Complete pathological remission after treatment with olaparib in a patient with PTEN-deficient sarcomatoid prostate cancer
Received: 29-Sep-2018 Accepted Date: Oct 29, 2018; Published: 10-Nov-2018
Citation: Jue Wang. Complete pathological remission after treatment with olaparib in a patient with PTEN-deficient sarcomatoid prostate cancer. J Mol Cancer. 2018;1(3):17-19
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Precision oncology utilizes the individual tumor genomic information to guide the optimal cancer treatment. In this case report, we describe a 67-year-old white male with T3N0M1, Gleason score 9 (5+4), prostate adenocarcinoma, who developed progressive urinary obstruction after seven months treatment of luteinizing hormone-releasing hormone analog. A Computed Tomography (CT) scan detected large masses within the pelvis, involving the bladder and rectum. He underwent resection of tumor with pathology finding of carcinosarcoma transformation (sarcomatoid prostate cancer). Comprehensive genomic profiling identified multiple genomic alterations including TMPRSS2-ERG rearrangement, PTEN deletion, RB loss, and TP53 alteration. Based on the novel findings from next generation sequencing, he was treated with the oral Poly ((Adenosine di-phosphate) ADP)-Ribose Polymerase (PARP) 1 inhibitor olaparib. CT scan after three months of therapy showed interval necrotic tumor changes of the heterogeneous prostate mass. The patient underwent pelvic exenteration to manage rectovesical fistula. Pathology showed complete pathological remission without residual tumor. This case highlights the potential of clinical next-generation sequencing in guiding the treatment decision of rare cancer when standard options fail, and thereby improving patient outcomes.
Keywords
Sarcomatoid prostate cancer; poly (ADP)-ribose polymerase (PARP) 1 inhibitor; Olaparib, PTEN; TMPRSS2-ERG rearrangement; TP53, Complete pathological remission.
Sarcomatoid prostate carcinoma (carcinosarcoma) (SCP) is a rare and historically mysterious neoplastic process involving the prostate [1-5]. This finding suggests either increased awareness of sarcomatoid prostate cancer in urology community or treatment effects of current management. Analysis of the available case series and reports on sarcomatoid prostate cancer revealed that the majority of cases of sarcomatoid prostate cancer occurred after the patient had undergone hormone or radiation therapy for adenocarcinoma [2,3]: suggesting SCP is not unique histology; it is likely a phenotype of dedifferentiation; possibly by mechanism of Epithelial-mesenchymal Transformation (EMT). A recent literature search of sarcomatoid prostate cancer revealed substantial increases in both published manuscripts, and reported cases of this cancer since 1970s [2,3].
Treatment of metastatic sarcomatoid prostate carcinoma is challenging [2-5]. There is no consensus among oncologists regarding the treatment of sarcomatoid prostate cancer owing to the rarity of this disease and the difficulty in conducting clinical trial to produce high quality clinical evidence. Although molecular targets have not consistently proven to be reliable predictors of response to therapy. Despite this caveat, with rare cancers, such as sarcomatoid prostate carcinoma, the opportunity for enrollment into clinical trials are exceedingly limited. Genomic profiling of tumor may represent the best chance for oncologists in providing an informed opinion about therapies [5,6]. At University of Arizona Cancer Center (UACC) at Dignity Health St. Joseph’s Hospital and Medical Center, we developed the Prostate Cancer Molecular Tumor Board (MTB) to track, and interpret clinical genomic profiling and potential targeted therapeutic recommendations. Poly-(ADP-ribose) Polymerase (PARP) is responsible for repairing single strand breaks in DNA. In the situation of PARP inhibition, cells switch over to Homologous Recombination (HR) for DNA repair. BRCA1- and BRCA2-mutated cells, which are HR deficient, are hypersensitive to PARP inhibition through the mechanism of synthetic lethality [7-10]. Similarly, a synthetic lethality using DNA-damage repair inhibitors has also been proposed to apply to the common (Phosphatase and tensin homolog) PTEN-deletion tumors, which are reported to have defects in homologous recombination [11].
We present here a 67-year-old male with treatment refractory metastatic sarcomatoid prostate cancer with molecular profiling findings of PTEN deletion, RB (Retinoblastoma) loss, TMPRSS2-ERG rearrangement, and TP53 alteration. Given the presence of potentially actionable alterations, and the patient’s progressive of disease through multiple lines of therapies, he was started on olaparib, to which, he had a complete pathological remission. To our knowledge, this is the first report of complete response to PARP inhibitor-based therapy for a patient with sarcomatoid prostate cancer.
