Clinical outcomes of ondansetron administration with elective cesarean section
Received: 20-Jan-2019 Accepted Date: Feb 13, 2019; Published: 28-Feb-2019
Citation: Ranalli L, Dvorchak B.Clinical outcomes of ondansetron administration with elective cesarean delivery. J Nurs Res Pract. 2019;3(1): 11-14
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Intraoperative vital sign variability such as hypotension and bradycardia continue to remain a concern for patients undergoing cesarean delivery under spinal anesthesia. Recent literature has suggested that administering a 5-hydroxytryptamine3 antagonist such as ondansetron prior to spinal anesthesia for cesarean delivery can mitigate intraoperative vital sign variability and reduce vasopressor utilization.
Purpose and objectives: The purpose of this project was to examine the optimal perioperative timing of ondansetron administration and associated maternal clinical outcomes with elective cesarean delivery. The main objectives were to determine if intraoperative hemodynamic variables and vasopressor administration significantly differed regarding ondansetron timing.
Method: A retrospective medical record review was conducted from 68 patients with cesarean delivery under spinal anesthesia to compare the timing of ondansetron administration (pre-spinal versus post-spinal) with intraoperative vital sign variability and vasopressor utilization.
Results: There were no significant differences between pre-spinal and post-spinal ondansetron groups regarding systolic blood pressure (p=0.11), diastolic blood pressure (p=0.56), mean arterial pressure (p=0.75), or heart rate (p=0.75). Also, there were no significant differences regarding intraoperative phenylephrine (p=0.86) and ephedrine (p=0.08) administration.
Implications: Although statistical significance was not found, the systolic blood pressure was consistently higher and less vasopressor medication was administered in the pre-spinal ondansetron group. Results such as these, in combination with recently published literature should be taken into consideration to guide obstetric anesthesia practitioners regarding optimal perioperative timing of ondansetron until a practice standard is set forth.
Keywords
Ondansetron; Spinal anesthesia; Cesarean delivery
Introduction
ue to the ability to avoid general anesthesia, spinal anesthesia is often the anesthetic of choice for cesarean delivery (CD) [1]. However, side effects associated with spinal anesthesia include a 50% risk of hypotension and 13% incidence of bradycardia [2]. Hypotension can lead to an altered level of consciousness, nausea/vomiting, decreased fetal blood flow, and increased risk of maternal aspiration. Vasopressors (vasoconstrictive medications) such as ephedrine and neosynephrine are commonly utilized during spinal anesthetics in an attempt to prevent or treat hypotensive episodes [1]. The resultant hypotension from spinal anesthesia likely stems from decreased systemic vascular resistance, parasympathetic predominance, and stimulation of the Bezold-Jarisch reflex (BJR). Activation of the BJR leads to a decreased heart rate (HR) and vasodilation [3].
Clinical Outcomes of Ondansetron Administration with Elective Cesarean Delivery
Antagonism of the receptor 5-hydroxytryptamine3 (5-HT3) has limited the occurrence of the BJR.3 Ondansetron is a commonly administered 5-HT3 antagonist within the perioperative care of CD patients. Ondansetron is labeled as a category B drug by the FDA and is considered safe in pregnancy [4]. Category B drugs have not proven to put the fetus at risk but there are no high-level studies that exist [5]. Professional associations such as the American Society of Anesthesiologists (ASA) put forth practice guidelines specifically for obstetric anesthesia that recommend perioperative timing of a multitude of drugs that include histamine-2 antagonists, gastrokinetics, and sodium citrate. However, the timing of ondansetron administration has yet to be included into the ASA obstetric practice guideline recommendations [6]. Therefore, the perioperative timing of ondansetron administration is mainly decided by the physician or nurse anesthesiologist assigned to provide care for the patient, as there is currently no administration standard established.
