Clinical Impact of Rare Aortic Arch Variant
Received: 17-May-2021 Accepted Date: Jun 16, 2021; Published: 23-Jun-2021, DOI: 10.37532/1308-4038.14(6).130-131
Citation: Magnuson CA, Quinn MM. Clinical Impact of Rare Aortic Arch Variant. Int J Anat Var. 2021;14(6):97-98.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
This case report describes the clinical implications of peripheral arterial disease in a patient with a rare variant of the aortic arch branches. The patient was a 68-year old-female with an extensive cardiovascular history including multiple severe peripheral arterial stenoses, systemic and pulmonary hypertension, hyperlipidemia, coronary artery disease, multiple myocardial infarctions, atrial fibrillation, and possible aortic valve disease. The focus of the case report will be how the patient’s anatomical variant played a large role in maintaining cerebral circulation in the presence of significant stenoses of the right vertebral artery, left subclavian artery, and left common carotid artery.
Introduction
This case report focuses on the clinical implications of an aortic arch variant in the context of a peripheral arterial disease, coronary artery disease, and many other significant non-cardiovascular comorbidities. The variant found in this patient played a prominent role in maintaining cerebral perfusion as her peripheral artery disease progressed in both of her subclavian arteries, left common carotid artery, and right vertebral artery; all of which have an important role in providing continuous blood flow to the brain. This case report also discusses how aortic arch variants develop and the atypical clinical presentations that may arise as a result of the variants.
Case Report
Our patient was a 68-year-old female who passed away as a result of ischemic cardiomyopathy. She had a cardiovascular medical history that consisted of coronary artery disease, systemic hypertension, pulmonary hypertension, hyperlipidemia, atrial fibrillation, and peripheral vascular disease. In addition to her cardiovascular history, she also suffered from COPD, type II diabetes mellitus, osteoarthritis, and pyelonephritis.
After being diagnosed with peripheral arterial disease (PAD) of her subclavian artery 5 years prior to her death, aortic arch angiography revealed a branching variation that played a significant role in retaining cerebral circulation despite the complete occlusion of the left subclavian artery (LSC), which would normally stop or even reverse blood flow of the left vertebral artery (known as subclavian steal syndrome)[6]. The patient underwent a carotid-subclavian bypass surgery utilizing a Dacron graft to revascularize her left upper limb.
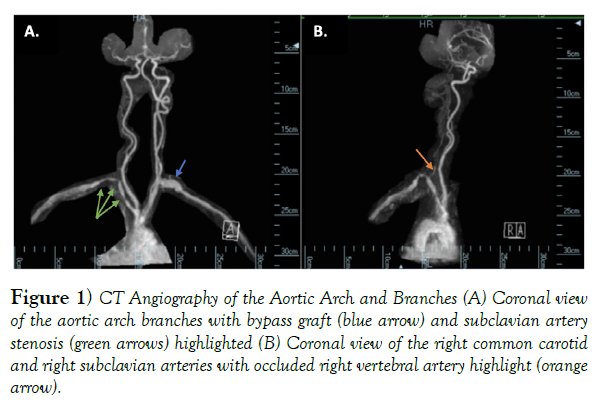
In the subsequent years, she suffered two episodes of myocardial infarction, which lead to global hypokinesis of her left ventricle as seen on echocardiography and ultimately, her passing. In addition, her peripheral vascular disease progressed post-surgery to result in multiple stenoses of the right subclavian artery (RSC), near-complete occlusion of the right vertebral artery (RVA), and severe stenosis of the left common carotid artery (LCC) just proximal to the bifurcation of the left external carotid artery seen by CT angiography in (Figure 1).
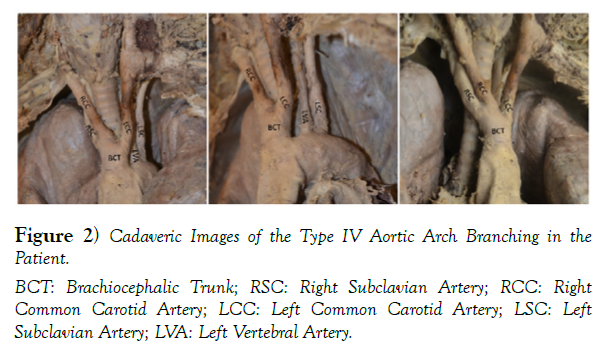
Prior to her death the patient registered her body to the Division of Anatomy’s Body Donor Program at The Ohio State University. With her generous gift, we were able to learn and see her rare anatomical variation, which can be seen in (Figure 2).
