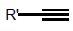Conjugates toward medicines
Received: 20-Dec-2017 Accepted Date: Dec 26, 2017; Published: 29-Dec-2017
Citation: Pearson D, Li H. Conjugates toward medicines. J Pharmacol Med Chem 2017;1(1):32-39.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The more and more drugs in basic research, drug discovery, and drug development have been diverting to various types of structural perspectives, in which conjugated constructions emerge with remarkable functions superior to conventional drugs. These conjugated drugs include small molecules and macro compounds. The main difference between conjugated and conventional structural modifications is multi-functionalities. These multi-functionalities combine one or more bioavailable moieties: a drug segment, a ligand segment, and a linker segment. The drug segment plays the critical therapeutic role, the ligand segment is in charge of the selectivity, and the linker segment connects the drug and the ligand together. The simplest conjugate would be bi-functionalized molecule that biologic properties has been changed upon intent design. This review will summarize the most recent advancements in the medicinal conjugations under development and at various other stages of development as they are investigated as for novel drugs, drug candidates, and drug leads.
Keywords
Conjugation; Linker; Multiple functions; Pre-drug; Drug delivery; Drug targeting; Antibody; Nano medicine; Aptamer
In drug discovery and development, it is impractical to test large quantities of drug candidates during clinic studies because that the expense of the clinic studies would be enormous. Only those possessing comprehensive properties as drugs should be moved to this stage, which is largely relied on structural modification or optimization. Conventional drugs are composed of single molecule or macro compound, or various size compounds in between. These drugs, such as active pharmaceutic ingredient (API), have to be subjected accordingly to delivery techniques. In the last few decades, more and more drugs in basic research, drug discovery, and drug development have been diverting to various types in structural perspective, in which conjugated constructions are emerging. In principle, a conjugated constructed drug is composed of three parts, the therapeutic part or the ‘warhead’, the additive segment or ligand, and the linker. The therapeutic part is the “warhead” for the conjugated constructed drug. The additive segment or ligand possesses specific functionalities upon the purpose of the designs. The linker tethers the first two moieties together. The linker can be classified as degradable or nondegradable. Degradable linkers are relatively chemically and enzymatically sensitive either out of the cell or in the cytoplasm or lysosomes, including pH responding hydrolysis or proteolytic dissociation, to cleave the therapeutic segment from the parent conjugate. In contrast, a non-degradable linker resulting no dissociation so that the entire compound behaves as a whole therapeutic entity.
Literature Review
The features of degradable and non-degradable linker depend on whether the therapeutic agent remains stable when and after the conjugate binds to the target. The creation of a clinically successful conjugated medicine has been challenging. For the therapeutic to work well, each of the parts must be carefully considered in the molecular design [1]. The most common bioconjugations are small molecule (such as vitamins, fluorescent dyes, cytotoxine, etc.) coupled to protein, oligonucleotides, carbohydrates, polymers, metals, or antibodies [2]. A number of antibody-drug conjugates (ADC) used to target tumors named vaxculature, Brentuximab vedotin and Gemtuzumab ozogamicin are active areas of research in the pharmaceutical industry [3]. In contrast to peptides and/or proteins, molecules like oligosaccharides, nucleic acids, synthetic polymers, carbon nanotubes, or polyethylene glycol used as bioconjugations are relatively inexpensive, but their cost are increasing rapidly [4]. Bioconjugation has also gained importance in nanotechnology applications, such as in bioconjugated quantum dots [5,6]. In this review conjugated molecules are classified into following sections in terms of conjugate medicine. It will be shown that conjugate distinguishes is not so strict in between but crossed related.
Conjugation Chemistry
The constitution of conjugate has been resolving by various of approaches in chemistry. Different linkers have been constructed to connect small molecule drugs to other small or large molecules. The conjugation chemistry regulates the comprehensive profiles of the characters, nevertheless, it is essential to the safety as well as efficacy of the conjugate. Antibody-drug conjugate has been used as medicine, and typically represents conjugate drugs. The requirement to design an ideal conjugate is it has to be relative stable throughout its active time before and after administration. The ligand binds to the surface of the target cell to ensure that the drug can be delivered to the initial site of interaction, and eventually internalized into the cell.
The most well developed approaches of conjugation chemistry are highly dependent on the type of functional groups on the back bone or core frame of the moieties. The functional groups provide sufficient flexibility and distant between the moieties so that not to interfere with the function of the therapeutic site. However, as a whole entity, the rigidity must be considered to ensure the synergistic or coincident interactions between the ligand and the receptor, or conjugate and the target.
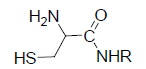
In peptide and protein conjugation, cysteines and lysines are reacted with hydrazones, disulfides, and thioethers, because these functionalities can serve as good nucleophiles. In the developments of ADCs, more than half of the thiol groups of the cysteine residues, with one quarter conjugated through surface lysines [7], so does lysine residues, which make the conjugation heterogeneously.
The conjugating conditions are required to have a high conversion rate (>99%), faster, milder, with good solubility, particularly in biologic environments. Extensive studies have been performed on the conjugating conditions during the last few decades. On the other hand, the conjugated bonds must be easily cleaved whenever not needed in vitro, but the bonds must be stable enough to allow for controlled cleavage.
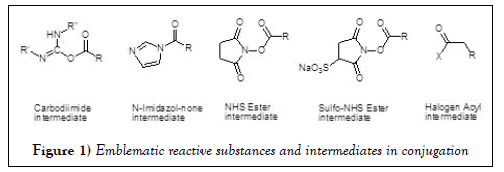
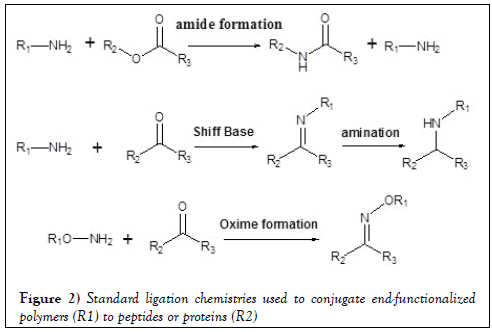
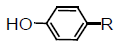
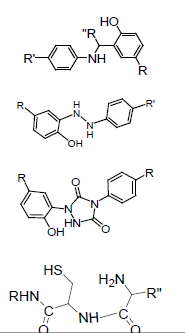
The emblematic reactive substances and intermediates in conjugation are summarized in Figures 1 and 2. Some of the common intermediates for coupling bears NHS-, and Sulfo-NHS-active esters. One of the advantages of these groups is the availability in aqueous solutions (Figure 1). Amide formation is the most conventional coupling approach along with primary and/or secondary amine nucleophile coupled to active esters, or cyanates, or carbonyl derivatives (Figure 1). Activated acyl derivatives of carboxylic acids can react with target nucleophilic groups to give the acetylated products listed below (Table 1).