Background
Recent studies support the administration of ondansetron prior to performing the spinal anesthetic in patients undergoing CD. [1-3,7-10] Study variables have included a focus on intraoperative hemodynamics such as variations in systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and HR. Regarding hemodynamic variability, multiple studies have resulted in obstetric patients undergoing CD experiencing significantly less intraoperative hypotension or bradycardia when the patient was administered ondansetron prior to receiving spinal anesthesia [1,2,7-10]. Additionally, studies also focused on the amount of intraoperative vasopressor administration between pre-spinal and control groups. Several studies resulted in a significantly lower utilization of intraoperative vasopressors such as ephedrine and neosynephrine in patients that received ondansetron prior to receiving their spinal anesthetic [1,9,10]. Furthermore, additional studies evaluated fetal outcomes from patients that received ondansetron prior to spinal anesthesia versus a control group. Fetal outcomes were found to be significantly improved in patients that received the ondansetron prior to spinal anesthesia [9,10].
Purpose
The purpose of this chart review was to evaluate the optimal perioperative timing of ondansetron administration and associated maternal clinical outcomes, which included the intraoperative blood pressure and HR fluctuations during elective CD. The main objective was to compare intraoperative hemodynamic variability, vasopressor usage, and ondansetron timing among spinal anesthesia patients with CD.
Materials and Methods
Setting
This study was performed within an 11-bed facility located in the southwest region of the United States. The facility performs approximately 1,200 CD per year. Approval for the chart review was obtained from the University of Alabama institutional review board and by the medical facility. No ethics approval was deemed necessary due to the retrospective nature of this study.
Participants
Inclusion criteria consisted of age ≥ 20 years, spinal anesthesia, and scheduled for elective lower-segment CD. Additional inclusion criteria required that the patient received ondansetron 4 mg IV within the final 10-minutes (min) of the surgical procedure (group 1) or within 5-min prior to spinal anesthesia (group 2). For consistency, patients must have received a 1-liter lactated ringer’s bolus prior to the spinal anesthetic. Exclusion criteria included patients that converted to general anesthesia, preexisting hypertensive disorder of pregnancy, cardiovascular insufficiency, intraoperative blood loss ≥ 1000 mL, or had been prescribed 5-HT3 reuptake inhibitors.
GPower 3.1.9.3 (Heinrich-Heine-Universität, Düsseldorf, Germany) was utilized to conduct a power analysis in order to calculate the required number of patients required per group 1 and group 2. Parameters of a moderate effect were utilized, power 80%, p value 0.05, and two groups with mean of 83 in group 1 and 77 in group 2. A paired sample size of at least 34 subjects was required in each group totaling an n = 68, which correlates to previous studies [9,13].
Procedure
A retrospective electronic medical record review was conducted to evaluate ondansetron timing of administration and clinical outcomes with elective cesarean delivery. Intraoperative anesthesia records were reviewed electronically through the Cerner-Millenium Powerchart. Electronic records from a total of 7 obstetric anesthesia practitioners were utilized. Recent charts (2017-2018) were reviewed for inclusion criteria until the appropriate number of patients were reached in the pre-spinal ondansetron group (n=34) and the post-spinal ondansetron group (n=34).
Demographic variables including patient age, height, weight, gestational age, total preoperative fluid bolus, and procedural estimated blood loss were recorded for each patient. The hemodynamic variables including SBP, DBP, MAP, and HR were documented at baseline and at 5-min intervals following the spinal anesthetic for a total of 45 min. Intervals regarding blood pressure assessment varied between practitioners with a range of 1-5 min between each reading. Therefore, 5-min intervals were selected for comparison. Additionally, since the duration of intraoperative time for CD varied, hemodynamic variables assessed upon arrival to the post-anesthesia care unit (PACU) were also analyzed. Total intraoperative vasopressor usage (ephedrine versus neosynephrine) was recorded for each patient. Total intraoperative dosages of ephedrine (mg) and phenylephrine (mcg) were compared between both groups.
Data analysis
Study variables such as age, height, weight, gestational age, and total fluid administration were analyzed by a two-sample Student’s t-test. Total spinal anesthetic dose was analyzed by the Fisher’s exact test. Variables such as total ephedrine dosing, neosynephrine dosing, and intraoperative estimated blood loss was analyzed via the Wilcoxon rank sum test. Hemodynamic parameters such as SBP, MAP, DBP, and HR were analyzed by utilizing a two-way repeated measures ANOVA. The p-values were adjusted by using the Bonferroni method. SPSS Statistics Version 25 was utilized to conduct data analysis. A p-value<0.05 was to be considered statistically significant.