Figure 1) CT Angiography of the Aortic Arch and Branches (A) Coronal view of the aortic arch branches with bypass graft (blue arrow) and subclavian artery stenosis (green arrows) highlighted (B) Coronal view of the right common carotid and right subclavian arteries with occluded right vertebral artery highlight (orange arrow).
Discussion
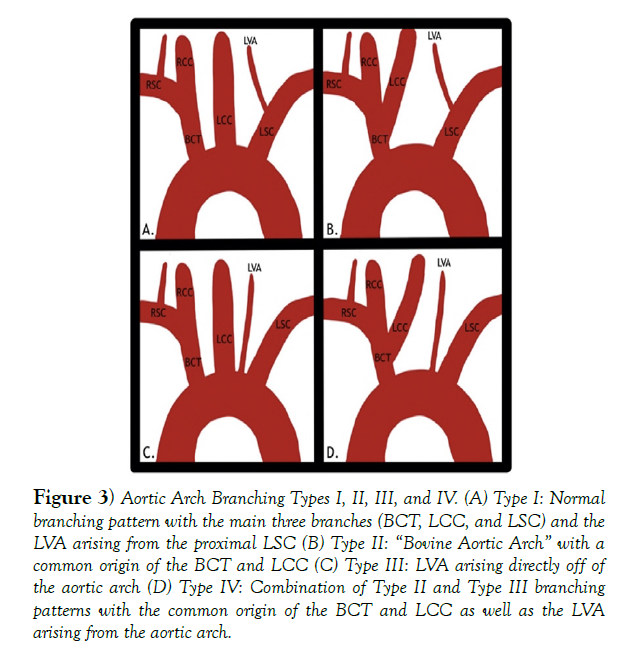
The In the majority of the general population, the aortic arch of the thoracic aorta that gives rise to the 3 major arteries that supply both upper limbs as well as the head and neck in a predictable pattern, but there are 7 major branching patterns that can occur, four of which can be seen in (Figure 3). In the most common type I pattern, the 1st branch is the brachiocephalic trunk, which divides into the right subclavian artery to provide vascular supply to the right upper limb and the right common carotid artery to provide vascular supply to the right half of the head and neck. The 2nd major branch off of the aortic arch is the left common carotid artery, which provides vascular supply to the left half of the head and neck. The final major branch off of the aortic arch is the left subclavian artery (LSC), which supplies blood to the left upper limb [1,3]. Another key artery that can exhibit variation is the left vertebral artery (LVA), which in a normal individual arises from the proximal left subclavian artery1.
The patient described in this case report exhibited a type IV aortic arch pattern, which is a combination of the type II “bovine aortic arch” with a common origin of the brachiocephalic trunk and the LCC and the type III variation where the LVA arises directly from the aortic arch between the LCC and LSA. Her branching pattern has to reported to only be in 1-2% of the population and is usually asymptomatic [3,4].
Figure 3) Aortic Arch Branching Types I, II, III, and IV. (A) Type I: Normal branching pattern with the main three branches (BCT, LCC, and LSC) and the LVA arising from the proximal LSC (B) Type II: “Bovine Aortic Arch” with a common origin of the BCT and LCC (C) Type III: LVA arising directly off of the aortic arch (D) Type IV: Combination of Type II and Type III branching patterns with the common origin of the BCT and LCC as well as the LVA arising from the aortic arch.
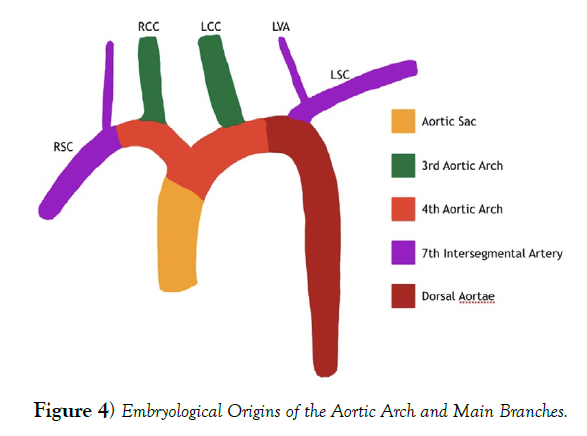
The adult aortic arch and its branches are formed by 5 different cell lineages in a relatively small area. The adult aortic arch is formed by cardiac neural crest (CNC) cells during the 4th and 5th weeks of development. The CNC cells also become the aortic arch arteries are responsible for regulating branch patterning through signals from the endodermal and ectodermal lining of the aortic arch arteries. The aortic arch and its major branches are formed by the 3rd and 4th aortic arch arteries as well as the aortic sac. The 3rd aortic arch artery becomes the common carotid arteries and the proximal internal carotid. The 4th aortic arch arteries persist on both sides with the left forming the aortic arch between the LCC and the LSC, while the right side forms the proximal portion of the RSC. The aortic sac is just distal to the outflow tract. It forms the left and right horns, which gives rise to the brachiocephalic artery and proximal aortic arch. It is also important to note that 7th intersegmental arteries gives rise to the vertebral arteries and the respective subclavian artery just distal to the 4th aortic arch artery. These embryological origins contribute different parts to the developing aortic arch branches leading to the adult version of the aortic arch with the most common branching pattern, which can be seen in (Figure 4), and these different origins have resulted in many branching patterns that have been described in the adult population [3,4].
