Combined toxicity of cypermethrin, a pyerthroid insecticide and metal to earthworms Eisenia fetida
2 Department of Histology, Faculty of Medicine, University of Benghazi, Benghazi, Libya, Email: abeer_haa2000@yahoo.com
Received: 07-Jun-2018 Accepted Date: Jun 22, 2018; Published: 10-Jul-2018
Citation: Aebeed AS, Amer AH. Combined toxicity of cypermethrin, a pyerthroid insecticide and metal to earthworms Eisenia fetida. Med Tox Curr Res. 2018;1(1):4-10.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Effects of Sub-Lethal dose of a mixture of cypermethrin and heavy metal on growth, reproduction and juvenile number of epigeic earthworm, Eisenia fetida were investigated in artificial soil glass containers (pH to 5-6.5 temperature of 20 ± 2°C) under laboratory conditions. E. fetida exposed to 25+200 mg/L Cypermethrin+Copper, Cypermethrin+chromium 25+200 mg/L and 25+50 mg/L Cypermethrin+lead. The results indicated that all metals at their tested sub lethal doses Cypermethrin and heavy metal had miled to relatively significant effect on the body weight, cocoon production and juvenile number after 28, 49 and 70 days exposure of E. fetida. The present novel study also indicated the use of E. fetida as a biomarker in assessment of insecticide and metal pollution.
Introduction
The increased use of various types of pesticides in the modern world has led to much greater emphasis on the possibility of serious environmental contaminations arising from their use in soil, these uses of such chemicals on soils causes decrease in soil fertility, alteration of soil strictures disturbance of the balance between flora and fauna residing in the soil, leading to a threat for living organisms.
Laboratory and Field experiment concerning the impacts of both organic and metals toxicity in a continuous process in order to detect their interaction behavior in the environment which usually results due to several environmental factors leading to the reverse of their intended use.
Cyperkill (Cypermethrin) is apyrethroid. It was first synthesized in 1974 [1]. Cyperkill is a synthetic chemical similar to the prethrins in pyrethrum extract (which comes from the chrysanthemum plant). Pyrethroids, including cyperkill were designed to be effective longer than pyrethrins [1]. The effect of cyperkill on wildlife is very evident as it is highly toxic to fish as well as bees, cockroaches and other insects. Cyperkill exhibited a nervous system response, which cause restlessness, in coordination, prostration, and paralysis in the laboratory testing animals [2].
Mice exposed to small doses (0.3 to 4.3 mg/g) of cyperkill displayed symptoms including writhing, convulsions and salivation [3]. Rats exposed to cyperkill exhibited similar symptoms including tremors, seizures, writhing, and salivation as well as burrowing behavior [4]. Newborn rats were more sensitive to cyperkill than adult rats; the liver enzymes that break down cyperkill in the body are not completely developed in the newborn rats [5]. People handling or working with pyrethrins and pyrethroids (including cyperkill) sometimes developed tingling, burning, dizziness and itching [1,4].
The urge for increasing agricultural yield to feed the increasing population has brought a large number of chemicals in protecting the crop against insect pests. However, a grave environmental problem have aroused because of the indiscriminate usage of these chemicals in agricultural fields [6-8]. Insecticides are known to be toxic to many non-target organisms and also cause serious sub [9-11] increases and decreases in reproductive potential and growth rate [12-14]. Cypermethrin is a pyrethroid insecticide that is used to control insect pests Cox [15] by disrupting normal functioning of the nervous system [16]. Though it has been shown that they are harmful to beneficial insects, pyrethroids are effective at low rates and relatively inexpensive [17]. However the effects of pyrethroids on earthworms, a major group of non been fully explored. Cypermethrin, one of the major pyrethroid insecticides currently used worldwide against insect pests Usmani [18] study to determine its effects on a non fetida, an epigeic earthworm known for its efficacy in nutrient recycling and widely used for vermicompost production throughout the world.
Chromium is one of the major soil pollutants, but its toxicity in soil organism is less studied. So among the various metals that contaminate terrestrial ecosystems, Copper, chromium and Lead were chosen to be used in this study. The toxicity of chromium in soil organisms is less studied. The effects of the mixture of Insecticide and heavy metal on soil fauna is even less.
Earthworm is well studied as a model for heavy metal toxicity. There were many literatures concerned with metal uptake and accumulation in earthworms. Much of them measured metal content, growth, worms density Pizl [19] accumulation rate Vijver [20] and excretion rate Lock [21].
Among the earthworms, two species are well known as soil animals for testing soil pollutants including pesticides and heavy metals, these are the European Species E. fetida and the Aporrectodea Caliginosa which is widely found in Libyan habitat. Although, several researches were conducted on these later species in the zoology lab and have been published in several journals.
However, in this present study E. fetida saving, 1826 was selected because it is a standard test organism used in terrestrial ecotoxicology and because it can be easily bred on a variety of organic wastes with short generation time therefore, different endpoints can be observed in short time such as mortality, change in body weight, cocoon numbers as well as fecundity. Furthermore, E. fetida was chosen for this study because its growth and reproduction are well documented [22,23] and it is considered suitable model species [24].