Results
Demographics
Overall, no significant differences were found regarding demographics between group 1 and group 2. Demographics included age, height, weight, gestational age, intraoperative crystalloid, spinal dosages, and estimated blood loss. These results are summarized in Tables 1 and 2.
| Variable: Mean (SD) | Group 1 | Group 2 | P-value |
|---|---|---|---|
| Age (yrs) | 31.5 (5.12) | 30.2 (4.92) | 0.3 |
| Height (cm) | 163.7 (4.87) | 163.1 (5.22) | 0.63 |
| Weight (kg) | 93.4 (20.12) | 87.94 (19.08) | 0.25 |
| Gestational Age (wks) | 38.7 (1.19) | 38.9 (1.01) | 0.38 |
| Crystalloid (mL) | 964.7 (299.4) | 955.9 (209.2) | 0.89 |
Table 1: Demographic Table
| Variable: Mean (range) | Group 1 | Group 2 | P-value |
|---|---|---|---|
| Age (yrs) | 31.5 (21-42) | 30.2 (20-41) | 0.3 |
| Height (cm) | 163.7 (155-174) | 163.1 (152-175) | 0.63 |
| Weight (kg) | 93.4 (65-152) | 87.94 (61-152) | 0.25 |
| Gestational Age (wks) | 38.7 (35-41) | 38.9 (36-41) | 0.38 |
| Crystalloid (mL) | 964.7 (500-1600) | 955.9 (500-1300) | 0.89 |
| EBL (mL): Median (range) | 600 (500-800) | 600 (500-900) | 0.21 |
| (Wilcoxon rank sum test) | |||
| Spinal Bupivicaine Dosage (mg) | |||
| 10.5 | 2/34 (6%) | 2/34 (6%) | 0.85 |
| 11.25 | 1/34 (3%) | 3/34 (9%) | (Fisher's exact test) |
| 12 | 31/34 (91%) | 29/34 (85%) | |
Table 2: Demographic Table
Hemodynamic variables
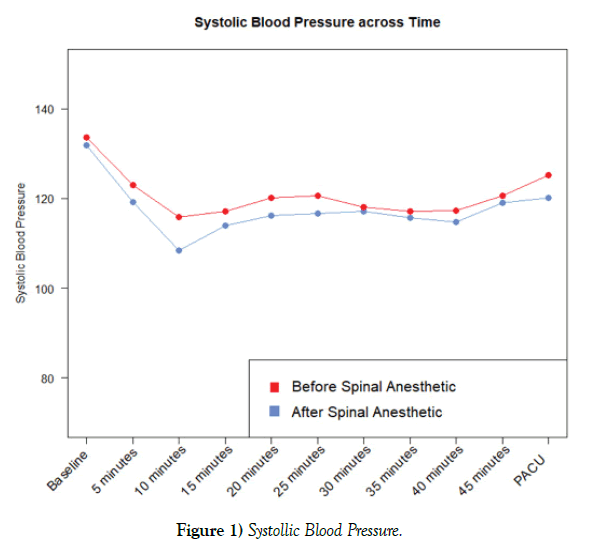
The mean intraoperative SBP for group 1 at baseline was 131.9 mmHg (SD 13.5) as compared to 133.6 mmHg (SD 13.1) for group 2. At 5-min postspinal anesthesia, the mean was 119.2 (SD 20.6) for group 1 (post-spinal) and 122.9 (SD 17.8) for group 2 (pre-spinal). At 10-min, the mean was 108.5 (SD 17.1) for group 1 and 115.8 (SD 16.5) for group 2. At 15-min, the mean was 113.9 (SD 16.8) for group 1 and 117.1 (SD 12.8) for group 2. At 20-min, the mean was 116.2 (SD 14.5) for group 1 and 120.1 (SD 12.0) for group 2. At 25-min, the mean was 116.6 (SD 15.4) for group 1 and 120.6 (SD 13.2) for group 2. At 30-min, the mean was 117.1 (SD 17.1) for group 1 and 118.1 (SD 11.8) for group 2. At 35-min, the mean was 115.6 (SD 16.2) for group 1 and 117.1 (SD 11.2) for group 2. At 40-min, the mean was 114.7 (SD 13.7) for group 1 and 117.3 (SD 12.8) for group 2. At 45-min, the mean was 119 (SD 10.6) for group 1 and 120.6 (SD 11.3) for group 2. Upon arrival to the PACU, the mean was 120.1 (SD 11.2) for group 1 and 125.2 (SD 13.7) for group 2. Overall, no significant differences regarding SBP were found when comparing group 1 and group 2 (p=0.11). However, differences in SBP was most evident 10-min following spinal anesthesia administration when the mean of group 2 was 115.8 as compared to 108.5 for group 1 (p=0.08) (Figure 1).