Materials and Methods
Pathological diagnosis and review of tumor samples were performed by members of pathology, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona. Immunohistochemically studies that pertinent to sarcomatoid carcinoma were used. Genomic profiling was performed in a (Clinical Laboratory Improvement Amendments) CLIA-certified, (College of American Pathologists) CAP-accredited reference laboratory (Foundation Medicine). DNA extracted from formalin-fixed paraffin-embedded tumor samples was analyzed by hybridization capture of 3,769 exons from 315 cancer-related genes and 47 introns of 19 genes commonly rearranged in cancer. At least 50 ng of DNA per sample was isolated and sequenced to high, uniform coverage (average 756X) on the Illumina HiSeq2500 instrument. Genomic Alterations (GAs) (base substitutions, small insertions/deletions, rearrangements, and copy number alterations) were determined and reported. Actionable genomic alterations were defined as those with targeted anti-cancer drugs currently on the market or in registered clinical trials. Tumor Mutational Burden (TMB) was calculated from a minimum of 1.11 Mb of sequenced DNA and reported as mutations/Mb.
Case description
67 year old white male who initially presented to our cancer center with symptoms of urinary obstruction. His PSA was 2 ng/mL. Digital examinations showed enlargement of prostate. He subsequently underwent Transrectal Ultrasound (TRUS) guided prostate biopsy, showing adenocarcinoma of the prostate, Gleason score 9 (5+4). A Computed Tomography (CT) scan showed evidence of extracapsular extension, lymph node involvement, and TC-99 MDP (Technetium-99 Medronic acid) bone scintigraphy showed bone metastatic lesions. After treatment with a luteinizing hormone-releasing hormone analog for seven months, his PSA (Prostate Specific Antigen) became undetectable. Unfortunately, he developed recurrence of urinary obstruction seven months later, with radiographically detected masses within the pelvis and bladder with rectum involvement. On 12/10/2015, he underwent transurethral resection of the prostate tumor which showed sarcomatoid prostatic adenocarcinoma (carcinosarcoma) involving the prostatic urethra and bladder neck and large mass extended into bladder. The malignant cells do not exhibit significant staining with antibodies directed against CK7, CK20 or desmin. The tumor exhibits focal reactivity with antibodies directed against cytokeratin AE1/AE3 as well as smooth muscle actin. The staining pattern is consistent with prostatic carcinosarcoma (sarcomatoid prostate cancer). Subsequently the patient progressed through multiple lines of therapies including chemotherapies (docetaxel) and chemoradiation.
Comprehensive Genomic Profiling (CGP) by Next-Generation Sequencing
The tumor tissue was further evaluated with next generation gene sequencing to evaluate for specific mutations. With Hybridization capture of 3,769 exons from 315 cancer-related genes and introns of 28 genes commonly rearranged in cancer; ≥ 50 ng of DNA, sequenced to high (average 756X), uniform coverage. Genomic alterations were identified including PTEN, RB deletion, TMPRSS2-ERG translocation, and TP53 mutation.
Treatment and outcome
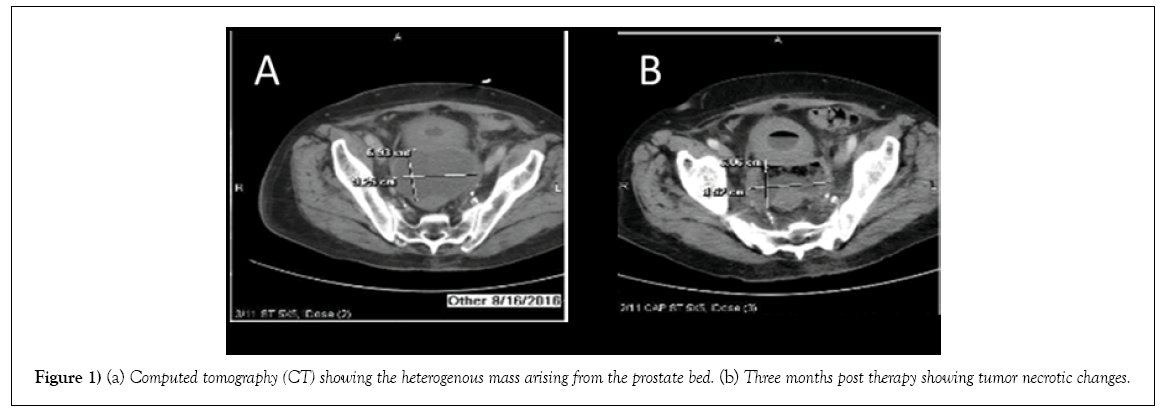
His case was discussed at UACC (University of Arizona Cancer Center) molecular tumor board. Based on the findings of PTEN loss and TMPRSS2- ERG translocation which suggested that he may have increased sensitivity to a Poly (ADP)-ribose Polymerase (PARP) 1 inhibitor. Given the presence of potentially actionable alterations, and the patient’s progressive of disease through multiple lines of therapies, he provided consent and was started olaparib 400 mg PO twice a day therapy on August 3, 2016. The treatment was well tolerated with only occasional mild diarrhea, and fatigue. A repeated CT scan of abdomen and pelvis after one month of treatment showed heterogenous pelvic mass arising from the superior prostate the measuring 8.7x6.1x8.8 cm, which was decreased from previous measurement of 10.1x9.6x12.9 cm. A repeated CT after three months of therapy showed interval necrotic tumor changes of prostate mass (Figure 1).