| Nucleophile | Substitute | Product | Medicating |
|---|---|---|---|
| R-NH2, R-NH-R, R-OH, R-SH, R-NHNH2, R-ONH2 | Carbodiimide N-Imidazol– none, NHS Ester, Sulfo-NHS Ester, Halogen Acyl, Urea, Thoreau, Cyanates |
Amides conjugates, Esters conjugates, Thioesters conjugates, Hydrazides of small molecules, Carbohydrates, Nucleotide and nucleosides | Pro-drug Targeting Delivery Imaging Vaccine Therapy |
| Aldehyde, Ketone | Oxime Diazenes, Diazenes Imines or Schiff Base |
||
Electrophile |
Tyrosine Derivatives
|
 |
|
Azide and Alkyl terminated 3+2
Staudinger ligation(7) Click Chemistry |
|
 |
Table 1: Emblematic reactive substances and intermediates in conjugation.
In addition to conventional approaches, a few novel techniques for selective reactions resulting in homogeneous conjugated productions have been developed, providing additional options under different conditions [8], which have the potential to provide the products with improved therapeutic properties, particularly for macromolecules [9].
Among these, Staudinger ligation are a relatively new development that uses phosphorene, but its effects on biological processes is not clear [8]. Click chemistry provides an even more convenient approach to conjugating because of its reaction conditions are more feasible both in aquatic and organic solvents. Nevertheless, this 2+3 cyclicadition creates triazole ring that may elicit immunogenic interfering [10] along with undesired antigenic.
Another valuable reaction in peptides or proteins is thioester-linked intermediate product undergoing subsequently a S→N-acyl rearrangement, giving a common amide bond at the reaction site. The ligation is highly specific and hence, only a single product is produced, even additional cysteine residues presented. This method typically requires a N-terminal cysteine at the ligation site, but recently it was demonstrated that auxiliary groups like N-ethanethiol, N-2-mercaptobenzylamine or N-(1-phenyl-2- mercaptoethyl) sulfhydryl can also be used [11-13]. The auxiliary groups act as surrogates for the N-terminal cysteine and facilitate the chemical ligation, making ligation possible at a non-cysteine residue [11,14]. Nucleophilics and additions molecules are summarized schematically below (Figure 3).
In actual application, selective N-terminal conjugation bases on the different reactivity of internal and terminal amino acids. Site-specific functionalization can be realized by enzymatic approach in terms of bioengineering, incorporated with unnatural amino acids, carbohydrates at desired positions to react with functional groups like azides, ketones, aldehydes, or alkynes [15], which are enable the use of novel conjugation chemistry, enzymatic ligation and the click chemistry for instance [16].
Nucleophilic strength:
R S- > R NH2 > R COO- = R O
Summary of Linkers
Linkers not only act as connection, but they may also affect biological activities. Lipid chains show remarkable cell internalizing characters, because it is compatible with cell membranes and easily inserts into cell due to its high hydrophobicity. This forces the other parts of the conjugate to be internalized by way of diffusion. On the contrary, polyethylene Glycol (PEG) enhances hydrophilicity, and increase the water solubility of the conjugate. Both types of linkers may be flexible chains, and may impact the stability of active structure, i.e. insisting protease, nuclease. Linker chains with disulfide bond can be cleaved by internal cell glutathione or reductants. Ester bonds are biodegradable and they are easily hydrolyzed by enzymatic disassociation with lipase.
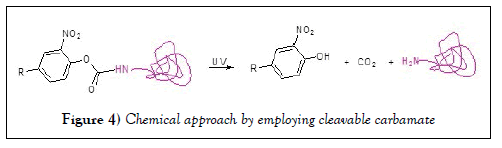
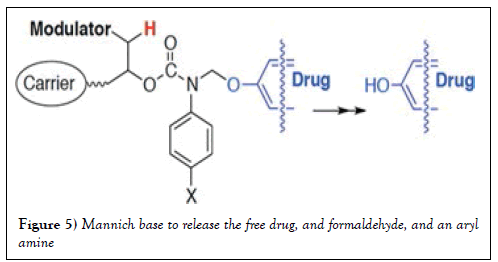
A new chemical approach by employing cleavable carbamate group extended the life time of the conjugate, which provides more options to design a conjugate [17], as showed in Figure 4. In this structure, linker undergoes β-elimination to release the active drug that could be small or big, it can be easily broken down by UV light. Such photo responsive conjugation empowers the development of novel method for drug discovery, disease diagnosis, and high-throughput screening. However, it requires amino group on the drug to introduce the carbamate, nevertheless, not all small molecules have amine. Carbonate and nitrile phenol ester had been then developed for a versatile cross-linker that can be used for a wide range of applications where photocleavage ensures clean and controllable release of biological active molecules from the conjugate. One profound design was adapted the similar mechanism; carbamate linkers can connect phenolic hydroxyl groups. The methylene is necessary in between the drug segment and the carbamate nitrogen that is also connected to an electron-withdrawing group to stabilize the carbamate structure from pH sensitive dissociation, and causes β-elimination, (Figure 5) intermediated a Mannich base to release the free drug, and formaldehyde, and an aryl amine [18,19].
Discussion
In drug development, it is not so fruitful to discover a bioactive molecule that may be a good candidate or lead compound in medicine development. One up-to-date solution that has been used during the last decade is conjugated medicines that consist of modified generic drugs with less drawbacks than the current treatments. In these conjugated medicines, modulators, linkers, and therapeutic heads are chemically connected through covalent bonds. The objectives to design these conjugates are summarized below. There may be no significant distinction between these classified categories, these compounds have crossed characteristics and activities.