The objective of this study is a continuation assessment of soil pollutants to the soil animals represented by E. fetida which aims to assess the impact of soil contaminates at sub lethal concentrations at different endpoints including: The effects of pesticides and metals on worm growth, and reproduction, and evaluating toxicity mixtures of pesticides-metals combinations.
Material and Methods
The European strain E. fetida were brought from the Czech Republic as a research sample and reared in the zoology lab for more than two years Wu [25]. E. fetida was chosen for this study as it has more reproductive potential as compared to the local species. The E. fetida was maintained in a glass aquaria on a culture media as described by Organization for Economic Cooperation and Development OECD [26], at room temperature of 20 ± 2°C. The food consisted of an artificial soil mixed with barley grains powder as a food supplement every week throughout the test period. The moisture conditions of the rearing soil were started at approximately 60% water holding capacity. The moisture, thereafter, was maintained by regularly sprinkling water on the soil. Fungal growth was removed when observed on the soil surface. Rearing soil was changed every eight weeks until the worms required for experiment were with an average weight of 7 to 9 grams.
In this experiment, the adult worms were exposed to the artificial soil contaminated with one concentration of Lead+Cyperkill, Copper +Cyperkill and Chrome+Cyperkill at 50+25 ppm, 200+25 ppm and 200+25 ppm respectively. The artificial soil used OECD [26] consisted of 70% quartz sand, 20% kaolin clay, 10% sphagnum peat and calcium carbonate to adjust the pH to 5-6.5. A weight of 250 grams of soil was transferred into glass containers (12 cm W, 15 cm L, 20 cm H) to which 100 ml of each of Lead+Cyperkill, Copper+Cyperkill and Chromium +Cyperkill were added and mixed thoroughly. Each treatment was replicated three times and control treatment with three replicates was set using plain water. Ten adult E. fetida then transferred into each test container, after their initial body weight as a whole replicate were taken KERN and Sohn GmbH.
The used doses were selected on the bases of a preliminary trail test with the consideration of finding prolonged effects rather than a cute direct mortality.
The further worms body weight were again taken after 28, 49 and 70 days post treatment. 5 grams of barely grain powder were spread on top of each test container as food, supplement and soil moisture content was checked once a week and 5 ml water was added when needed.
The parameters measured in this experiment were: Worm body weight change. Cocoon production by worms after 28 and 70 days post treatment. And the number of juveniles hatched from the cocoon after 70 days post treatment.
All data were subjected to SPSS, whereas, ANOVA were used to find the significant difference, and T-test for the mean differences.
Results
Change in body weight
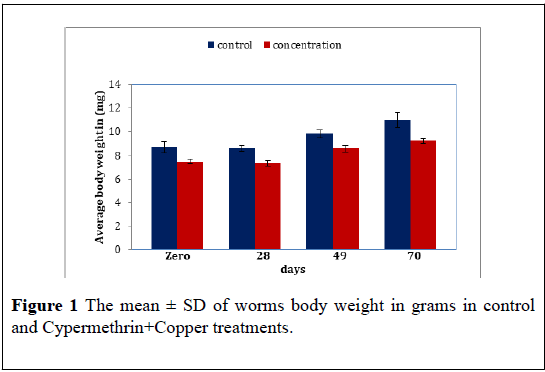
Cypermethrin+Copper: The average body weight of worms exposed to the Cypermethrin+Copper mixture (200+25 ppm) over 10 weeks. The result reveal significant difference in body weight between the Cypermethrin+Copper treated and control (F=30.78, P<0.05) further there in also significant difference in the worms body weight as time pars (F=16.79, P<0.05) The mean ± S.D of the body weight reveal an increase in worms weight along time thus the mean ± S.D were 7.50 ± 0.36 7.36 ± 0.41 8.53 ± 0.40 and 9.20 ± 0.34 for Cypermethrin+Copper treated compared to 8.66 ± 0.73 8.56 ± 0.32 9.80 ± 0.60 and 10.93 ± 1.10 for control at zero, 28 days 49 days and 70 days (Figure 1) (Table 1).
| Time | Control | Cypermethrin+Copper |
|---|---|---|
| Zero | 8.66 ± 0.737 | 7.500 ± 0.360 |
| After 28 | 8.567 ± 0.321 | 7.367 ± 0.416 |
| After 49 | 9.800 ± 0.600 | 8.533 ± 0.404 |
| After 70 | 10.933 ± 1.101 | 9.200 ± 0.346 |
Table 1: The mean ± S.D of E. fetida body weight in grams of control and Cypermethrin+Copper treatments. P mean difference is significant at the 0.05 level.
