Complex and unusual variation of renal vessels – embryological basis and clinical significance
Chandrika G. Teli* and Usha Kothandaraman
Department of Anatomy, Meenakshi Medical College Hospital & Research Institute, Enathur, Kancheepuram, Tamil Nadu, India
- *Corresponding Author:
- Dr. Chandrika G. Teli
Department of Anatomy, Meenakshi Medical College Hospital & Research Institute, Enathur, Kancheepuram, Tamil Nadu, India
Tel: +91 812 1987031
E-mail: chandrikatelikate@gmail.com
Date of Received: July 2nd, 2011
Date of Accepted: July 30th, 2012
Published Online: November 12th, 2012
© Int J Anat Var (IJAV). 2012; 5: 87–89.
[ft_below_content] =>Keywords
renal artery,renal vein,ureter,hydroureter,uroradiological procedures
Introduction
Classically, a single renal artery supplies and single renal vein drains each kidney. Variations regarding the origin and number have been reported by many researchers [1–3]. Modern surgical and radiological techniques dictate a reappraisal and definition of the renal vascular anatomy. In this regard, the role of the primary tributaries of the renal vein and their relationship to the branches of the renal artery is important. The invasive interventions such as renal transplantation, interventional radiologic procedures and urologic operations require awareness of the possible variations of the renal arteries is necessary for adequate surgical management [4,5].
Case Report
In a 10%formalin fixed female cadaver approximately of 60 years, during the routine dissection, variations were found bilaterally. The presence of unexpected blood vessels to and from the kidney was observed.
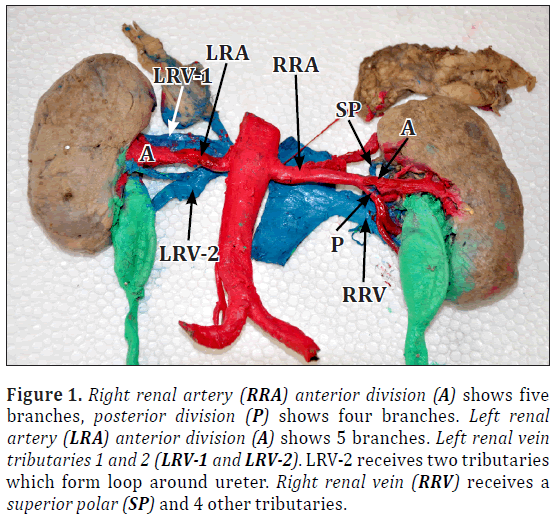
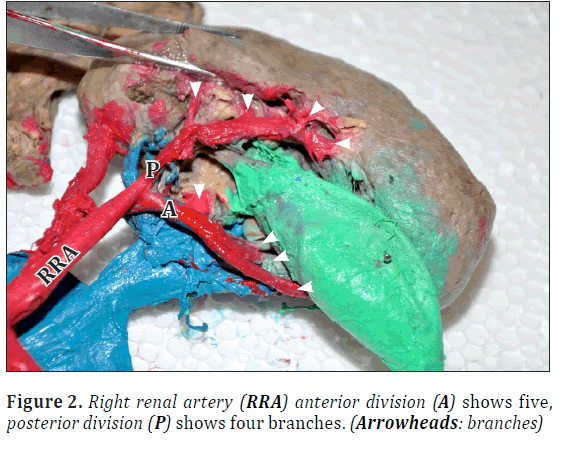
Right renal artery had anterior and posterior divisions. Anterior division gave off five branches including one superior polar branch. Posterior division showed four branches near hilum (Figures 1, 2).
Figure 1: Right renal artery (RRA) anterior division (A) shows five branches, posterior division (P) shows four branches. Left renal artery (LRA) anterior division (A) shows 5 branches. Left renal vein tributaries 1 and 2 (LRV-1 and LRV-2). LRV-2 receives two tributaries which form loop around ureter. Right renal vein (RRV) receives a superior polar (SP) and 4 other tributaries.