The mean intraoperative DBP for group 1 at baseline was 77.9 mmHg (SD 10.6) as compared to 78.1 mmHg (SD 13.8) for group 2. At 5-min post-spinal anesthesia, the mean was 70.9 (SD 15.4) for group 1 and 70.7 (SD 14.9) for group 2. At 10-min, the mean was 62.9 (SD 13.9) for group 1 and 63.6 (SD 12.3) for group 2. At 15-min, the mean was 65.5 (SD 11.5) for group 1 and 64.9 (SD 9.1) for group 2. At 20-min, the mean was 64.8 (SD 13.1) for group 1 and 64.9 (SD 11.1) for group 2. At 25-min, the mean was 63.9 (SD 11.3) for group 1 and 61.5 (SD 9.2) for group 2. At 30-min, the mean was 63.2 (SD 13.0) for group 1 and 58.7 (SD 9.6) for group 2. At 35-min, the mean was 60.7 (SD 15.7) for group 1 and 55.8 (SD 9.0) for group 2. At 40-min, the mean was 57.1 (SD 11.9) for group 1 and 54.1 (SD 9.8) for group 2. At 45-min, the mean was 59.3 (SD 10.2) for group 1 and 59.6 (SD 9.0) for group 2. Upon arrival to the PACU, the mean was 62.7 (SD 8.5) for group 1 and 66.6 (SD 8.6) for group 2. Overall, no significant differences were found regarding DBP between group 1 and group 2 (p=0.56).
The mean intraoperative MAP at baseline was 95.9 mmHg (SD 8.9) as compared to 96.6 mmHg (SD 12.1) for group 2. At 5-min post-spinal anesthesia, the mean was 87.1 (SD 16.1) for group 1 and 88.1 (SD 14.8) for group 2. At 10-min, the mean was 77.9 (SD 14.2) for group 1 and 81.1 (SD 12.6) for group 2. At 15-min, the mean was 81.6 (SD 12.4) for group 1 and 82.5 (SD 8.8) for group 2. At 20-min, the mean was 81.9 (SD 12.5) for group 1 and 83.4 (SD 10.0) for group 2. At 25-min, the mean was 81.5 (SD 10.7) for group 1 and 78.5 (SD 9.0) for group 2. At 30-min, the mean was 81.1 (SD 12.7) for group 1 and 78.5 (SD 9.0) for group 2. At 35-min, the mean was 78.9 (SD 14.7) for group 1 and 76.1 (SD 8.2) for group 2. At 40-min, the mean was 76.3 (SD 9.9) regarding group 1 and 75 (SD 9.4) for group 2. At 45-min, the mean was 78.9 (SD 8.2) for group 1 and 79.9 (SD 8.7) for group 2. Upon arrival to the PACU, the mean was 81.8 (SD 8.6) for group 1 and 86.1 (SD 9) for group 2. Overall, no significant differences resulted regarding MAP between group 1 and group 2 (p=0.75).
The mean intraoperative HR for group 1 at baseline was 89.7 beats per min (bpm) (SD 12.5) as compared to 89.7 bpm (SD 15.6) for group 2. At 5-min post-spinal anesthesia, the mean was 89 (SD 14.1) for group 1 and 89.3 (SD 15.1) for group 2. At 10-min, the mean was 87.9 (SD 14.7) for group 1 and 90.4 (SD 17.8) for group 2. At 15-min, the mean was 82.9 (SD 15.8) for group 1 and 87.2 (SD 17.3) for group 2. At 20-min, the mean was 83.9 (SD 15.6) for group 1 and 88.9 (SD 18.2) for group 2. At 25-min, the mean was 87.3 (SD 15.9) for group 1 and 91.3 (SD 15.1) for group 2. At 30-min, the mean was 93 (SD 15.1) for group 1 and 93 (SD 14.8) for group 2. At 35-min, the mean was 96.9 (SD 13.4) for group 1 and 93.8 (SD 12.5) for group 2. At 40-min, the mean was 92.7 (SD 14.3) for group 1 and 94.2 (SD 10.7) for group 2. At 45-min, the mean was 87.8 (SD 12.4) for group 1 and 91 bpm (SD 11.4) for group 2. Upon arrival to the PACU, the mean was 84.2 (SD 11.2) for group 1 and 87.3 (SD 13.4) for group 2. Overall, no significant differences regarding HR were found between group 1 and group 2 (p=0.75).