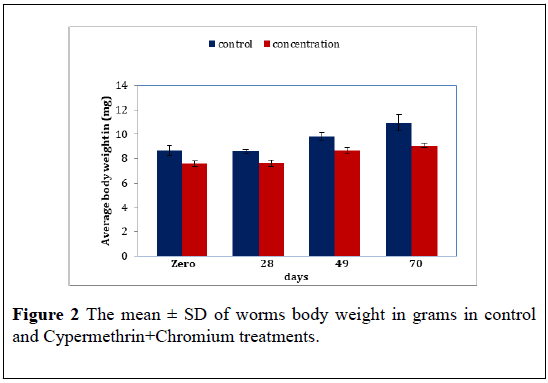
Cypermethrin+Chromium: The average body weight of worms exposed to the Cypermethrin+Chromium mixture (200+25 ppm) over 10 weeks. The result reveal significant difference in body weight between the Cypermethrin+Chromium treated and control (F=18.54, P<0.05). Further there in also significant difference in the worms body weight as time pars (F=9.85, P<0.05) The mean ± S.D of the body weight reveal an increase in worms weight along time thus the mean ± S.D were 7.56 ± 0.60 7.63 ± 0.51 8.63 ± 0.90 and 9.06 ± 0.70 for Cypermethrin+Chromium treated compared to 8.66 ± 0.73 8.56 ± 0.32 9.80 ± 0.60 and 10.93 ± 1.10 for control at zero, 28 days 49 days and 70 days (Figure 2) (Table 2).
| Time | Control | Cypermethrin+Chromium |
|---|---|---|
| Zero | 8.66 ± 0.737 | 7.567 ± 0.602 |
| After 28 | 8.567 ± 0.321 | 7.633 ± 0.513 |
| After 49 | 9.800 ± 0.600 | 8.633 ± 0.901 |
| After 70 | 10.933 ± 1.101 | 9.067 ± 0.702 |
Table 2: The mean ± S.D of E. fetida body weight in grams of control and Cypermethrin+Chromium treatments. P mean difference is significant at the 0.05 level.
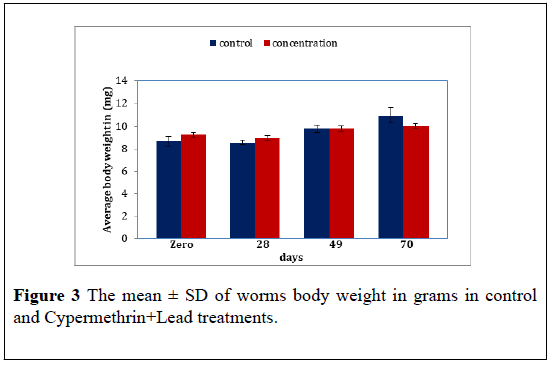
Cypermethrin+Lead: The average body weight of worms exposed to the Cypermethrin+Lead mixture (50+25 ppm) over 10 weeks. The result reveal no significant difference in body weight between the Cypermethrin +Lead and control (F=0.007, P>0.05) further there in also significant difference in the worms body weight as time pars (F=7.47, P<0.05) The mean ± S.D of the body weight reveal an increase in worms weight along time thus the mean ± S.D were 9.26 ± 0.96 8.96 ± 0.66 9.80 ± 0.51 and 10.03 ± 0.40 for Cypermethrin+Lead treated compared to 8.66 ± 0.73 8.56 ± 0.32 9.80 ± 0.60 and 10.93 ± 1.10 for control at zero, 28 days 49 days and 70 days (Figure 3) (Table 3).
| Time | Control | Cypermethrin+Lead |
|---|---|---|
| Zero | 8.66 ± 0.737 | 9.267 ± 0.960 |
| After 28 | 8.567 ± 0.321 | 8.967 ± 0.665 |
| After 49 | 9.800 ± 0.600 | 9.800 ± 0.519 |
| After 70 | 10.933 ± 1.101 | 10.033 ± 0.404 |
Table 3: The mean ± S.D of E. fetida body weight in grams of control and le Cypermethrin+Lead treatments. P mean difference is significant at the 0.05 level.
Cocoon production
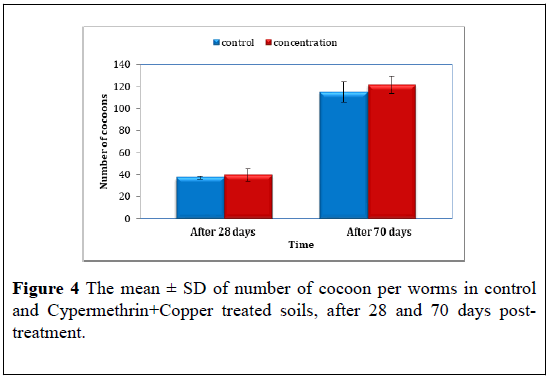
Cypermethrin+Copper: The effect of the Cypermethrin+Copper on the worm cocoon production. the number of cocoon produced by control worms were compared with the cocoon numbers produced by the Cypermethrin+Copper treated worms using t-test, the results clearly showed on significant difference (F=0.72, P>0.05) after 28 days period, the mean ± S.D of cocoon produced by control worms were 37.00 ± 2.00 compared to 39.33 ± 3.05 of copper-cyperkill at the 28 days post treat.
At the end of the experiment 70 days, no significant difference were reported (F=0.99, P>0.05) the mean ± S.D of cocoon were 114.67 ± 15.88 for control compared to 121.33 ± 11.06 for Cypermethrin+Copper treated (Figure 4) (Table 4).
| The mean ± S.D cocoon number | ||
| Time | After 28 days | After 70 days |
| control | 37.00 ± 2.00 | 114.67 ± 15.885 |
| Cypermethrin+Copper | 39.33 ± 3.055 | 121.33 ± 11.060 |
Table 4: The mean ± S.D of number of cocoon in control and Cypermethrin+Copper treated soils, after 28 and 70 days post treatment.