Pro-drugs
Pro-drugs are intensively modified therapeutic molecules that consist of multifunctionalities. These pro-drugs are coupled to at least one additional moiety to form a conjugate. These compounds are able to dissociate or release the therapeutic moiety after delivery to prevent the original therapeutic problem. As a consequence of this, these conjugates have been widely used in generic drug development. Conjugated functional groups may improve biological properties of the molecule, such as bioavailability, pharmacokinetics, pharmacodynamics, metabolism, stability, and targeting. Using anti-cancer drugs as an example, an antibody and cytotoxic reagent conjugated through a linker have specific impacts to the overall therapeutic efficacy, including the process of internalization and the efficiency of intracellular transferring of receptor-conjugate complex. In designing anti-cancer conjugate, the tumor specific metabolites result ADC dissociation. Trastuzumab emtansine is an example of such an anti-cancer prodrug, it is dissociated in the cytoplasm of tumor cell [20]. So far, three ADCs were approved by FDA: Adcetris® (brentuximab vedotin developed by Seattle Genetics), Kadcyla® (ado-trastuzumab emtansine developed by Genentech), and ZEVALIN® (ibritumomab tiuxetan developed by Spectrum Pharmaceuticals) that was used to treat certain non-Hodgkin lymphomas, differs from the previous two in that rather than delivering an anti-neoplastic payload to cancer cells, it delivers yttrium, a cytotoxic radioactive compound directly to the cancer cells. A fourth pro-drug, Inotuzumab ozogamicin, was approved by the European Commission, it is a monotherapy to treat adult patients suffered by refractory or relapsed CD22-positive B-cell precursor acute lymphoblastic. Inotuzumab ozogamicin has also been approved recently by FDA [21].
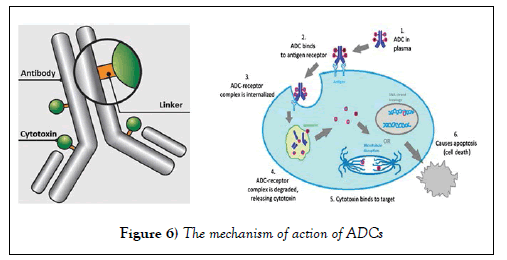
In recent years there have been about 40 ADCs in clinical trials [22]. Lonza Group Ltd. (Basel, Switzerland) has been able to start large-scale production of antibody drug conjugates for an emerging class of products utilized mainly for the treatment of cancer. ADCs are made by coupling highly active chemical substances with a biotechnologically manufactured antibody [23]. The mechanism of action of ADCs is that the antibody portion of an ADC hones onto a cell-surface antigen that is specific to a certain type of cancer cell. Upon binding, the ADC-antigen protein complex becomes internalized into the cancer cell. When the complex is degraded in the lysosome, it releases the cytotoxin which then binds to its target to cause cancer cell apoptosis [24]. This mechanism is elucidatedas Figure 6.
Cholic acid and peptides conjugates are one type of pre-drugs that are designed and investigated for peptide drug oral delivery from our study. These compounds have demonstrated that the ester bond linking the bi-functional moieties are internalized into the cancer cell membrane and then dissociated efficiently [25], suggesting that the peptide drugs may be administrated through the gastrointestinal track by employing specific transporter mediated delivery, such as cholic acid receptor. Similarly, another example of a prodrug consists of lipid conjugated drugs in our project were able to penetrate into brain cells in an in vivo trial, on which [26] the conjugates are connected with lipid chains or cholesterol hydrophobic group, made up of 10 borane cages for positron capture therapy against brain cancer.
Polymeric and nano medicines
Covalent attached synthetic polymers are another type of conjugates in medicine. Nucleic acids, peptides, proteins, enzymes, carbohydrates biologic molecules, even viruses are most likely in the nano scale. Because of the similarities in macro molecular mass and geometric size, polymeric and nano medicines are classified as one family. Polymer therapeutic is used as a general term because its major character is bioactive. These large conjugates are not encapsulated, but connected covalently to the polymeric backbone in various rate and positions [27,28], thus they differ in molecular structures in apparently large range [29-31].
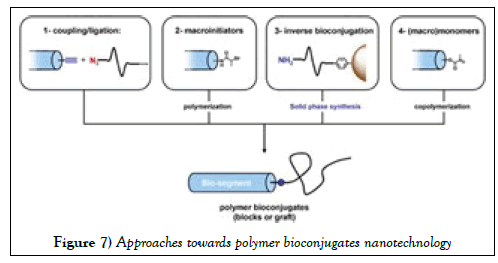
In contrast, thermosensitive nanocarriers are the “smart” drug delivery systems that have shown tremendous promise in the field of controlled drug delivery due to their special property. Thermosensitive nanocarriers have long circulative features, they can accumulate in pathological sites because of increased permeability and retention induced by attaching target ligands onto the surface of the nanocarriers, the conjugate dissociating rates can be adjusted in response to thermal variability of the environment. In this case, the therapeutic drug is encapsulated in the aggregated particles rather than covalently connected. These drugs can contain particles such as liposome, micelle, poly cross-linked matrix, or dendrimer matrix that are sensitive to temperature [32]. Polymer bioconjugates can be typically classified into four main strategies or approaches: first, the coupling strategies, in which biological segments are covalently linked to preformed polymers using one or multiple reactive sites; second, the macroinitiator strategies, in which the synthetic polymer segment is grown in situ from a biological structure, third, the inverse approaches, in which the biomolecules are constructed on a preformed synthetic polymer, and the fourth, the monomer approaches, which rely on short polymerizable bio-segments [33-35].
Schematic illustration of the four principal strategies or approaches towards polymer bioconjugates nanotechnology is showed in Figure 7. These approaches are related via synthesis and usage of a wide range of nanomaterials. These nanomaterials are crystalline allotropic forms of carbon such as fullerene, nanotube, graphene, carbin, or nanodiamond. These materials represent an important source for medical applications [36-39].