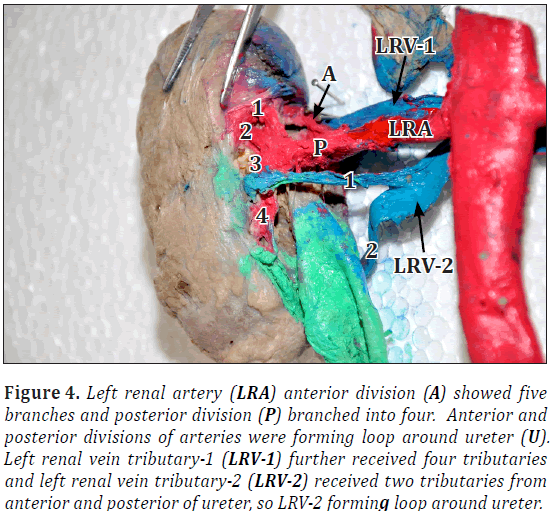
Left renal artery had anterior and posterior divisions. Anterior division showed five branches and posterior branched into four. Anterior and posterior divisions of arteries were forming loop around ureter (Figures 1, 3).
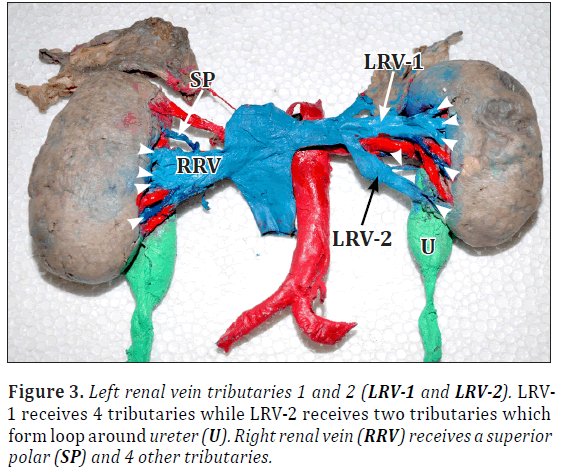
Left renal vein received two tributaries. Left renal vein-1 (LRV1) further received four tributaries and left renal vein-2 (LRV2) received two tributaries from anterior and posterior of ureter, so LRV2 forming loop around ureter (Figures 3, 4).
Right renal vein showed total five tributaries including one from superior pole (Figure 4). Bilaterally ureters were thick and dilated throughout the length (Figures 1, 4). Urinary bladder also showed increased wall thickness.
Discussion
Renal Artery
The anatomical knowledge of arterial segments is important while performing nephrectomies [6]. Variation in renal arteries is not uncommon. Shoja et al. studied the perihilar branching pattern of renal artery. They observed fork pattern in 92.6% of kidneys, duplicate in 80.2%, triplicate in 12.4% and ladder pattern in 7.4% kidneys [7]. Prehilar multiple branching of renal arteries were reported by Rao et al. [2]. Other study observed prehilar multiple branching of renal arteries in 11 (11.66%) cases. These branches were directed towards apical, middle, inferior and posterior vascular segments of kidney [8]. As multiple branches of renal artery correspond to segmental branches, the risk of hemorrhage during renal transplantation, segmental ischemia and postoperative hypertension due to loss of parenchyma increases [5]. The surgical accessibility to clamping of segmental arteries from anterior and posterior approaches was determined by Weld et al. [9]. The vascular anatomy of kidney assessed in their study consisted of zero, one or two pre-segmental arteries in 49.3%, 31.5% and 19.2% of kidneys, respectively. The renal arterial supply was analyzed in 266 kidneys dissected from 133 fixed adult subjects. The anatomical findings included: 1 hilar artery in 53.3% of the cases, 1 hilar artery with 1 superior pole extra-hilar branch in 14.3%, 2 hilar arteries in 7.9%, 3 hilar arteries in 1.9%, superior polar artery in 6.8%, inferior polar artery in 5.3% and other variations in 8.5% [10].