Figure 1: (a) Computed tomography (CT) showing the heterogenous mass arising from the prostate bed. (b) Three months post therapy showing tumor necrotic changes.
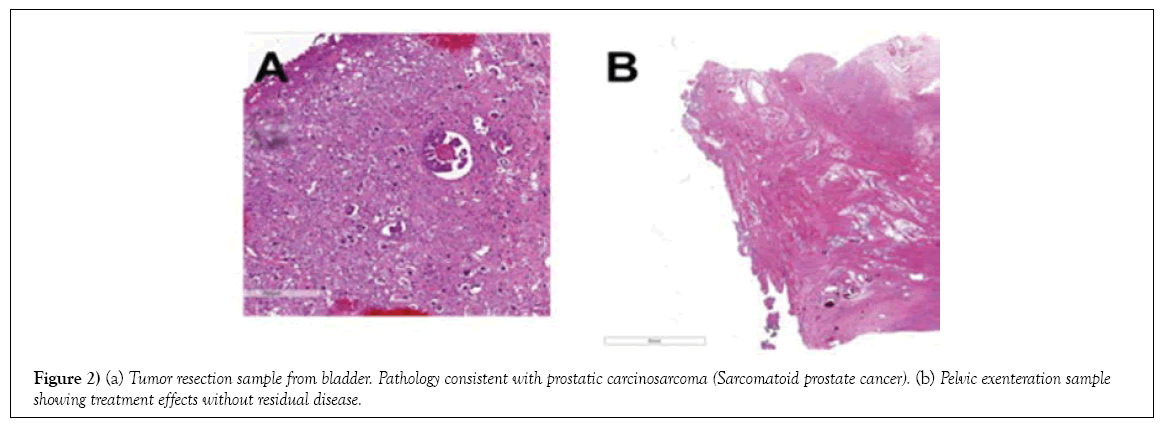
On December 27, 2016, the patient underwent pelvic exenteration to manage rectovesical fistula secondary to previous radiation therapy. Surgical pathology showed treatment effects, without residual disease (Table 1; Figure 2). His PSA at the time of cystoprostatectomy was <0.05 ng/mL.
| Date | Procedure | Pathology |
|---|---|---|
| 5/16/2014 | Prostate biopsy | Adenocarcinoma |
| 9/24/2015 | Transurethral resection of prostate | Adenocarcinoma, Gleason score 5+4 |
| 12/8/2015 | Transurethral resection of prostate | Sarcomatoid prostate cancer (Carcinosarcoma) |
| 12/27/2016 | Cystoprostatectomy | No evidence of viable neoplasm |
Table 1: Pathology Review
Discussion
Sarcomatoid carcinoma of the prostate is a rare and aggressive subtype of prostate cancer representing <1% of all prostate cancers [1-3]. Currently there is no standardized treatment regimen for SCP. We describe a patient with sarcomatoid prostate cancer with PTEN deletion, TMPRSS2-ERG translocation, RB loss, and TP53 mutation who was treated with olaparib after multiple lines of therapy and who had an impressive radiographic response and clinical benefit after 3 months of treatment. To our knowledge, this is the first reported case of sarcomatoid prostate cancer successfully treated with a PARP 1 inhibitor. Oncologists have been long observed considerable heterogeneity in clinical behavior and prognosis of prostate cancer. The results from our recent next generation gene sequencing profiling study [5] and genomic work up in this patient has provided fresh molecular insights into histogenesis of sarcomatoid prostate cancer. TMPRSS2-ERG is the most frequent genomic alteration described in localized prostate cancer, with 40–50% of patients harboring this translocation, and others harboring rearrangements involving other ETS family members [11]. Loss of PTEN and loss of TP53 are common genetic aberrations occurring in prostate cancer [12-15]. A preclinical study demonstrated that PTEN and TP53 contribute to the regulation of self-renewal and differentiation in prostate progenitors, presumptive tumor initiating cells for prostate cancer [16-19]. In a Phase I dose-escalation clinical trial [20], Sandhu et al. treated 21 treatment refractory metastatic prostate cancer patients with PARP inhibitor niraparib. The investigators reported stabilization of disease in 43% of patients with a median duration of response of 254 days. In total, 30% of patients had a decrease of Circulating Tumor Cells (CTCs) and one of the 21 patients had >50% PSA reduction. Although the investigators did not observe correlation between ERG rearrangements/loss of PTEN expression and antitumor activity, however, they did not report any information specifically on the patients with TP53 alteration, RB loss, PTEN deletion, and TMPRSS2- ERG translocation. Next-generation molecular profiling may have a role in such rare neoplasms, by searching for actionable mutations that could guide treatment decision when standard treatment fail upon recurrence and disease progression. Patients such as this case show that the usefulness of molecular guided guide therapy in management of rare tumor such as sarcomatoid prostate carcinoma. Exploration of the molecular characteristics of tumors with exceptional responses is also an important tool in improving the use of the available targeted therapies we have at hand.