Synthetic vaccine
Vaccines are traditionally produced from attenuated or deactivated organisms, which are subjected to constitute vaccine formulations for clinic treatment. If a vaccine is deemed successfully, it has to meet three important criteria, first, it must be able to elicit immune response, and provide long-lasting protection [40]; second, it has to be safe and effective in vaccinations; third, it should be moderately stable [41]. Additionally, a vaccine or an antigen has also to be delivered to particular immunogenic cells to elicit immune response. In the design of the delivery system, it has been considered to prevent induced possibility of diminishing immunity or immune tolerance [42]. Since the mutations have been developing always in different pathogens, including cancer cells, accordingly the regeneration of the new antigens is necessary. Synthetic or artificial vaccine is considerable to be manipulated due to the molecular antigen enables to conjugate to carrier protein chemically, giving an opportunity to evade this problem. It sights climax of the progress that has been succeeded to evolve synthetic or artificial vaccines by using various molecular antigens. The approaches to design conjugate vaccines are adaptable by employing large molecules like peptide/proteins, carbohydrates or small compounds as antigens [43]. A latent problem associated with synthetic vaccines would be that these vaccine conjugates may not need to be necessarily cleaved.
Carbohydrate has been well known as the major immunogenic epitope in extensive vaccines design. The molecular immune properties of glycoconjugate vaccines has heavily count on the structure of carbohydrate. A carbohydrate segment with a well-defined β-(1-3) glycan conjugates to the predetermined tyrosine or lysine residues onto the carrier protein CRM 197 is a successful anti- Candida glycoconjugate vaccine [10].
Higher molecular mass and larger geometric size make the conjugated molecules behave immunogenicity that can be designed rationally. On the assumption that the larger molecules as nanoparticles are able to target dendritic cells, and delivered to draining lymph nodes so that they can induct faster immune responses. It is the key to develop new generation vaccines to use conjugated structures inducing the antigen-specific cellular response.
Numerous antigens have been linked to small or larger ligands in order to develop vaccines that are modified chemically to introduce immunogenic moieties. The advantages of this type of vaccines are manipulative. It can be rationally designed, and synthesized chemically on a large scale with less cost. They can also be diversified in parallel and combinatory manners into potential drug candidates by widely screening.
Nanoparticle vaccine development involves using a template technique for the preparation of nanoparticles. Besides, nanoparticle conjugated antigen traffic systems have been used to develop new generation vaccines [44]. Efforts are in the works to design glycosylated peptides and mini protein segment (Figure 8) synthetic vaccines composed of multivalent antigens or antigenic functional groups in the treatment against HIV-1[45-47].
Imaging reagents
In the diagnostic applications, fluorescent imaging of biological systems in the second near-infrared window can probe tissue at centimeter depths and achieve micrometer-scale resolution at depths of several millimeters. It has been reported that recombinant human SUMO E1 proteins have shown to be a probe to target overexpressing hEphA2 in cancer cells. Another conjugated compound has also been developed to serve as a specific optical imaging agent, so called Renilla luciferase variant 8 and green fluorescent protein (EGFP) [48]. High levels of uptake of even simple PEGylated- CH1055 conjugated dyes have observed in brain tumors in mice, on which PEGylated tails acts a key role to help the drug cross the brain blood barrier. This compound applies also molecular imaging of tumors if connected to anti-EGFR antibodies [49].
Another type useful conjugate is nanoparticle magnetic resonance imaging (MRI) negative agent, its major effort is to increase cell-loading capacity but maintain less cytotoxicity. Recent reports have shown that synthesized gold/ iron bimetallic nanoparticles have been able to deposited on the surface a matrix, its diameter is about 4 nm. Phantom MRI image has revealed these nanoparticles versatility of providing capabilities for conjugate diagnostics, therapeutic applications, as well as imaging contrast reagents [50,51]. Precautions must be considered when trying to adopt heavy metals into nano-conjugates as medicines because of their metabolic and toxicity issues.
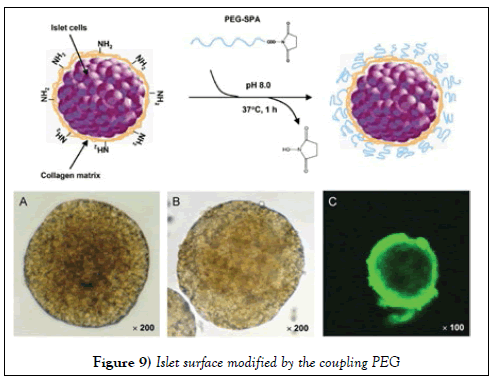
Poly ethylene glycol (PEG) coated living cells lead to reduced immune responses in blood transfusion or tissue transplantation. Such behavior was previously reported for red blood cells, T-lymphocytes or pancreas islets [52]. In Figure 9, it was displayed that an islet surface modified by the coupling PEG that possesses an active terminal ester.
Synthetic strategy for modifying the surface of pancreas islets with poly (ethylene glycol) (PEG). Images A and B show the morphology of the cells before and after PEGylation respectively. Image C shows a confocal laser scanning micrograph after conjugation with a fluorescein labeled PEG.
Drug delivery
Drug delivery is the vital application for conjugated compounds. Small nanoparticles made up of DNA conjugated by a complex of biodegradable poly β-amino ester with PEG can penetrate orthotopic brain tumors as well as healthy brain parenchyma rapidly, this rapid diffusion of DNA-loaded nanocomplexes have been observed in non-cancerous tissues ex vivo. It was demonstrated that the nanoconjugates evade adhesive trap in brain due to the dense of PEG coating [53]. As we well know that pharmacokinetic properties can be dramatically improved by conjugation technique. It has been reported that homogeneous 8-loaded ADCs with drug linkers designed to minimize hydrophobicity has been administered without impairing antibody pharmacokinetics. The drug-antibody ratio (DAR) in the conjugate enhance in vitro efficacy, so does the plasma clearance. While it also reduces in vivo efficacy and exposure however. The hydrophobicity of this ADC is favorable to accelerate clearance, this function owes to modulation of druglinker design, hydrophilic linkers and PEG connected ADCs with high DAR are superior in vivo study [54].
In a recently reported micelle formula approach, the drug warhead was not attached by a covalent bond. The matrix metalloproteinase (MMP)- responsive peptide GPLGVRGDG was introduced into the block copolymer via click chemistry, and ring-opening polymerization to prepare PEG– GPLGVRGDG–PDLLA. This complex was then self-assembled on to micellar nanoparticles (NPs) in load paclitaxel (PTX). The resulting complex was treated with MMP-2 which triggered dePEGylation due to cleavage of the peptide linkage. Moreover, the residual VRGDG sequences are retained on the surface of the NPs after dePEGylation, which facilitates in cellular uptake. The cytotoxicity of PTX loaded in the NPs against 4T1 cells was significantly enhanced [55] (Figure 10).