The embryological explanation of these variations was discussed by Keibel and Mall [11]. In an 18 mm fetus, the developing mesonephros, metanephros, suprarenal glands and gonads are supplied by nine pairs of lateral mesonephric arteries arising from the dorsal aorta. Felix divided these arteries into three groups as follows: the 1st and 2nd arteries as the cranial group, the 3rd to 5th arteries as the middle group and 6th to 9th arteries as the caudal group. The middle group gives rise to renal arteries. Persistence of more than one renal arteries of the middle group results as multiple renal arteries [11].
Renal Vein
Typical arrangement of the left renal vein is described in percentages ranging from 79 to 91% [12,13]. Two renal veins are described in about 3.1% of cases [14]. Additional veins are seen much more frequently on the right side than on the left side [15,16]. The different development of the right and left sub–supracardinal anastomosis is thought to count for the inverse relationship between supernumerary renal veins [17]. Although multiple renal veins are more common on the right side, the variations in the course are almost exclusively located on the left [17], with a reported incidence of a single vein on the right side of 26% and only 2.6% on the left [15]. While on the right side the sub–supracardinal anastomosis is incorporated into the inferior vena cava, which might be a factor favoring the persistence of both right primitive renal veins, on the left side, the sub–supracardinal anastomosis fully regresses [17].
Consequently, the persistence of more than one primitive renal vein appears improbable. In this case the regression of the sub–supracardinal anastomosis was partial, leading to the persistence of a loop between two tributaries outside the hilum, which joined together further from the hilum to form the main renal vein. Satyapal have demonstrated that 39% of the renal veins consists of two primary tributaries only, in our case, these two tributaries were large veins (measuring 1.2 and 1.1 cm in diameter – which is close to the average diameter of the main renal vein, described by Satyapal et al. and Ribeiro, forming a loop around the main renal artery branches and also around the ureter. Such variation of veins has been described previously by only one author [18] associated with additional renal artery, normal sized ureter. We reported prehilar branching of renal arteries and prehilar primary tributaries of renal veins, both looping around ureter and hydroureters bilaterally. This type of variation might have clinical implications because of the possibility of a compression of these structures by this venous loop, but could not be clinically correlated since medical history was admittedly not available.
Conclusion
Describing anatomical variations such as the one in the present study is not only of academic interest, but also important to help radiologists on the correct interpretation of image examinations and for surgeons. We believe that complex anatomical variations with direct clinical implications such as the described in the present study are worth publication.
References
- Shakeri AB, Tubbs RS, Shoja MM, Pezeshk P, Farahani RM, Khaki AA, Ezzati F, Seyednejad F. Bipolar supernumerary renal artery. Surg Radiol Anat. 2007; 29: 89–92.
- Rao M, Bhat SM, Venkataramana V, Deepthinath R, Bolla SR. Bilateral prehilar multiple branching of renal arteries: a case report and literature review. Kathmandu Univ Med J (KUMJ). 2006; 4: 345–348.
- Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM. Additional renal arteries: incidence and morphometry. Surg Radiol Anat. 2001; 23: 33–38.
- Petru B, Elena S, Dan I, Constantin D. The morphology and the surgical importance of the gonadal arteries originating from renal artery. Surg Radiol Anat. 2007; 29: 367–371.
- Sampaio FJ, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992; 14: 113–117.
- Standring Susan, Ellis H, Healey JC, eds. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. London, Elsevier – Churchill Livingstone Publishers. 2005; 1274–1275.
- Shoja MM, Tubbs RS, Shakeri A, Loukas M, Ardalan MR, Khosroshahi HT, Oakes WJ. Peri-hilar branching patterns and morphologies of the renal artery: a review and anatomical study. Surg Radiol Anat. 2008; 30: 375–382.
- Budhiraja V, Rastogi R, Asthana AK. Renal artery variations: embryological basis and surgical correlation. Rom J Morphol Embryol. 2010; 51: 533–536.