Vasopressor usage
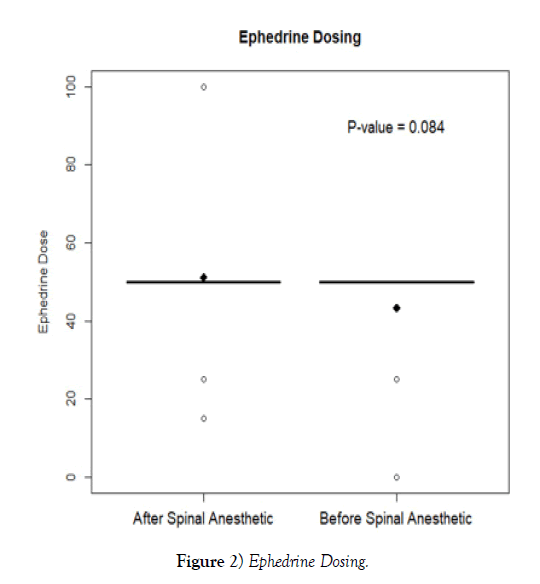
Regarding vasopressor usage, the mean intraoperative phenylephrine IV dose in group 1 was 111.7 mcg (SD 201.1) as compared to 102.9 mcg (SD 170.6) in group 2 (p = 0.86). When evaluating ephedrine, group 1 averaged an intraoperative IV dose of 51.2 mg (SD 14.1) versus 43.4 mg (SD 16.4) for group 2 (p=0.08).
Discussion
Overall, the results did not reach levels of statistical significance regarding hemodynamic variability or vasopressor utilization between both groups, which differed from multiple previous studies [1-3,7-10]. However, the results of the chart review were similar to the outcomes of a prospective doubleblinded randomized study conducted by Ortiz-Gomez et al. [11] which evaluated differing dosages of ondansetron prior to spinal anesthesia versus an IV saline group. Overall, there were 4 groups in total with a similar number of participants per group (n = 32) as compared to the medical record review. Group 1 (n=32) received IV saline prior to receiving their spinal anesthetic. The experimental pre-spinal ondansetron groups were differentiated by dose. For example, group 2 (n=32) received ondansetron 2 mg IV prior to spinal anesthesia, group 3 (n=32) was administered ondansetron 4 mg IV, and group 4 was given ondansetron 8 mg IV (n=32). Similar to the results of the medical record review, no significant differences were found regarding hypotension when comparing the experimental groups and IV saline group (p = 0.77). Also, no significant differences were found regarding ephedrine (p = 0.11) and neosynephrine requirements (p=0.89) [11].
However, the chart review ultimately differed from much of the literature that has resulted in significantly improved clinical outcomes when providing ondansetron prior to spinal anesthesia for cesarean delivery [1-3,7-10]. El Khouly and Meligy conducted a double-blinded randomized controlled trial comparing one group that received pre-spinal ondansetron intravenous (IV) administration (n=50) versus another group that received only pre-spinal saline IV (n=50). Patients were subsequently randomized to be administered ondansetron 4 mg IV or normal saline IV 5-min before the conduction of spinal anesthesia. Overall, significantly lower SBP was found within the saline group at the 10-min (p=0.012), 30-min (p=0.001), and 60-min (p = 0.005) after spinal anesthesia. Also, the patient heart rates were found to be significantly decreased in the saline group at 20-min (p = 0.012) and 50- min (p = 0.021) after the spinal anesthetic. Furthermore, total ephedrine administration was significantly increased regarding the IV saline group versus the preoperative ondansetron group (p=0.005) [1].