In the next baccatin generation molecules, the PTX wrapped liposomes composed of RGD-PEG-Chol were constituted by a method of film formation. The liposome size is about 100 nm; this leads to enhancement of cytotoxicity of paclitaxel to B16F10 cell significantly. Flow cytometry revealed the increasing cellular uptake of drug encapsulated in the liposome. Using peptide modified liposome provides a supplementary practicable approach to prepare these materials that are controllable in performances as new drug delivery method [56].
Drug targeting
As described above, antibody-drug conjugates are exceedingly effective drugs designed for targeting therapy of cancer [57]. Comparing with traditional chemotherapy, ADCs are more specific to cancer cells, and more safe to normal cells [58-61]. These conjugates family has impeccable structures that refers to ligand based molecular design for conjugate medicines [62]. Because the healthy cells are less severely affected it would be a good choice to against cancer by using these conjugated molecules [63,64]. One more representative ADC is CD46 antibody connected to the microtubule inhibitor, monomethyl auristatin F. Figure 11 shows toxicity specifically against myeloma. This conjugate has also been revealed the potent to treat primary multiple myeloma ex vivo [65]. The distinctive sizes of moieties in ADCs and the conjugating ratio and connecting sites can effect entire molecule’s behavior. Mylotarg is the first ADC approved by FDA, which is a conjugate between a recombinant large IgG4 kappa antibody and a small cytotoxic antibiotic-calicheamicin via a bifunctional linker of N-acetyl-gamma calicheamicin. This conjugated molecule has about 50% of loaded antibody, five folds’ moles of calicheamicin than antibody [66].
Therapeutic reagents
As full entity, protected and specific delivery of nucleic acids to malignant cells remains a highly desirable approach for cancer therapy. Therapeutic efficacy of a tenfibgen (TBG)-shell nanocapsule for tumor-directed delivery of single stranded DNA/RNA chimeric oligomers shows to target CK2αα’ and xenograft tumors in mice. This encapsulated chimeric oligomer targets CK2αα’ suggesting the development of a systemic treatment of prostate tumor with significant promise [67].
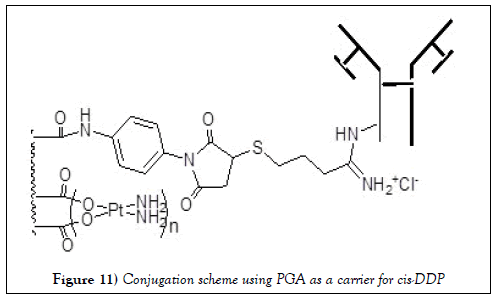
It was reported recently that the monoclonal antibody cetuximab targeting epidermal growth factor receptor (EGFR) was conjugated to cisplatin (cisdiamminedichloroplatinum), it was proven to treat brain tumors by targeting human EGFR. It behaves as a full drug and still can undergo dissociation at the binding position to release cisplatin [63].
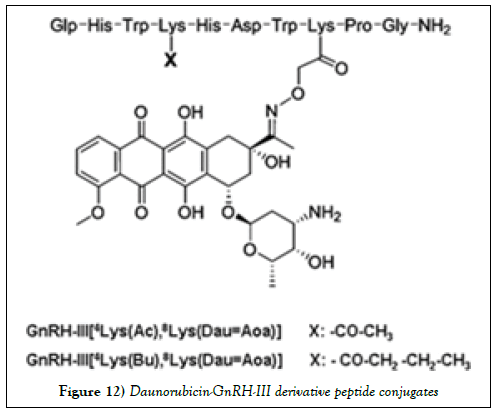
Peptide-based drug is an another promising therapeutic approach for cancer, which can provide increased selectivity and decreased side effects as compared to current anticancer drugs. A daunorubicin- Gonadotropin-releasing hormone-III (GnRH-III) conjugate complex attached to the 8Lys of a GnRHIII derivative via an oxime linkage has proved to have significant benefits over free daunorubicin that typically has be administered at the maximum tolerated dose in vivo to have an antitumor effect on subcutaneously developed HT-29 colon tumors. GnRH-III peptide represents a suitable targeting moiety, in the treatment of hormone independent tumors that highly express GnRH receptors (Figure 12) (e.g. colon carcinoma) [68].
As a small conjugate, haluronan conjugates was designed to enhance cytotoxic drugs selectively penetrating hyaluronic acid (HA) receptor-expressing cancerous cells. As an example, HA-paclitaxel conjugate was used to treat intraperitoneal of ovarian cancer, it can be distributed all over connective, neural tissues, and epithelial [69].
In our study using neutron capture cancer therapy, boron 10 cluster-lipids and cholesterol conjugates were employed. The conjugates were efficiently transported into brain cell by undertaken liposome formulations [26]. High boron content liposomes are promising antitumor effect for neutron capture therapy, which has developed the distearoyl boron lipid (DSBL) liposomes as a vehicle for neutron capture cancer therapy. Significant antitumor effect was observed in mice injected with Mercaptoundecahydrododecaborate (BSH)- encapsulating 10% DSBL liposomes even at the dose of 15 mg/kg. The tumor completely disappeared after three weeks of th ermal neutron irradiation [70].
Except for anti-cancer reagents, anti-microbial conjugate medicine is also critical in drug discovery and development. When a fungus Penicillium citrinum fermented mass was conjugated with silver to produce nanobioconjugates (SNBCs), it is an eco-friendly process to synthesize a stable silver nanobioconjugate with antibacterial properties. It was found that SNBCs exert antimicrobial activity against Gram positive and Gram negative bacteria and fungus [71].
As mentioned before, lipopeptide is a new family of potent versatile bioconjugates acting with a variety of phytopathogens, and is considered environmentally acceptable. Lipopeptides are nontoxic, biodegradable, highly stable, ecofriendly, and nonpolluting biomolecules, therefore they have wide applications in plant disease management, in cosmetics, food preservation, as surfactants, anti-parasitic, anti-viral, and anti-tumor/cancer agents. This compound seems to be a promising biopesticides in agriculture as it can be used to replace harmful chemical pesticides and a potent tool to overcome chemical resistance of phytopathogens [72].