- Weld KJ, Bhayani SB, Belani J, Ames CD, Hruby G, Landman J. Extrarenal vascular anatomy of kidney: assessment of variations and their relevance to partial nephrectomy. Urology. 2005; 66: 985–989.
- Sampaio FJ, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992; 14: 113–117.
- Keibel F, Mall FP, eds., Manual of Human Embryology. Vol. 2. Philadelphia, J. B. Lippincott. 1912; 820–825.
- Reis RH, Esenther G. Variations in the pattern of renal vessels and their relation to the type of posterior vena cava in man. Am J Anat. 1959; 104: 295–318.
- Davis CJ Jr, Lundberg GD. Retroaortic left renal vein, a relatively frequent anomaly. Am J Clin Pathol. 1968; 50: 700–703.
- Goswami AP. Anatomical variation of the renal veins with varicosity presenting as pseudotumor of the kidney. J Urol. 1976; 116: 648–649.
- Satyapal KS, Rambiritch V, Pillai G. Additional renal veins: incidence and morphometry. Clin Anat. 1995; 8: 51–55.
- Dhar P. An additional renal vein. Clin Anat. 2002; 15: 64–66.
- Macchi V, Parenti A, De Caro R. Pivotal role of the sub-supracardinal anastomosis in the development and course of the left renal vein. Clin Anat. 2003; 16: 358–361.
- Ribeiro JAS, Ribeiro RA, Caetano AG, Rodrigues Filho AO, Fazan VPS. Complex distribution of renal vessels. Braz J Morphol Sci. 2007; 3: 157–159.
Chandrika G. Teli* and Usha Kothandaraman
Department of Anatomy, Meenakshi Medical College Hospital & Research Institute, Enathur, Kancheepuram, Tamil Nadu, India
- *Corresponding Author:
- Dr. Chandrika G. Teli
Department of Anatomy, Meenakshi Medical College Hospital & Research Institute, Enathur, Kancheepuram, Tamil Nadu, India
Tel: +91 812 1987031
E-mail: chandrikatelikate@gmail.com
Date of Received: July 2nd, 2011
Date of Accepted: July 30th, 2012
Published Online: November 12th, 2012
© Int J Anat Var (IJAV). 2012; 5: 87–89.
Abstract
The anatomical variations of renal blood vessels have interested anatomists for more than a century. We describe a complex variation of the renal blood vessels found during the dissection routine. A female cadaver, approximately of 60 years, embalmed with 10% formalin solution, presented bilateral multiple prehilar branching of renal arteries and multiple tributaries forming respective renal vein, made a loop around ureter. Bilateral hydroureter could not be correlated to the condition as the medical history was unavailable. This is a complex anatomical variation of the renal vessels which might have functional implications, once the vascular loop described might be a compression factor for the ureter.
-Keywords
renal artery,renal vein,ureter,hydroureter,uroradiological procedures
Introduction
Classically, a single renal artery supplies and single renal vein drains each kidney. Variations regarding the origin and number have been reported by many researchers [1–3]. Modern surgical and radiological techniques dictate a reappraisal and definition of the renal vascular anatomy. In this regard, the role of the primary tributaries of the renal vein and their relationship to the branches of the renal artery is important. The invasive interventions such as renal transplantation, interventional radiologic procedures and urologic operations require awareness of the possible variations of the renal arteries is necessary for adequate surgical management [4,5].
Case Report
In a 10%formalin fixed female cadaver approximately of 60 years, during the routine dissection, variations were found bilaterally. The presence of unexpected blood vessels to and from the kidney was observed.
Right renal artery had anterior and posterior divisions. Anterior division gave off five branches including one superior polar branch. Posterior division showed four branches near hilum (Figures 1, 2).