Cypermethrin+Chromium: The effect Cypermethrin+Chromium on the worm cocoon production the number of cocoon production by control worms were compared with the cocoon numbers produced by the Cypermethrin+Chromium treated using no significant difference (F=2.28, P>0.05) after 28 day period, the mean ± S.D cocoon produced by control worms were 37.00 ± 2.00 compared to only 39.67 ± 4.16 of Cypermethrin +Chromium at the 28 day post treat.
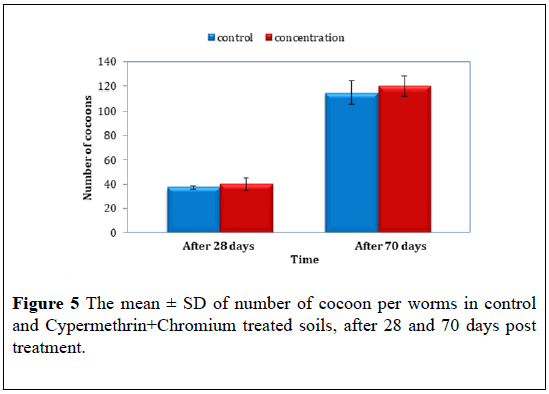
At the end of the experiment 70 days, no significant differences were reported (F=5.67, P>0.05) the mean ± S.D of cocoon were 114.67 ± 15.88 for control compared to 120.33 ± 15.68 for Cypermethrin+Chromium treated (Figure 5) (Table 5).
| The mean ± S.D cocoon number | ||
| Time | After 28 days | After 70 days |
| Control | 37.00 ± 2.00 | 114.67 ± 15.885 |
| Cypermethrin+Chromium | 39.67 ± 4.163 | 120.33 ± 5.686 |
Table 5: The mean ± S.D of number of cocoon in control and Cypermethrin+Chromium treated soils, after 28 and 70 days post treatment.
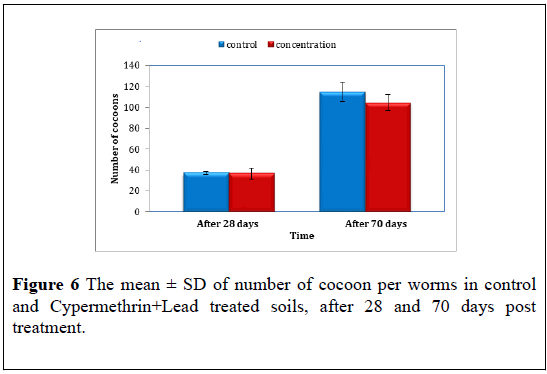
Cypermethrin+Lead: The results clearly showed a significant difference (F=9.98, P<0.05) after 28 days period, the mean ± S.D of cocoon produced by control worms were 37.00 ± 2.00 compared to 36.33 ± 11.59 of Cypermethrin+Lead treated at the 28 days post treat.
At the end of the experiment 70 days, a significant difference were reported (F=9.06, P<0.05) the mean ± S.D of cocoon were 114.67 ± 15.88 for control compared to 104.67 ± 3.51 for Cypermethrin+Lead treated (Figure 6) (Table 6).
| The mean ± S.D cocoon number | ||
| Time | After 28 days | After 70 days |
| control | 37.00 ± 2.00 | 114.67 ± 15.885 |
| Cypermethrin+Lead | 39.33 ± 3.055 | 121.33 ± 11.060 |
Table 6: The mean ± S.D of number of cocoon in control and Cypermethrin+Lead treated soils, after 28 and 70 days post treatment.
Number of juveniles per cocoon
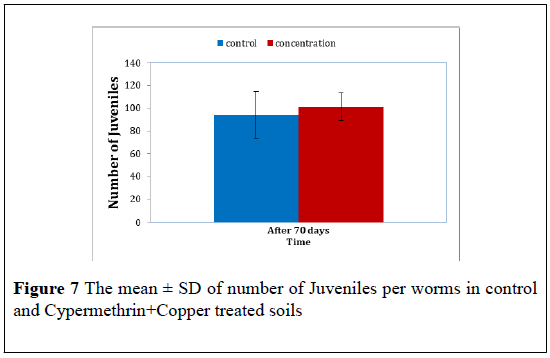
Cypermethrin+Copper: Cypermethrin+Copper the juvenile number produced by cocoons of the control worms and Cypermethrin+Copper treated worms were further counted. The mean ± S.D of these juvenile in presented in Table 7 the t-test revealed a significant difference juvenile number between treatment (F=11.16, P<0.05). The t-test revealed that the number of juvenile produced by Cypermethrin+Copper worms the mean ± S.D were 94.00 ± 35.53 for control juvenile and 101.00 ± 5.29 for Cypermethrin+Copper at 70 day post treated (Figure 7).
| The mean ± S.D juvenile number | |
| Time | After 70 days |
| Control | 94.00 ± 35.539 |
| Cypermethrin+Copper | 101.00 ± 5.292 |
Table 7: The mean ± SD of the number of juveniles in control and Cypermethrin+Copper treated worms after 70 days.