Interestingly, three types of Bacillus lipopeptides, Surfactins, Iturins and Fengycins, have expressed antagonistic activities within a wide range of phytopathogens such as bacteria, fungi, and oomycetes. Both Fengycin and Iturin are active against fungal, however Surfactins are active against broad spectrum of bacteria, and they are classified to larvicidal agent [73]. The first cyclic lipopeptide antibiotic Daptomycin was approved by FDA in US for the treatment of serious infections in blood and skin in 2003 [74]. Now, it has been approved in more than 70 countries.
Aptamer or oligonucleotide
Oligoaptamers have attracted ample interest in the areas of nanotechnology, biology, and biochemistry, medicines over the past decade [6,75-77]. One of the modification on monomeric building block is 2′-amino-LNA that has improved the therapeutic property of siRNA linked with aptamer [78-80], on which N-acetylgalactosamine and Galactose serve as binding ligands of asialoglycoprotein receptor [81,82].
In aptamer family, many conjugates in terms of ‘Aptabody’ have been developed for various medical applications. Among which, aptamer-hybrid nano-conjugate delivery technique was designed to selectively transport miRNA-29b to MUC1-expressing carcinoma cells. These novel aptamer nano-conjugates could serve for intracellular delivery of miRNAs to cancer cells to improve the therapeutic consequence of lung cancer [83,84]. Another modified slow off-rate aptamer (SOMAmer) with high binding affinity (Kd=0.2 nM) inhibits the signaling pathways of IL-6-dependent cell [85], it was screened from large random libraries. The structural properties are effected by hydrophobic functional groups including benzyl-, 2-naphthyl-, or 3-indolyl-carboxamide, linked at the 5-position of uridine via a carboxamide bond [86]. The hydrophobicity plays essential role of interactions with target molecules, and accordingly to form fresh secondary and tertiary intramolecular structural motifs [87,88]. Except for the improvement of binding affinities, SOMAmer enhances nuclease resistance, and the selection rates as well [89].
Similarly, SL105 and SL1026 are potent antagonists of the IL-6 signaling aptamer-small (PEG) molecular conjugates, they represent new drug candidates to treat IL-6- mediated diseases as shown in Figure 13 [90].
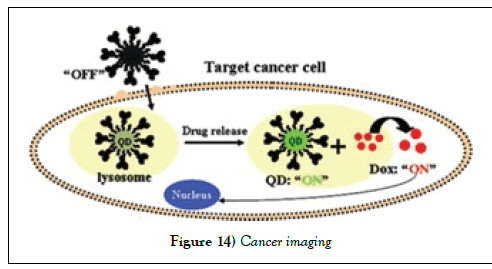
A new developed conjugate, Dot-aptamer-doxorubicin (QD-Apt(Dox)) is so called quantum dot conjugate. It acts as cancer imaging, therapy, and sensing compound. A10 RNA aptamer conjugates with fluorescent QD, which recognizes the prostate specific membrane antigen (PSMA) on the extracellular domain, targets the imaging regent (QD−Apt) to prostate cancer cells that express the PSMA protein (Figure 14).
Once QD-Apt then conjugates with cytotoxic drug, Dox, forms antineoplastic anthracycline drug and fluorescent multi-functional properties, in which, the double-stranded stem of the A10 aptamer acts targeting. The mechanism is the fluorescence resonance energy transfer within QD and Dox, and quench between Dox and aptamer. This multifunctional nanoparticle design is able to specifically deliver cytotoxicity to prostate cancer cells, and sense the fluorescence of QD for either cancer imaging, or therapy [91].
Conclusion
In this review, conjugated drugs have been classified as a group of medicines or new generation of medicine candidates from the standpoints of chemistry and the medications.
In the conjugated drugs, the original therapeutic compounds or new discovery leads may be administered by using advanced conjugate formulating techniques. Combining with chemical modification and rational design of the conjugation, the efficacies and safety of new and generic drugs will be potentially enhanced. Particularly small molecular conjugated drugs have certified that their modifications do not affect the core structures in terms of warheads. Because of multi functionality, the advantages of a conjugate drug may not simply only improve chemical, biologic and physic properties, but also create simultaneous interaction mechanisms which may highly benefit the efficacy of the drug from various perspectives.
REFERENCES
- Ritter A. Antibody-drug conjugates: Looking ahead to an emerging class of biotherapeutic: the cell-killing ability of a cytotoxin is joined with the specificity of a monoclonal antibody to produce the next generation of anticancer therapeutics. Creating a successful antibody-drug conjugate requires careful selection of the drug, antibody, and linker. Pharmaceutical Technol 2012;36:42.
- Koniev O, Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc Rev 2015;44:5495-551.
- Yang W, Thordarson P. Carbon nanotubes for biological and biomedical applications. Nanotechnol 2007;18:41.
- Gerber HP, Senter PD, Grewal IS. Antibody drug-conjugates targeting the tumor vasculature: Current and future developments. MAbs 2009;1:247-53.
- Thordarson P, Droumaguet B, Velonia K. Well-defined protein–polymer conjugates-synthesis and potential applications. Applied Microbiol Biotechnol 2006;73:243-54.
- Hernandez FJ, Kalra N, Wengel J, et al. Aptamers as a model for functional evaluation of LNA and 2′-amino LNA. Bioorg Med Chem Lett 2009;19:6585-7.
- Hermanson GT. Bioconjugate techniques Amsterdam; Boston: Elsevier Academic Press, USA. 2008.
- Lin FL, Hoyt HM, Van H, et al. Mechanistic Investigation of the Staudinger ligation. J American Chem Society 2005;127:2686-95.
- Laughlin ST, Baskin JM, Amacher SL, et al. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 2008;320:664-667.
- Adamo R, Hu QY, Torosantucci A, et al. Deciphering the structure-immunogenicity relationship of anti- Candida glycoconjugate vaccines. Chem Sci 2014;5:4302-11.
- Canne LE, Bark SJ, Kent S. Extending the applicability of native chemical ligation. J Am Chem Soc 1996;118:5891-6.
- Offer J, Dawson PE. N-alpha-2-mercaptobenzylamine-assisted chemical ligation. Org Lett 2000;2:23-26.
