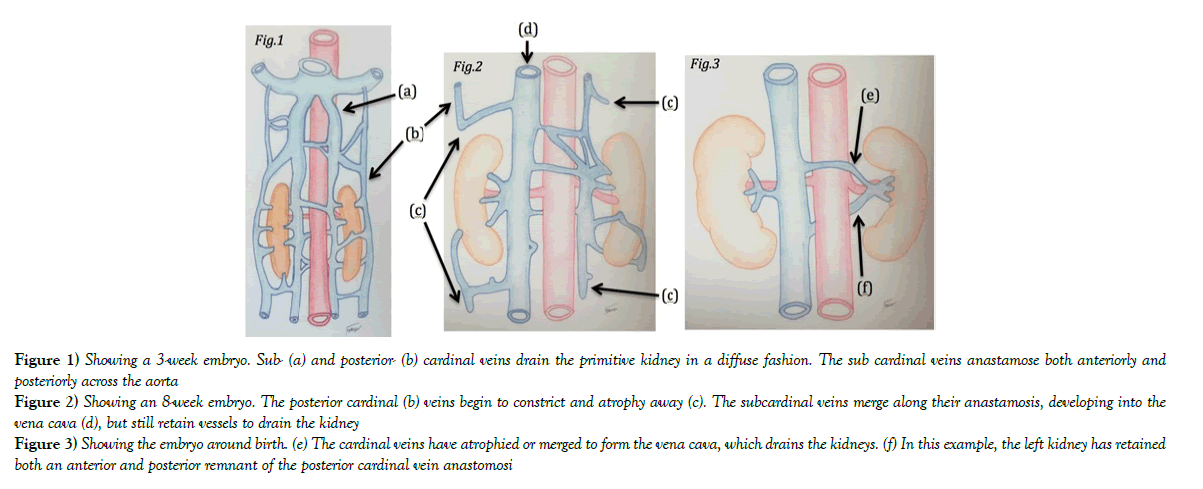
Circumaortic renal veins develop during the embryological stage. Normally the renal veins are formed from a poorly demarcated arrangement of venous networks, known as the cardinal system, which drain most of the embryological abdominal organs, including the mesonephric streaks. The cardinal veins can be described as having two main tributaries; the posterior cardinal (Figure 1a) and sub cardinal (Figure 1b).
They are both paired either side of the aorta, with communications passing both anteriorly and posteriorly. Over time, these vessels will grow in size and as they do, some areas will begin to constrict, blocking off drainage from that area. The vessel downstream of the constriction will then begin to atrophy away (Figure 2b-d), leading to a regression of most of the posterior cardinal vessels and other smaller tributaries. Meanwhile, the subcardinal veins start to anastamose as they grow, merging into a larger vessel next to the aorta which will eventually become the vena cava. Remnants of the left subcardinal vein still drain the kidney, but now towards the primitive vena cava (Figure 2). It is still possible at this point to retain the posterior anastomosis of the two subcardinal veins, and if they do not atrophy away then they will develop into a posterior renal vein when the foetus is fully developed (Figure 3f). Sometimes this will be the only vein (retroaortic renal vein), and sometimes it will develop alongside the more common anterior renal vein (Figure 3e) [7].
Figure 1) Showing a 3-week embryo. Sub- (a) and posterior- (b) cardinal veins drain the primitive kidney in a diffuse fashion. The sub cardinal veins anastamose both anteriorly and posteriorly across the aorta
Figure 2) Showing an 8-week embryo. The posterior cardinal (b) veins begin to constrict and atrophy away (c). The subcardinal veins merge along their anastamosis, developing into the vena cava (d), but still retain vessels to drain the kidney
Figure 3) Showing the embryo around birth. (e) The cardinal veins have atrophied or merged to form the vena cava, which drains the kidneys. (f) In this example, the left kidney has retained both an anterior and posterior remnant of the posterior cardinal vein anastomosi
Incidence
To find the incidence, four studies were added to a thorough metanalysis of venous abnormalities. The meta-analysis totalled 487 findings of circumaortic renal veins out of 19,060 cadaveric, operative and radiological studies. [8]. In addition, four other studies were found which were not included in the meta-analysis which detailed; 8 findings out of 74 venograms [9], 2 findings out of 152 angiograms [10], 19 findings out of 433 CT scans [11] and 7 findings out of 150 cadaveric studies [12]. Added to this can be the findings from St Georges Anatomy and Dissection department, as this report is the second cadaveric finding out of around 280 studies. This gives a sum total of 525 findings out of 20,149 studies, giving an incidence of 2.6%.
Clinical significance
Clinically, both of these variations can present with specific symptoms. Hyperfunctioning adrenals can include anxiety, sweating, non-responsive hypertension, diabetes and any other symptom related to an increase in any of the hormones produced by the gland. [2] Circumaortic renal veins rarely produce symptoms, however they have been attributed to producing haematuria as a by-product of renal hypertension [13].