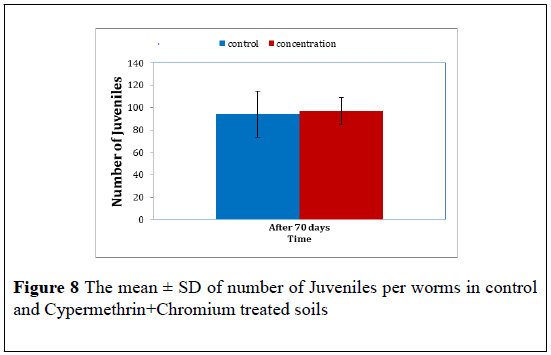
Cypermethrin+Chromium: The juvenile number produced by cocoons of the control worms and Cypermethrin+Chromium treated worms were further counted. The mean ± S.D of these juvenile in presented in Table 8 the t-test revealed no significant difference in juvenile number between treatment (F=7.05, P<0.05). The mean ± S.D were 94.00 ± 35.53 for control juvenile and 97.00 ± 5.56 for Cypermethrin+Chromium treated at 70 day post treated (Figure 8).
| The mean ± S.D juvenile number | |
| Time | After 70 days |
| Control | 94.00 ± 35.539 |
| Cypermethrin+Chromium | 97.00 ± 5.568 |
Table 8: The mean ± SD of the number of juveniles in control and Cypermethrin+Chromium treated worms after 70 days.
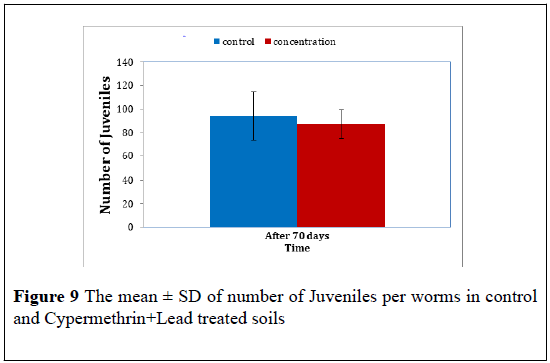
Cypermethrin+Lead: The juvenile number produced by cocoon of the control worms and Cypermethrin+Lead treated worms were further counted. The mean ± S.D of these juvenile in presented in Table 9 the ttest revealed a significant difference in the juvenile number between treatment (F=9.13, P<0.05). The t-test revealed that the number of juvenile produced by Cypermethrin+Lead worms were significant greater that produced by control worms. The mean ± S.D were 94.00 ± 35.53 for control juvenile and 87.00 ± 11.35 for Cypermethrin+Lead at 70 days post treated (Figure 9).
| The mean ± S.D juvenile number | |
| Time | After 70 days |
| Control | 94.00 ± 35.539 |
| Cypermethrin+Lead | 87.00 ± 11.358 |
Table 9: The mean ± SD of the number of juveniles in control and Cypermethrin+Lead treated worms after 70 days.
Discussion
The mixed cypermethrin and heavy metal showed no significant influence on the mortality of earthworms than that of their separate use. There was no mortality in any of the worms in control and cypermethrin heavy metal mixture. The temperature and moisture were maintained at 20 ± 2°C and 60-70% level respectively and food was provided as per [26,27] reported low toxicity of cypermethrin to earthworms and explained that this non toxicity can be due to the property of pyrethroids being adsorbed onto the organic matter of the soil particles which renders part of the dose unavailable to the worms. Since the LC50 value was many times greater than the maximum recommended agricultural dose for the compound, it was concluded by Ingesfield [27] that cypermethrin would not have adverse effects on natural populations of earthworms. Similar conclusion was made by [28].
The proposed objective in this study agrees with at that of Dasgupta [29] who stated that, mortality is generally accepted to be a rather insensitive parameter. Therefore sub lethal effect such as change in body weight and reproduction were more important to assess.
However, E. fetida body weight reduction due to heavy metal was observed in some studies [30].whereas, no impact on body weight or even body weight increase was observed in other studies [31]. This relationship between metals and body weight has explained by Spurgeon [24], who stated that the worms living in metal-contaminated soil reach the lower weight or need more time to reach the maximum weight than in nonpolluted sites.
The sensitivity tests on pesticides toxicity on earthworm showed that both cyperkill have toxicity to earthworms, and that is in agreement with other studies [32,33].
Alshawish [33] tested the effect of cyperkill, chorpyrifos, dicofol , mancozeb and haloxyfopetotyl on their chronic toxicity on Aporrectodea caliginosa in laboratory cultures. They concluded that cyperkill at 50 mg Kg was least toxic compared the pesticides. Their study then confirm the present results as the cyperkill revealed insignificant change in E. fetida body weight after 70 days.
From this study, it indicates that the mixed use of cyperkill with cupper has obviously decreased not only the body weight, but also the juveniles number. This toxicity can lead to adverse impacts on earthworm populations, threatening the normal functioning of soil ecosystems.
The results also showed that the mixed pesticide with heavy metal can lead to impacts on chronic response such as growth and reproduction. This finding came in agreement with Zhou [34] who concluded that pesticides mixture was significantly more toxic to E. Andrei than either pesticide alone.