- Marinzi C, Bark SJ, Offer J, et al. A new scaffold for amide ligation. Bioorg Med Chem 2001;9:2323-2328.
- Botti P, Carrasco MR, Kent SBH. Native chemical ligation using removable N-alpha-(1-phenyl-2-mercaptoethyl) auxiliaries, Tetrahedron Lett, 2001;42: 1831-1833.
- Jain N, Smith SW, Ghone S, et al. Current ADC Linker Chemistry. Pharm Res. 2015;32:3526-40.
- McCombs JS, Owen SC. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015;17(2):339-51.
- Santi DV, Schneider EL, Reid R, et al. Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates. Proc Nat Acad Sci USA 2012;109:6211–16.
- Schneider EL, Robinson L, Reid R, et al. β-Eliminative releasable linkers adapted for bioconjugation of macromolecules to phenols. Bioconjugate Chem 2013;24:1990–1997.
- Wang R, Yan F, Qiu D, et al. Traceless cross-linker for photocleavable bioconjugation. Bioconjugate Chem 2012;23.
- Chu Y, Polson A, Antibody-drug conjugates for the treatment of B-cell non-Hodgkin's lymphoma and leukemia. Future Oncol 2013;9:355.
- https://adcreview.com/news/u-s-fda-approves-inotuzumab-ozogamicin-treatment-patients-rr-b-cell-precursor-acute-lymphoblastic-leukemia/
- Challener AC. Pharmaceutical technology. 2016;40:34.
- Ondrey G. Chemical Engineering. 2017;114: pp12.
- Asher M. Maturing antibody–drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12: 329–32.
- Li H, Song H, Oie S, et al. Synthesis of cholic acid-peptide Conjugates with a negatively charged ester linkage for oral delivery, JOM Med Chem, 2017;7:769-776.
- Alanazi F, Li H, Halpern DS, et al. Synthesis, preformulation and liposomal formulation of cholesteryl carborane esters with various fatty chains, Int J Pharmaceutics 2003;255:189-97.
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003;2:347-60.
- Haag R, Kratz F. Polymer therapeutics: Concepts and applications. Angew Chem Int Ed 2006;45:198-1215.
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 2016;6:688-701.
- Li C, Wallace S. Polymer-drug conjugates: Recent development in clinical oncology. Adv Drug Deliv Rev 2008;60:886-98.
- Vicent MJ, Duncan R. Polymer therapeutics: Clinical applications and challenges for development. Adv Drug Deliv Rev 2009;61:1117-232.
- Shao P, Wang B, Wang Y, et al. The application of thermosensitive nanocarriers in controlled drug delivery. J Nanomaterials 2011.
- http://blogs.cimav.edu.mx/daniel.glossman/data/files/Nanotecnolog%C3%ADa/Nanomaterials%20Handbook.pdf
- Schrand AM, Huang H, Carlson C, et al. Are diamond nanoparticles cytotoxic? J Phys Chem B 2007;111:2-7.
- Chekman ІS. Nanopharmacology Кyiv: Zadru ha. 2011;424.
- Prylutska SV, Grynyuk II, Grebinyk SM, et al. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Mat.-wiss. u. Werkstofftech 2009;40:238-41.
- Prylutska SV, Burlaka AP, Prylutskyy Yu, et al. Comparative study of antitumor effect of pristine C60 fullerenes and doxorubicin. Biotekhnolohiia 2011;4:82-7.
- Prylutska SV, Grynyuk II, Matyshevs-ka OP, et al. Anti-oxidant properties of C60 fullerenes in vitro. Fullerenes, Nanotubes Carbon Nanostruct 2008;5-6:698-705.
- El-Say KM. Nanodiamond as a drug delivery system: Applications and prospective. J Appl Pharmaceut Sci 2011;1:29-39.
- Atkins HS, Morton M, Griffin KF, et al. Recombinant Salmonella vaccines for biodefence. Vaccine 2006;24:2710-7.
- Beverley PCL. Immunology of vaccination. Br Med Bull 2002;62:15-28.
- Moron G, Dadaglio G, Leclerc C. New tools for antigen delivery to the MHC class I pathway. Trends Immunol 2004;25:92-7.
- Jones LH. Recent advances in the molecular design of synthetic vaccines. Nature Chemistry 2015;7:952-60.
- Taki A, Smooker P. Small wonders—the use of nanoparticles for delivering antigen. Vaccines 2015;3:638-61.
- Wang L, Ni J, Singh S, et al. Binding of high-mannose type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12. Implications for HIV-1 vaccine design. Chem Biol 2004;11:127-34.
- Li H, Wang L. Design and synthesis of a template-assembled oligomannose cluster as epitope mimic for human HIV-neutralizing antibody 2G12. Org Biomol Chem 2004;2:483-8.
- Naicker KP, Li H, Heredia A, et al. Design and synthesis of α-Gal-conjugated peptide T20 as novel antiviral agent for HIV immuno-targeting. Org Biomol Chem 2004;2:660-64.
- Kim MA, Yoon HS, Park SH, et al. Engineering of monobody conjugates for human EphA2-specific optical imaging. PLoS One. 2017;12.
- Antaris AL, Chen H, Cheng K, et al. A small-molecule dye for NIR-II imaging. Nature Materials 2016;15:235-42.
- Sousa F, Sanavio B, Saccani A, et al. Correction to superparamagnetic nanoparticles as high efficiency magnetic resonance imaging T2 contrast agent. Bioconjugate Chem 2017;28:161-70.
- Sun X, Sun S. Preparation of magnetic nanoparticles for biomedical applications. Methods Mol Biol 2017;1570:73-89.
- Scott MD, Chen AM. Beyond the red cell: Pegylation of other blood cells and tissues. Transf Clin Biol 2004;1:40-46.
- Mastorakos P, Zhang C, Song E, et al. Biodegradable brain-penetrating DNA nanocomplexes and their use to treat malignant brain tumors. J Control Release 2017;7:2.
- Lee DY, Park SJ, Nam JH. A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: long-functioning PEGylated islets in vivo. Tissue Engg 2006;12:615-23.
- Ke W, Zha Z, Mukerabigwi JF, et al. Matrix metalloproteinase-responsive multifunctional peptide-linked amphiphilic block copolymers for intelligent systemic anticancer drug delivery. Bioconjug Chem 2017;28:2190-8.