Sahoo et al. [7] performed a randomized prospective double-blinded controlled trial analyzing pre-spinal IV ondansetron administration (n=26) versus IV saline (n=26) and subsequent impact on intraoperative blood pressure variability during cesarean delivery. Patients either received ondansetron 4 mg IV or normal saline IV 5-min prior to the performance of spinal anesthesia. No significant differences regarding patient demographics were found between the groups. Ultimately, the saline group experienced a significantly lower MAP between 14-35 min post-spinal administration versus the ondansetron group (p=0.025). However, no significant differences were found regarding HR variability or oxygen saturation when comparing both groups. The results were comparable to a prospective double-blinded randomized controlled by Fattahi et al., which compared a pre-spinal ondansetron IV group (0.15 mg/kg) to an IV saline group. Overall, the MAP within the ondansetron group was also found to be significantly increased as compared to the IV saline group (p=0.01) [8].
An improvement regarding intraoperative hemodynamic stability is not only beneficial for the mother but also the unborn fetus. Trabelsi et al. [9] performed a prospective randomized controlled double-blinded trial evaluating intraoperative maternal hemodynamic variability and neonatal delivery blood gas values between a pre-spinal ondansetron IV group (n = 40) versus a pre-spinal IV saline group (n=40). Findings were consistent with previous literature demonstrating significantly less intraoperative hypotension (p <0.001) and bradycardia (p=0.022) for those that received ondansetron prior to the performance of spinal anesthesia for CD. The pre-spinal ondansetron group was also beneficial to infants born from these mothers by evidence of significantly higher APGAR scores (p <0.001) and an increased physiologic umbilical venous pH (p=0.01) when compared to the neonates born from mothers who did not receive ondansetron preoperatively [9,12].
Limitations
The lack of statistical significance may have been indicator of not reaching sufficient power within the study. Although a power analysis was conducted for sample size, an increase in study population may have increased the power needed to reach statistical significance. Additional limitations that are inherent when a retrospective review of data includes a lack of randomization, lack of control, and potential for investigator bias. Although not feasible for this particular study, the ability to randomize the participants into blinded and controlled groups in a prospective manner would have increased the power of the study. Furthermore, unintentional investigator bias may have resulted from unconscious bias regarding design and analysis choices. Regarding practitioner bias, anesthesia professionals each have their own preferences for which vasopressor (ephedrine versus neosynephrine) they select and varying thresholds for when they decide to administer them intra operatively. For example, anesthesia personnel may prefer to be more proactive in their approach with vasopressor administration while others may prefer to be more reactive. Therefore, not being able to control the parameters for the administration of vasopressors may have hindered the results and cannot be generalized to CD patients’ standard of care.
Implications
Although statistical significance was not reached, there are aspects of the results that may be considered clinically significant. For example, the SBP remained consistently higher intraoperatively in group 2 as compared to group 1 (Figure 1). This was most evident 10-min following spinal anesthesia administration when the SBP mean of group 2 was 115.8 mmHg (SD 17.1) as compared to 108.5 mmHg (SD 17.1) for group 1 (p=0.08). Regarding vasopressor administration, the total average intraoperative dose of neosynephrine was higher in group 1 at 111.8 mcg (SD 201.1) as compared to 102.9 mcg (SD 170.6) for group 2. Additionally, the average intraoperative dose for ephedrine was also higher for group 1 at 51.2 mg (SD 14.1) as compared to 43.4 mg (SD 16.4) for group 2 (Figure 2). The common intraoperative bolus dose of ephedrine is 5-10 mg IV [14]. Therefore, it can be postulated that group 1 averaged 1-2 additional IV boluses of ephedrine as compared to group 2. This increased vasopressor utilization may have resulted in increased intraoperative blood pressures in group 1 and thus impacted the hemodynamic study variables.
Conclusion
For future design purposes, prospective randomized designs should be attempted to result in the highest levels of research possible. Organizational approval will be sought to take these preliminary results and aim to conduct a similar study that is prospective, randomized, and controlled in methodology. Obstetric anesthesia providers at the host facility will be educated on the results of this retrospective chart review. Obstetric practice guidelines such as those put forth by the American Association of Nurse Anesthetists (AANA) and the ASA will continue to be monitored for potential inclusion of best practice recommendations regarding ondansetron dosing for cesarean delivery. Until a standard is established, recent literature and future studies regarding perioperative timing of ondansetron administration for CD must continue to be evaluated by anesthesia professionals in order to guide best practice and outcomes.