The results also showed that the pesticides-heavy metal mixture can lead to a greater impact than the individual pesticides or heavy metal in earthworms.
The impacts of pesticide-heavy metal mixture on earthworm reproduction means that the effects of contamination can last for more than one generation, leading to significant decline and reductions of genetic diversity, which may subsequently cause disruption of the functioning of the soil ecosystem. Therefore, the ecological risk of mixed pesticide or heavy metal – pesticide mixture on soil organisms should be studied in detail before applying to sensitive environments.
The results observed here came in support of effect on growth of the earthworm E. fetida from soils treated by cyperkill reported by Mosleh [35], this test also support the finding of Zhou [36] the results of these chronic toxicity tests demonstrated that cyperkill could lead adverse impacts on both the growth and reproduction of adult and juvenile earthworms, while juveniles are more sensitive during the development stage.
The impacts of the metal lead on the worm growth were also evaluated and the result seems to confirm the finding of Zaltauskaite [29] the relationship between lead and body weight was negative, though 70 days and statistically insignificant body weight reduction due to metal exposure was observed in other studies [30,31].
In an experiment using the earthworm E. fetida, showed that cupper at 200 ppm affect body weight relative to the control, whereas chrome at 200ppm resulted in a reduction of body weight, tested the effect of Cd, Cu, Pb, Ni and Zn on the growth of E. fetida in laboratory cultures, the concluded that Cadmium was the most toxic metal, with significant decrease in growth 50 μg dry soil.
Our results demonstrated that the effect of mixture of copper, lead and chromatic with pesticides on the growth, cocoon production and juvenile number E. fetida was slightly less than additive. The results clearly demonstrated that heavy metal mixed into artificial soil had a negative on the growth E. fetida the results of which are comparable to those reported.
For a very long time the impact of pollutants mixture in soil, such as pesticide-pesticide or pesticide-heavy metals were either underestimated or simply not much works were undertaken to determine their impacts. Lately, however, greater emphases were directed on the possible interactions in the soil or in the organisms inhabiting the soil and the consequent negative effect on the soil ecosystem. For this Zhou [34], elaborated that the increase in toxicity mixture means that the use of toxicity data from a single pesticide experiments may underestimate the ecological risk of pesticides that are actually present in the soil.
Consequently, the study of pesticide or pollutants mixtures is even more important in evaluating the ecological risk of pollutants on the ecosystem.
For Libyan case several works were conducted concerning the toxic effects of pesticides and/or heavy metals separately on several soil animals mainly Woodlice, Porcellio scaper, Procellio laevis, Hemilipestis reumori, Armadello officinalis and the earthworm Aporrectodea calignosa.
However, the effects of the mixture of these pollutants were not studied so far. Consequently it seems very important to undertake the impact of paired or multiple pesticide-pesticide and pesticide-heavy metal mixture not only on the mentioned above species do meaning the agro system of Libyan soil specially in the eastern regions, but also to extend the study to include other soil animals that might be present and to include the majority of pesticides that have not so far involved into the test.
Cypermethrin at concentration 15 μg soil onwards but there was no doserelated response within the range of concentration tested. Growth has been reported to be a sensitive parameter to evaluate the toxicity of insecticides on earthworms [37,38]. The weight loss may indicate feeding inhibition, with reducing consumption rate and thus affecting growth rate [35,40].
The individual toxicity tests on earthworm showed that the toxicity of Cd and Cr in nature soil is moderately. When heavy metals coexist in the environment, it is important to confirm the exact interaction among these metals because it will significantly affect their bioaccumulation processes in organisms and toxicological effects on different biological levels [25].
Some studies on interaction between heavy metals have been carried out Pan [37] found that the combination of Cd and Pb had synergistic toxicological effects on enzymes activities in soil. Jing [38] found that more cadmium in the mixture decreased the toxicity and more copper increased the toxicity to the nematode Caenorhabditis elegans. The combined toxicity tests on earthworm in this study showed that the interaction type of Cr and Cd is antagonistic. The results of factorial analysis showed that the Cd had the highest influences on combined effects of both metals. The mechanisms of combined effects between Cd and Cr are complex, which might be influenced by the competition adsorption of both metals in soil and bio-membrane and their bioavailability. Further studies are needed to intensively identify the mechanism of the combined toxicological effects.
The weight loss may indicate feeding inhibition, with the earthworms regulating the intake of insecticides by reducing consumption rate and thus affecting growth rate [35,39]. However no significant alteration in the change of biomass of the earthworms could be detected in the present study as compared to control even with the exposure of the highest sublethal dose used, i.e., 0.25 mg/kg cypermethrin corresponding to 50% of the LC50 value of the insecticide indicating its less toxic nature in comparison to carbamates and organophosphates.
Reduction in growth of earthworm by sub-lethal doses of insecticides has also been observed in E. fetida. Yasmin [11] exposed to several carbamate and organophosphate insecticides. Significant alterations in the reproductive performance of the test worms in terms of cocoons/worm/ week and juvenile production per ten worms compared to control was detected when exposed to the highest sub lethal dose used, i.e., 0.25 mg/kg cypermethrin.