- Shi Z, Fengbo W, Li B. Synthesis, characterization, and evaluation of a novel amphiphilic polymer RGD-PEG-chol for target drug delivery system. Scientific World J 2014.
- https://adcreview.com/editorial/next-advancements-cancer-drug-development-antibody-drug-conjugates/
- http://www.pharmtech.com/antibody-drug-conjugates-marriage-biologics-and-small-molecules
- Laurent D, Bernhard S. Antibody-drug conjugates: Linking cytotoxic payloads to monoclonal antibodies. Bioconjugate Chem 2010;21:5-13.
- Kovtun YV, Audette CA, Ye Y. et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 2006;66:3214-21.
- Kovtun YV, Goldmacher VS. Cell killing by antibody–drug conjugates. Cancer Letters. 2007;255:232-40.
- Armellino D, Boghaert ER, Khandke K, et al. Hamann chemotherapy with CMC-544: A CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 2004;103:1807-14.
- Barth RF, Wu G, Meisen WH, et al. Design, synthesis, and evaluation of cisplatin-containing EGFR targeting bioconjugates as potential therapeutic. Onco Targets Therapy 2016:9:2769-81.
- Delaria K, Foletti D, Witt JM, et al. Site-specific conjugation improves therapeutic index of antibody drug conjugates with high drug loading Pavel Strop. Nature Biotechnology. 2015;33:694.
- Sherbenou DW, Aftab B, Su Y, et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J Clinical Investigation 2016;126:4640-53.
- Arnum PV. Antibody drug conjugates: A marriage of biologics and small molecules. Patricia Van Arnum, antibody drug conjugates: A marriage of biologics and small molecules. Antibody drug conjugates offer a niche opportunity in drug development and contract manufacturing. Pharmaceutical Technology. 2008;326.
- Trembley JH, Unger GM, Korman VL, et al. Tenfibgen ligand nanoencapsulation delivers bi-functional anti-CK2 RNAi oligomer to key sites for prostate cancer targeting using human xenograft tumors in mice. 2014;9.
- Kapuvári B, Hegedüs R, Schulcz A, et al. Improved in vivo antitumor effect of a daunorubicin-GnRH-III bioconjugate modified by apoptosis inducing agent butyric acid on colorectal carcinoma bearing mice. Invest New Drugs 2016;34:416-23.
- Stefano ID, Battaglia A, Zannoni GF, et al. Hyaluronic acid–paclitaxel: eVects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts, Cancer Chemother Pharmacol 2011;68:107-16.
- Koganei H, Ueno M, Tachikawa S, et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjugate Chem 2013;24:124-32.
- Goswami AM, Sarkar TS, Ghosh S. An ecofriendly synthesis of silver nano-bioconjugates by Penicillium citrinum (MTCC9999) and its antimicrobial effect. AMB Express 2013;3:16.
- Raj MK, Kanwar, Shamsher S. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res Internat 2015;1.
- Roongsawang N, Washio K, Morikawa M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int J Mol Sci 2011;12:141-172.
- Nakhate PH, Yadav VK, Pathak AN. A review on daptomycin; the first US-FDA approved. Lipopeptide antibiotics. J Scientific Innovative Res 2013;2:970-80.
- Gao Y, Liu XL, Li XR. Research progress on siRNA delivery with nonviral carriers. Int J Nanomed 2011;6:1017–25.
- Karlsen KK, Wengel J. Locked nucleic acid and aptamers. Nucleic Acid Ther 2012;22:366–70.
- Spinelli N, Defrancq E, Morvan F. Glycoclusters on oligonucleotide and PNA scaffolds: Synthesis and applications. Chem Soc Rev 2013;42:4557-73.
- Kumar R, Ries A, Wengel J. Synthesis and excellent duplex stability of oligonucleotides containing 2′-Amino-LNA functionalized with galactose units. Molecules 2017;22:852-865.
- Perepelyuk M, Maher C, Lakshmikuttyamma A, et al. Aptamer-hybrid nanoparticle bioconjugate efficiently delivers miRNA-29b to non-small-cell lung cancer cells. Internat J Nanomed 2016:7.
- Yu C, Hu Y, Duan J, et al. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS ONE 2011;6:9.
- Gupta S, Hirota M, Waugh SM, et al. Chemically modified DNA aptamers bind interleukin-6 with high affinity and inhibit signaling by blocking its interaction with interleukin-6 receptor. J Biol Chem 2014;289:8706-19.
- Vaught JD, Bock C, Carter J, et al. Expanding the chemistry of DNA for in vitro selection. J Am Chem Soc 2010;132:4141-51.
- Gelinas AD, Davies DR, Edwards TE, et al. Crystal structure of interleukin-6 in complex with a modified nucleic acid ligand. J Biol Chem 2014;289:8720-34.
- Davies DR, Gelinas AD, Zhang C, et al. Unique motifs and hydrophobic interactions shape the binding of modified DNA ligands to protein targets. Proc Natl Acad Sci 2012;109:19971-76.
- Gold L, Ayers D, Bertino J, et al. Aptamer-based multiplexed proteomic technology for bio-marker discovery. PLoS One 2010; 5:e15004.
- Hirota M, Murakami I, Ishikawa Y, et al. Chemically Modified Interleukin-6 aptamer inhibits development of collagen-induced arthritis in cynomolgus monkeys. Nucleic Acid Ther 2016;26:10-9.
- Shabanpoor F, McClorey G, Saleh AF, et al. Bi-specific splice-switching PMO oligonucleotides conjugated via a single peptide active in a mouse model of Duchenne muscular dystrophy. Nucleic Acids Res 2015;43:29-39.
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, et al. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004;73:39-85.
- Sessler JL, Lawrence CM, Jayawickramarajah J. Molecular recognition via base-pairing. Chem Soc Rev 2007;36:314-25.
- Boersma AJ, Megens RP, Feringa BL, et al. DNA-based asymmetric catalysis. Chem Soc Rev 2010;39:2083-92.
- Yang D, Huang M, Chang M. Aptamer-conjugated DNA icosahedral nanoparticles as a carrier of doxorubicin for cancer therapy. CSACS Nano 2011;5:6156-63.