Conclusion
We describe here a case of sarcomatoid prostate carcinoma with complete response after three months of olaparib therapy in a patient with PTEN loss, RB deletion, TMPRSS2-ERG translocation, and TP53 mutation. Together with preclinical studies, our case provides clinical evidence of a potential role for PARP inhibitor treatment for PTEN-deficient tumors. Further prospective studies are needed to confirm the usefulness of novel biomarkers for PARP inhibitor sensitivity.
REFERENCES
- Quay SC, and Proppe KH. Carcinosarcoma of the prostate: case report and review of the literature. J Urol. 1981;125:436-38.
- Roy Sb, Cheney S, Brunnhoelzl D, et al. Natural history, treatment, and survival of patients with sarcomatoid carcinoma of the prostate: An analysis of 199 cases. J Clin Oncol. 2017;35:278.
- Ross M, Roy SB, Brunnhoelzl D, et al. Sarcomatoid Carcinoma of the Prostate. Ann Mens Health Wellness. 2018;2:1008.
- Wang J, Wang FW. The Impact of Radical Prostatectomy on the Survival of Patients with Carcinosarcoma of the Prostate. JCT. 2011;2:475-80.
- Millis SZ, Ross JS, Stephens PJ, et al. Comprehensive genomic sequencing of prostate sarcomatoid carcinoma tumors identifies differences in genomic alterations compared to prostate adenocarcinoma tumors. J Clin Oncol. 2017;35:226.
- Wang J. Translating Therapeutic Innovations into the Management of Metastatic Castration-Resistant Prostate Cancer. Ann Mens Health Wellness. 2017;1:1007.
- Wang J. Applying precision medicine approach to metastatic castration-resistant prostate cancer: urgent education need on genomic oncology. J Mol Oncol Res. 2017;1:79-83.
- McCabe, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly (ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109-15.
- Dhawan M, Ryan CJ, Ashworth A. DNA repair deficiency is common in advanced prostate cancer: new therapeutic opportunities. Oncologist. 2016;21:940-5.
- Wang J, Freeman B, Mathew P. Chapter 13 The Emerging Role of PARP Inhibitors in the Treatment of Prostate cancer. In R. Mohan(ed) Prostate Cancer – Leading-Edge Diagnostic Procedures and Treatments. InTech - open science/open minds 2016; 213-26.
- Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315-22.
- Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644-8.
- Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209-21.
- Uzoh CC, Perks CM, Bahl A, et al. PTEN-mediated pathways and their association with treatment-resistant prostate cancer. BJU Int. 2009;104:556-61.
- Gupta A, Yang Q, Pandita RK, et al. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle. 2009;8:2198–2210.
- Martin P, Liu YN, Pierce R, et al. Prostate epithelial Pten/TP53 loss leads to transformation of multipotential progenitors and epithelial to mesenchymal transition. Am J Pathol. 2011;179:422-35.
- Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:53ra75.
- Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011;8:302-6.
- Brenner JC, Ateeq B, Li Y, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664-78.
- Sandhu SK, Schelman WR, Wilding G. The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a Phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882-92.