The present study indicates its possible hazardous nature. Various scientists have also reported similar result regarding insecticides influencing reproduction of earthworms [11,32,39,41-44].
Conclusion
Thus it can be concluded from the present study that sub-lethal dose of cypermethrin significantly affects reproductive performance of the test specimen and their widespread application in agro-ecosystems must be carefully monitored. The present study also indicated the Growth and reproductive parameters of earthworms exposed to agro pesticides seems to be useful bio-indicators of soil pollution. The mixture of pesticides – heavy metal causes greater effects on endpoints such as growth and reproduction on earthworms E. fetida than the individual pesticides or metals. Consequently, it should be very important to evaluate pesticideheavy metal mixture on soil animals before their use even is each pesticide alone seem to be safe. Furthermore, the misuse and overuse of both organic pesticides and toxic metals separately or in combination can have severe implication on both human and environment especially the soil fauna and this result showed that.
Acknowledgements
Thanks are to Dr. Gebriel. M. Shamia for his help in the statistical analysis.
REFERENCES
- WHO. Environmental Health Criteria. Cypermethrin . Geneva: United Nations Environmental programme, the International lab our Organization, and the world Health Organization. Zoomorphologic1989;98:135-167
- Gammon, D.W. Two classes of pyrethriod action in the cockroach. Pestic. Biochem. Physiol. 1981;15:181-191
- Lawrence J L and Casida J E . Pyrethroid toxicology: mouse intracerebral structure-toxicity relationships. 1982
- Casarett, Louis J,Klaassen, Curtis D,Amdur, et al. Casarett and Doull's toxicology:the basic science of poisons .1996
- Cantalamessa F. Acute toxicity of two pyrethroids, permthrin and cypermethrin in neonatal and adult rats. Archives of Toxicology. 1993;67:510-513
- Tillman, P. G. and Mulrooney. Effect of selected insecticides on the natural enemies Coleomegilla maculate and Hippodamia convergens (Coleoptera:Coccinellidae),Geocoris punctipes (Hemiptera: Lygaeidae), and Braconmellitor, Cardiochiles nigriceps, and Cotesia marginiventris(Hymenoptera:Braconidae) in cotton . J. Econ. entomology 2000;93: 1638-1643
- Zhu, G Wu, H Guo et al . Microbial degradation of fipronil in clay loam soil . Water Air Soil Pollution. 2004;153(1): 35-44
- Reinecke S A and Reinecke A J. The impact of organophosphate pesticides in orchards on earthworms in the Western Cape, South Africa. Ecotoxicology Environment. Safety2007;66:244-251.
- Nath, B S Suresh, A Varma, et al. Changes in protein metabolism in hemolymph and fat body of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) in response to organophosphorous insecticides toxicity. Ecotoxicology. Environ. Safety1997;36:169-173
- Suh, C. P. C.Orr, Van Duyn, etal. Effect of insecticides on Trichogramma exiguum (Hymenoptera; Trichogrammatidae) preimaginal development and adult survival . J. Econ. Entomology2000; 94: 1122-1129.
- Yasmin, S. and D.Souza. Effect of Pesticides on the reproductive output of Eisenia fetida . Bull. Environment Contam. toxicology 2007;79: 529-532.
- Takada, Y.Kawamura, S.Tanaka, . Effects of various insecticides on the development of the egg parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2001;94(6): 1340-3.
- Willrich, M. M and Boethel , D. J. Effects of diflubenzuron on Pseudoplusia includens (Lepidoptera: Noctuidae) and its parasitoid Copidosoma floridanum (Hymenoptera: Encyrtidae). Environmental Entomology2001;30(4): 794-797.
- Yasmin S and D Souza. Effects of pesticides on the growth and reproduction of earthworm: A Rev. Appl. Environ. Soil Sci.2010.
- Cox C . J. Pest. Insecticide Factsheet Cypermethrin1996;16: 15-20
- Vijenberg H P M. and Van den Bercken . J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit. Rev. Toxicol1990;21:105-126
- Nowak J T, Mc cravy, K W Fettig, et al . Susceptibility of adult hymenopteran parasitoids of the nantucket pine tip moth (Lepidoptera: Tortricidae) toNoctuidae). J. Econ. Entomol 2001;94:868-873
- Usmani K A and Knowles. Toxicity of pyrethroids and effect of synergists to larval and adult Helicoverpa zea, Spodoptera frugiperda and Agrotis ipsilon (Lepidoptera: Noctuidae). J. Econ. Entomol. 2001;94(4): 868-73
- Pizl V and Josens G. The influence of traffic pollution on earthworms and their heavy metal contents in an urban ecosystem. Pedobiologia 1995;39:442-453
- Vijver M G, Vink J P M ,Van Gestel et al .Oral sealing using glue: a new method to distinguish between intestinal and dermal uptake of metals in earthworms, Soil Biology and Biochemical 2003;35:125-132
- Lock K and Janssen C R . Zinc and cadmium body burdens in terrestrial oligochaetes: use and significance in environmental risk assessment. Environment Toxicology and Chemical 2001;20:2067-2072
- Venter J M and Reinecke A J. The life-cycle of the compost worm Eisenia fetida (oligochaeta). S Afr J Zool 1988;23:161-165.