This can occur when a renal vein is compressed for some reason, and the blood cannot escape, builds up, and damages the kidney [14]. In this patient, the posterior vein was the larger, and so entrapment between the aorta and the vertebral column could have had a profound effect on the venous outflow from the kidney.
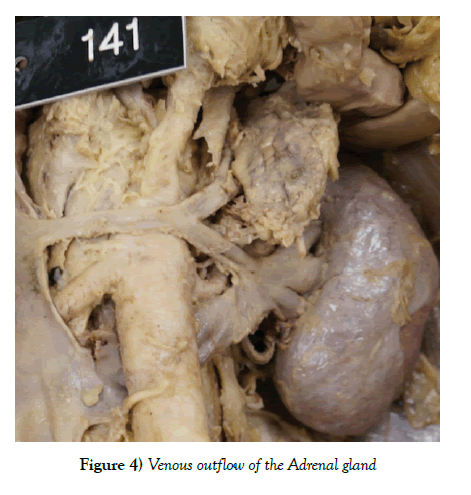
Surgical problems can also arise from either of these anatomical variations. One is that, if not identified, then during any abdominal surgery, the unnoticed vein could get damaged, leading to a large rate of blood loss directly from the IVC. [15] Another problem involves adrenal vein sampling. If there is doubt as to whether an adrenal gland is hyperfunctioning, a catheter can be passed through the vena cava and renal vein in order to get a blood sample directly from the venous outflow of the adrenal gland (Figure 4). Had this person required it, there may have been navigational errors due to the anatomical variation (Figure 5).
Conclusion
Taken together, an adrenal incidentaloma rate of 4% and a circumaortic renal vein rate of 2.6% suggests that, without other factors being considered, there is a 0.01% chance of both anomalies being present. No other studies of both being present have been found, although it is worth noting that many of the large studies have not specifically looked for this combination. Since the medical records of the cadaver were not available, it is impossible to tell whether the adrenal gland was hyperfunctioning, or whether there was any evidence of venous hypertension. Nonetheless, this case demonstrates how clinically silent anatomical variations can occur together and the importance of this to surgeons and medical practitioners.
REFERENCES
- Royal S, Callen P CT. Evaluation of Anomalies of the Inferior Vena Cava and Left Renal Vein. AM J Roentgenol. 1979;132:759-63.
- Dent P, Palazzo F. Adrenal Tumours in Sam A, Hameed S Recent Advances in Endocrinology and Diabetes, JP Medical. 2017;43-56.
- Li B, Guo Q, Yang H, et al. Giant non-functional adrenal adenoma: A case report. Oncol Let. 2013;5:378-80.
- Francis IR, Smid A, Gross MD, et al. Adrenal masses in oncologic patients: functional and morphologic evaluation. Radiology. 1988;166: 353-6.
- Jenny Yoo, Kelly McCoy, Sally Carty, et al. Adrenal Imaging Features Predict Malignancy Better than Tumour Size. Ann Sur Oncol 2015; 22:721-7.
- Dhaval Patel, Matthew DT, Soumen K, et al. Gonzalez and Electron Kebebew Unique and novel urinary metabolic features in malignany versus benign adrenal neoplasms. Clinical Cancer Research. 2017;23:5302-10.
- Hikspoors JPJM, Soffers JHM, Mekonen HK, et al. Development of the human infrahepatic inferior caval and azygos venous systems. J Anatomy. 2015;226:113-25.
- Yi SQ, Ueno Y, Naito M, et al. The three most common variations of the left renal vein: a review and meta-analysis. Surg Radiol Anat. 2012;34:799-804.
- Beckmann CF, Abrams HL. Circumaortic venous ring: Incidence and significance. American J Roentgenology. 1979;132:561-5.
- Hayashi M, Kume T, Nihira H. Abnormalities of Renal Venous System and Unexplained Renal Hematuria. J Urol. 1980;124:12-6.
- Reed MD, Friedman AC, Nealey P. Anomalies of the Left Renal Vein: Analysis of 433 CT Scans. J Comp Ass Tomography. 1982;6:1124-6.
- Kaufman JA, Waltman AC, Rivitz SM, et al. Anatomical observations of the renal veins and inferior vena cava at magnetic resonance angiography. Cardio Vascular and Interventional Radiology. 1995;18:153-7.
- Gibo M, Onitsuka H. Retroaortic left renal vein with renal vein hypertension causing hematuria Clinical Imaging. 1998;22:422-4.
- Kurklinsky AK, Thom WR. Nutcracker Phenomenon and Nutcracker Syndrome. Mayo Clinic Proceedings. 2010;85:552-9.
- Ros Pablo, Mortele KJ. CT and MRI of the Abdomen and Pelvis: A Teaching File Lippincott Williams & Wilkins. 2007;421.