Anatomical variations like the one seen in this patient can go unnoticed for the majority of a patient’s life and are usually diagnosed incidentally [3-5] which was the case in this individual. However, it is important for clinicians to understand the variations that can be seen and the potential implications of the variants that can be seen throughout the human body. Aortic arch variants as this example have been shown to be associated with an increased risk of thoracic aorta dilation and dissection4 as well as increased risk of cerebral aneurysms. However, these variants can lead to unusual presentations and symptoms with the development of other arterial disorders [3-6].
In this patient, the advanced PAD was a likely result of the combination of multiple factors including smoking, diabetes mellitus, hyperlipidemia, and hypertension as all have been known to increase the incidence of PAD7. Her comorbidities led to complete occlusion of the LSC, which required a bypass graft from the LCC. Another stenosis was identified in the LCC distal to the bypass graft near the bifurcation of the internal and external carotid arteries during follow up after the bypass graft procedure. Additionally, multiple stenoses were seen in the RSC as well as severe obstruction of the RVA.
All of these areas of stenosis would have result in decreased cerebral blood flow in addition to impaired flow to both upper extremities. Due to the stenoses of the LCC and RVA, the predominant source of left-sided cerebral circulation was from the LVA while the right internal carotid artery was required to compensate for the diminished flow through the RVA1. Interestingly, the variant origin of the left vertebral artery actually had a beneficial effect in this patient. Had the LVA originated in its normal position off the proximal LSC, the complete occlusion would have cut off its source of circulation while also potentially siphoning additional flow from the cerebral circulation as a result of “subclavian steal syndrome” leading to an even further reduction in blood flow to the brain [1,6]. Due to the variation, the LVA was allowed to function relatively unaffected to preserve the dominant circulation to the left side of the cerebral circulation while the right side was almost completely supplied by the right internal carotid artery.
Early in this patient’s life, this anatomical variation likely had little to no impact on her everyday life [3-5]. As her list of illnesses grew longer, this variation potentially had a positive impact as her PAD progressed due to retained cerebral circulation via the LVA that was unaffected by the patient’s PAD. Had her LVA been in its normal position, the neurological presentation of her PAD would have likely been more prominent as additional sources of cerebral blood flow were pinched off by the atherosclerotic lesions in her LCC and RVA [1,3,6]. This patient case emphasizes the importance of understanding the basic embryology of the cardiovascular system as well as the anatomical variants that can be seen in the general population [2,7].
Acknowledgement
Support for this project was provided by The Ohio State University, Division of Anatomy Body Donor Program, with special thanks to the generous donors and their loved ones.
REFERENCES
- IbingraStandring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 41st Ed., New York, Elsevier Limited. 2016:1097-1102.
- Sadler T, Langman J. Langman's Medical Embryology. 2015:202-214.
- Karacan A, Türkvatan, Karacan K. Anatomical variations of aortic arch branching: evaluation with computer tomographic angiography. Cardiology in the Young. 2014:485-493.
- Mylonas SN, Barkans A, Ante M, et al. Prevalence of Bovine Aortic Arch Variant in Patients with Aortic Dissection and its Implications in the Outcome of Patients with Acute Type B Aortic Dissection. European Journal of Vascular and Endovascular Surgery. 2018:385-391.
- Polak J, Ciuk S, Kucybala I, et al. Cerebral Aneurysms: Are They Associated with Anatomic Variations of Carotid and Main Cerebral Arteries? World Neurosurgery. 2019:604-608.
- Potter BJ, Pinto DS. Subclavian Steal Syndrome. Circulation. 2014:2320-2323.
- Selvin E, Erlinger TP. Prevalence of and Risk Factors for Peripheral Arterial Disease in the United States. Circulation. 2010:738-743.