Figure 1: Right renal artery (RRA) anterior division (A) shows five branches, posterior division (P) shows four branches. Left renal artery (LRA) anterior division (A) shows 5 branches. Left renal vein tributaries 1 and 2 (LRV-1 and LRV-2). LRV-2 receives two tributaries which form loop around ureter. Right renal vein (RRV) receives a superior polar (SP) and 4 other tributaries.
Left renal artery had anterior and posterior divisions. Anterior division showed five branches and posterior branched into four. Anterior and posterior divisions of arteries were forming loop around ureter (Figures 1, 3).
Left renal vein received two tributaries. Left renal vein-1 (LRV1) further received four tributaries and left renal vein-2 (LRV2) received two tributaries from anterior and posterior of ureter, so LRV2 forming loop around ureter (Figures 3, 4).
Right renal vein showed total five tributaries including one from superior pole (Figure 4). Bilaterally ureters were thick and dilated throughout the length (Figures 1, 4). Urinary bladder also showed increased wall thickness.
Discussion
Renal Artery
The anatomical knowledge of arterial segments is important while performing nephrectomies [6]. Variation in renal arteries is not uncommon. Shoja et al. studied the perihilar branching pattern of renal artery. They observed fork pattern in 92.6% of kidneys, duplicate in 80.2%, triplicate in 12.4% and ladder pattern in 7.4% kidneys [7]. Prehilar multiple branching of renal arteries were reported by Rao et al. [2]. Other study observed prehilar multiple branching of renal arteries in 11 (11.66%) cases. These branches were directed towards apical, middle, inferior and posterior vascular segments of kidney [8]. As multiple branches of renal artery correspond to segmental branches, the risk of hemorrhage during renal transplantation, segmental ischemia and postoperative hypertension due to loss of parenchyma increases [5]. The surgical accessibility to clamping of segmental arteries from anterior and posterior approaches was determined by Weld et al. [9]. The vascular anatomy of kidney assessed in their study consisted of zero, one or two pre-segmental arteries in 49.3%, 31.5% and 19.2% of kidneys, respectively. The renal arterial supply was analyzed in 266 kidneys dissected from 133 fixed adult subjects. The anatomical findings included: 1 hilar artery in 53.3% of the cases, 1 hilar artery with 1 superior pole extra-hilar branch in 14.3%, 2 hilar arteries in 7.9%, 3 hilar arteries in 1.9%, superior polar artery in 6.8%, inferior polar artery in 5.3% and other variations in 8.5% [10].
The embryological explanation of these variations was discussed by Keibel and Mall [11]. In an 18 mm fetus, the developing mesonephros, metanephros, suprarenal glands and gonads are supplied by nine pairs of lateral mesonephric arteries arising from the dorsal aorta. Felix divided these arteries into three groups as follows: the 1st and 2nd arteries as the cranial group, the 3rd to 5th arteries as the middle group and 6th to 9th arteries as the caudal group. The middle group gives rise to renal arteries. Persistence of more than one renal arteries of the middle group results as multiple renal arteries [11].
Renal Vein
Typical arrangement of the left renal vein is described in percentages ranging from 79 to 91% [12,13]. Two renal veins are described in about 3.1% of cases [14]. Additional veins are seen much more frequently on the right side than on the left side [15,16]. The different development of the right and left sub–supracardinal anastomosis is thought to count for the inverse relationship between supernumerary renal veins [17]. Although multiple renal veins are more common on the right side, the variations in the course are almost exclusively located on the left [17], with a reported incidence of a single vein on the right side of 26% and only 2.6% on the left [15]. While on the right side the sub–supracardinal anastomosis is incorporated into the inferior vena cava, which might be a factor favoring the persistence of both right primitive renal veins, on the left side, the sub–supracardinal anastomosis fully regresses [17].