- Presley M , Mceiroy T C and Diehl et al. Soil moisture and temperature interact to alect growth, survivorship, fecundity and fitness in the earthworm Eisenia fetida. Comp. Biochem. Phgsiol1996;114:319-326
- Spurgeon D J and Hopkin. Effects of metal-contaminated soils on the growth, Sexual development, and early cocoon production of the earthworm Eisenia fetida with particular reference to zinc. Ecotoxicology Environment Safety1996;35:86-95
- Bing Wu, Zhengtao Liu, Yun Xu et al. Combined toxicity of cadmium and lead on the earthworm Eisenia fetida (Annelida, Oligochaeta). Ecotoxicology and Environmental Safety.2012;82:122-126
- OECD, Guidelines for testing chemicals, No, 222. Earthworms, Reproduction Tests (Eisenia Fetida/Andrei), Organization for Econmic Cooperation and Development, paris, france2004
- Ingesfield C . Toxicity of the Pyrethroid Insecticides Cypermethrinand WL85871 to the Earthworm, Eisenia foetida Savigny. Bull. Environ. Contaminated toxicology1984;33:568- 570
- Dasgupta, R Chakravorty, P P Kaviraj. Studies on Relative Toxicities of Six Insecticides on Epigeic Earthworm, Perionyx excavatus. Environment Contam Toxicol 2010;85:83–86
- Zaltauskaite J , Sodiene I. Effects of total cadmium and lead concentrations in soil on the growth, reproduction and survival of earthworm Eisenia fetida. 2010;56:10-16
- Berthelot Y, Valton E, Auroy A, et al. Intergration of toxicological and chemical tools to assess the bioavability of metals and energetic compounds in contaminated soils. Chemosphere. 2008;74:166-177
- Van Gestel C A M , Dirven-van Breemen, E M Baerselman R . Accumulation and Elimination of cadmium, chromium and zinc and effects on groth and reproduction in Eisenia Andrei (Oligochaeta, Annelida). The Science of the Total Environment1993;585-597
- Booth, L. H, Ò Halloran K A .Comparison of biomarker responses in the earthworm Aporrectodea Caliginosa to the organophosphorus insecticides diazinon and chlorpyrifors. Environmental Toxicology and chemistry.2001;20: 2494-2502
- Alshawish, S A , Mohamed ,A I, Nair G. Prolonged toxicity of sub-lethal dosages of chemical pesticides on the body mass and cocoons of Aporrectodea caliginosa (Savigny 1826) (Oligochaeta Lumbricidae) inhabiting Benghazi . Libya. Proceeding of the National Academy of Sciences India Section B (Biological Sciences)2004;74 (part2) :123-133
- Zhou, S P, Duan.C.Q, Minchelle,W.H.Yang, F.Z.Wamg.X.H . Individual and combined toxic effects of cypermethrin and chlorpyrifos on earthworm. Journal of Environmental Sciences2011; 23(4):676-680
- Mosleh Y Y ,Paris-Palacios S, Couderchet et al. Acute and sub-lethal effects of two insecticides on earthworms (Lumbricus terrestris) under laboratory conditions. Environment Toxicology. 2003;18(1): 1–8
- Zhou S P , Duan C Q , Wang X H et al. Assessing Cypermethrin-contaminated soil with three different, earthworm test methods. J of Environ Sci2008;20(11):1381-1385
- Pan J and Yu L . Effects of Cd or/and Pb on soil enzyme activities and microbial community structure, Ecology of England2011.;37:1889–1894
- Xiao N Jing, B Ge, F Liu X. The fate of herbicide acetochlor and its toxicity to Eisenia fetida under laboratory conditions. Chemosphere. 2006;62(8):1366–1373
- Jonker , M J Piskiewicz ,A M Ivorra et al . Toxicity of binary mixtures of cadmium–copper and carbendazim–copper to the nematode Caenorhabditis elegans . Environment Toxicology and Chemical2004;23:1529–1537
- Mosleh Y Y, Paris-Palacios S, Couderchet, et al . Biological effects of two insecticides on earthworms (Lumbricus terrestris L.) under laboratory conditions. Mededelingen Rijksuniversiteit te Gent. Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen2002;67(2): 59–68
- Booth, L H Hodge, S O’Halloran K. Use of cholinesterase in Aporrectodea caliginosa (Oligochaeta; Lumbricidae) to detect organophosphate contamination. Comparison of laboratory tests, mesocosms, and field studies. Environ Toxicol Chem 1994;19:417-422
- Toxicology. The Basic Science of poisons.5th ed. Toront: McGraw-Hill companies, Inc
- Mosleh, Paris-Palacios YY, Couderchet S, et al. Effects of the herbicide isoproturon on survival, growth rate, and protein content of mature earthworm (Lumbricus terrestris L.) Under laboratory conditions. Environmental Toxicology. 2003;18:1-8.
- Mosleh, Paris-Palacios YY, Couderchet S. Acute and sublethal effects of two insecticides on earthworms (Lumbricus terrestris L.) under Laboratory Conditions. Environmental Toxicology. 2003;18:1-8.