Conflict of Interest
There are no conflicts of interests to report from the authors. No funding was necessary for the completion of this project.
Acknowledgement
I would like to thank Manager of BioClinical Statistics at Midwestern University Dr. Amy Stein, PhD for her vital role in the data analysis of this project.
REFERENCES
- Khouly El, Meligy AM. Randomized controlled trial comparing ondansetron and placebo for the reduction of spinal anesthesia- induced hypotension during elective cesarean section delivery in egypt. International Journal of Gynecology and Obstetrics. 2016;135:205-209.
- Marashi SM, Soltani-Omid S, Mohammadi SS, Aghajani Y, Movafegh A. Comparing two difference doses of intravenous ondansetron with placebo on attenuation of spinal-induced hypotension and shivering. Anesthesiology and Pain Medicine. 2014;4(2), e12055.
- Heesen M, Klimek M, Hoeks SE, Rossaint, R. Prevention of spinal anesthesia-induced hypotension during cesarean delivery by 5-hydroxytryptamine-3 receptor antagonists: A systemic review and meta- analysis and meta-regression. Anesthesia & Analgesia. 2016;123(4), 977-988.
- Mahadevan U, Sunanda K. American gastroenterological association institute medical position statement on the use of gastrointestinal medications in pregnancy. Gatroenterology. 2006;131(1):278-282.
- United States Department of Health and Human Services. FDA pregnancy categories. https://chemm.nlm.nih.gov/pregnancycategories. htm. Accessed November 11th, 2017.
- American Association of Nurse Anesthetists. Analgesia and anesthesia for the obstetric patient: Practice guidelines. https://www.aana.com/ docs/default-source/practice-aana-com-web-documents-(all)/analgesia- and-anesthesia-for-the-obstetric-patient.pdf?sfvrsn=be7446b1_8. Accessed February 21, 2019.
- American Society of Anesthesiologists. Practice guidelines for obstetric anesthesia: An updated report by the american society of anesthesiologists task force on obstetric anesthesia and the society for obstetric anesthesia and perinatology. Anesthesiology. 2016;124(2):270-300.
- Tubog TD, Kane TD, Pugh MD. Effects of ondansetron on attenuating spinal anesthesia-inducted hypotension and bradycardia in obstetric and nonobstetric subjects: A systematic review and meta-analysis. AANA Journal. 2017;85(2), 113-122.
- Sahoo T, SenDasgupta C, Goswami A, Hazra A. Reduction in spinal- induced hypotension with ondansetron in parturients undergoing caesarean section: A double-blind randomized, placebo-controlled study. International Journal of Obstetric Anesthesia. 2012;21:24-28.
- Fattahi Z, Hadavi SMR, Sahmeddini MA. Effect of ondansetron on post- dural puncture headache (PDPH) in parturients undergoing cesarean section: A double-blind randomized placebo-controlled study. Journal of Anesthesia. 2015;29(5):702-707.
- Trabelsi W, Romdhani C, Elaskri H, et al. Effect of ondansetron on the occurrence of hypotension and on neonatal parameters during spinal anesthesia for elective caesarean section: A prospective, randomized, controlled, double-blind study. Anesthesiology Research and Practice. 2015.
- Wang M, Zhuo L, Wang Q, Shen MK, et al. Efficacy of prophylactic intravenous ondansetron on the prevention of hypotension during cesarean delivery: A dose-dependent study. International Journal of Clinical and Experimental Medicine. 2014;7(12):5210-5216.
- Ortiz-Gomez JR, Palacio-Abizanda FJ, Morillas-Ramirez F, Fornet-Ruiz I, Lorenzo-Jimenez A, Bermejo-Albares ML. The effect of intravenous ondansetron on maternal haemodynamics during elective caesarean delivery under spinal anaesthesia: A double-blind, randomized, placebo- controlled trial. International Journal of Obstetric Anesthesia. 2014;23:138- 143.
- Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in caesarean section. British Journal of Anaesthesia. 2006;96(1):95-99.