Consequently, the persistence of more than one primitive renal vein appears improbable. In this case the regression of the sub–supracardinal anastomosis was partial, leading to the persistence of a loop between two tributaries outside the hilum, which joined together further from the hilum to form the main renal vein. Satyapal have demonstrated that 39% of the renal veins consists of two primary tributaries only, in our case, these two tributaries were large veins (measuring 1.2 and 1.1 cm in diameter – which is close to the average diameter of the main renal vein, described by Satyapal et al. and Ribeiro, forming a loop around the main renal artery branches and also around the ureter. Such variation of veins has been described previously by only one author [18] associated with additional renal artery, normal sized ureter. We reported prehilar branching of renal arteries and prehilar primary tributaries of renal veins, both looping around ureter and hydroureters bilaterally. This type of variation might have clinical implications because of the possibility of a compression of these structures by this venous loop, but could not be clinically correlated since medical history was admittedly not available.
Conclusion
Describing anatomical variations such as the one in the present study is not only of academic interest, but also important to help radiologists on the correct interpretation of image examinations and for surgeons. We believe that complex anatomical variations with direct clinical implications such as the described in the present study are worth publication.
References
- Shakeri AB, Tubbs RS, Shoja MM, Pezeshk P, Farahani RM, Khaki AA, Ezzati F, Seyednejad F. Bipolar supernumerary renal artery. Surg Radiol Anat. 2007; 29: 89–92.
- Rao M, Bhat SM, Venkataramana V, Deepthinath R, Bolla SR. Bilateral prehilar multiple branching of renal arteries: a case report and literature review. Kathmandu Univ Med J (KUMJ). 2006; 4: 345–348.
- Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM. Additional renal arteries: incidence and morphometry. Surg Radiol Anat. 2001; 23: 33–38.
- Petru B, Elena S, Dan I, Constantin D. The morphology and the surgical importance of the gonadal arteries originating from renal artery. Surg Radiol Anat. 2007; 29: 367–371.
- Sampaio FJ, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992; 14: 113–117.
- Standring Susan, Ellis H, Healey JC, eds. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. London, Elsevier – Churchill Livingstone Publishers. 2005; 1274–1275.
- Shoja MM, Tubbs RS, Shakeri A, Loukas M, Ardalan MR, Khosroshahi HT, Oakes WJ. Peri-hilar branching patterns and morphologies of the renal artery: a review and anatomical study. Surg Radiol Anat. 2008; 30: 375–382.
- Budhiraja V, Rastogi R, Asthana AK. Renal artery variations: embryological basis and surgical correlation. Rom J Morphol Embryol. 2010; 51: 533–536.
- Weld KJ, Bhayani SB, Belani J, Ames CD, Hruby G, Landman J. Extrarenal vascular anatomy of kidney: assessment of variations and their relevance to partial nephrectomy. Urology. 2005; 66: 985–989.
- Sampaio FJ, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992; 14: 113–117.
- Keibel F, Mall FP, eds., Manual of Human Embryology. Vol. 2. Philadelphia, J. B. Lippincott. 1912; 820–825.
- Reis RH, Esenther G. Variations in the pattern of renal vessels and their relation to the type of posterior vena cava in man. Am J Anat. 1959; 104: 295–318.
- Davis CJ Jr, Lundberg GD. Retroaortic left renal vein, a relatively frequent anomaly. Am J Clin Pathol. 1968; 50: 700–703.
- Goswami AP. Anatomical variation of the renal veins with varicosity presenting as pseudotumor of the kidney. J Urol. 1976; 116: 648–649.
- Satyapal KS, Rambiritch V, Pillai G. Additional renal veins: incidence and morphometry. Clin Anat. 1995; 8: 51–55.
- Dhar P. An additional renal vein. Clin Anat. 2002; 15: 64–66.
- Macchi V, Parenti A, De Caro R. Pivotal role of the sub-supracardinal anastomosis in the development and course of the left renal vein. Clin Anat. 2003; 16: 358–361.
- Ribeiro JAS, Ribeiro RA, Caetano AG, Rodrigues Filho AO, Fazan VPS. Complex distribution of renal vessels. Braz J Morphol Sci. 2007; 3: 157–159.