Continuous versus intermittent paracentesis in severe ovarian hyperstimulation syndrome: A multicenter randomized clinical trial
Received: 01-Nov-2017 Accepted Date: Nov 29, 2017; Published: 02-Dec-2017
Citation: Dawood AS, Omar MK. Continuous versus intermittent paracentesis in severe ovarian hyperstimulation syndrome: A multicenter randomized clinical trial. J Reprod Biol Endocrinol. 2017;1(2):23-28.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Ovarian a syndrome (OHSS) is the most dangerous complication of assisted reproductive technologies. The syndrome has 3 degrees mild, moderate and severe. The severe form is characterized by marked enlargement of ovaries and ascites. Fluid management in sever OHSS was the main purpose of all relevant studies.
OBJECTIVE: To detect the efficacy, safety, applicability and patient satisfaction for the continuous paracentesis method in relation to intermittent method in management of severe cases of OHSS.
PATIENTS and METHODS: Patients with severe OHSS (n=118) were allocated into 2 groups continuous paracentesis group I, and intermittent paracentesis group II.
RESULTS: The demographic and cycle characteristics were compared. There were significant differences between both groups as regard duration of hospitalization, symptom relief, time to complete drainage of ascites and patient satisfaction, where marvelous improvement of symptoms and clinical signs were noticed within 24 h in group of continuous paracentesis.
CONCLUSION: Continuous paracentesis proved effective and safe for cases with severe OHSS and shortened duration of hospitalizations and drugs used with immediate symptom relief.
Keywords
Ovarian; Hyperstimulation; Paracentesis; Ascites
Abbreviations
OHSS Ovarian hyperstimulation syndrome; IUI Intrauterine insemination; IVF In vitro fertilization; HCG Human chorionic gonadotropin; ICU Intensive care unit; DVT Deep vein thrombosis; RCOG Royal college of obstetrics and gynecology; FFP Fresh frozen plasma; VEGF Vascular endothelial growth factor; rLH recombinant luteinizing hormone
Ovarian hyperstimulation syndrome (OHSS) is caused by over stimulating the ovaries with gonadotropins, whether during ovulation induction or during controlled ovarian hyperstimulation before intra-uterine insemination (IUI), In-vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI). It is a purely iatrogenic condition which could be largely prevented. OHSS may be occur early, due to the human chorionic gonadotropin (HCG) injection or late, due to HCG secreted by the trophoblast of the developing pregnancy [1]. Many proposed preventive measures were evaluated, but no single method proved to be superior to others [2-4]. In severe form of OHSS tense ascites, leads to abdominal discomfort and dyspnea and associated with decreased renal function. Tense ascites is associated with nausea and vomiting leading to more dehydration, hypoproteinimia and thrombosis. If the condition not promptly managed, maternal mortality is high. Management of such situation requires tertiary care hospitals with well-equipped ready intensive care unit (ICU) [5]. One of the proposed solutions for ascites was paracentesis especially if the patient is haemodynamically stable. Paracentesis may be done trans-abdominally or trans- vaginally and, in either case, should be ultrasound guided. Paracentesis should be gradual and slow better through closed system catheter with a locking device as Rapid drainage may induce a rapid deterioration in intravascular volume. Continuous tapping is very useful for avoiding repeated punctures [6,7].
In this study, the effectiveness of paracentesis by intermittent and continuous methods in severe OHSS cases was compared at 2 centers the fertility unit of Tanta University and Qurrat Ain fertility center as multicenter randomized controlled study.
Patients and Methods
Study design and settings
A multicenter randomized controlled clinical trial conducted at fertility unit of Department of Obstetrics and Gynecology, Tanta University, Tanta and Qurrat Ain fertility center, Elmahalla Elkubra, Egypt in the period from July, 1, 2015 to June, 30, 2017.
Recruitment
Hundred and thirty two patients were included in this study from both centers. Patients were enrolled in the study according to inclusion criteria including: Patients diagnosed to have severe form of OHSS by the following criteria according to proposed Royal College of Obstetrics and Gynecology (RCOG classification) of severity of OHSS: (a) Clinical ascites, (b) Ovarian size>12 cm, (c) Hemoconcentration; (hematocrit>45%) (d) Oliguria, and (e) Electrolyte imbalance [8]. The exclusion criteria were: Critical cases requiring ICU admission and mechanical ventilation, patient refusing participation in the study and patients with evident deep vein thrombosis (DVT).
Sample size calculation
The sample was calculated by Epi info 0.7 programs with confidence level 95% at power of 85%. The hypothesis (H0) proposed that continuous paracentesis is better than intermittent type; the calculated sample size was 132 patients.
Randomization and allocation
After stabilization of patients with crystalloid fluids, human albumin, Starch solution, patients were randomly allocated into 2 groups:
Group I
These patients were subjected to continuous controlled paracentesis.
Group II
Patients were subjected to intermittent paracentesis. Randomization was done by computer assisted program with random numbers. Allocation was done by simple alternate type.
Intervention
Group I: Continuous paracentesis
In this group patients were given thiopental sodium 500 mg IV as an anesthetic or analgesic, then by sharp scalpel no. 23 a small incision 0.5 mm was done at the right or left lumbar regions in the mid-clavicular line. The blunt 5 mm trocar and cannula of laparoscope was introduced into the peritoneal cavity and the trocar is removed with insertion of Nelaton’s catheter drain no.16 inside the cannula into peritoneal cavity then the cannula of laparoscope was removed leaving the Nelaton’s catheter drain inside the abdomen. The drain was immediately connected to a collection bag with a control clips applied to control the fluid drainage outflow as shown in Figure 1. The catheter was then fixed to the skin by proline 2/0 suture.
Group II: Intermittent paracentesis
In this group local anesthetic xylocaine was injected superficially at the site of paracentesis then sedative was given as diazepam 5 mg then a tapping needle was inserted either abdominally or vaginally under ultrasound guidance. The process was repeated every 3 days or earlier according to recollection of ascites. The amount aspirated was 1000-1500 ml every setting.
Parameters of assessment
All demographic data of enrolled patients were collected. The cycle characteristics as the stimulation protocol, dose of gonadotropins, type of triggering factor, E2 level and size of ovary. The patient condition at presentation including: main presentation, electrolytes, serum albumin, and hematocrit value. All patients were followed either inpatient or outpatient till complete recovery, duration of treatment till complete resolution and patient satisfaction were recorded for all patients plus requesting follow up investigations including electrolytes, serum albumin, hematocrit value after 5 days of treatment. The study primary outcomes were resolution of OHSS and time to complete resolution of ascites while the secondary outcomes were the need for ICU, occurrence of complications and patient satisfaction.
Ethical approval
This study was approved by the ethical committee of Tanta University and was given the code number 31532. All patients were thoroughly informed about the procedures and a written consent was obtained.
Statistical methods
The data were analyzed using SPSS version 18, USA. The tests used were mean, standard deviation, Chi X2 and P value. P value less than 0.05 was considered significant.
Results
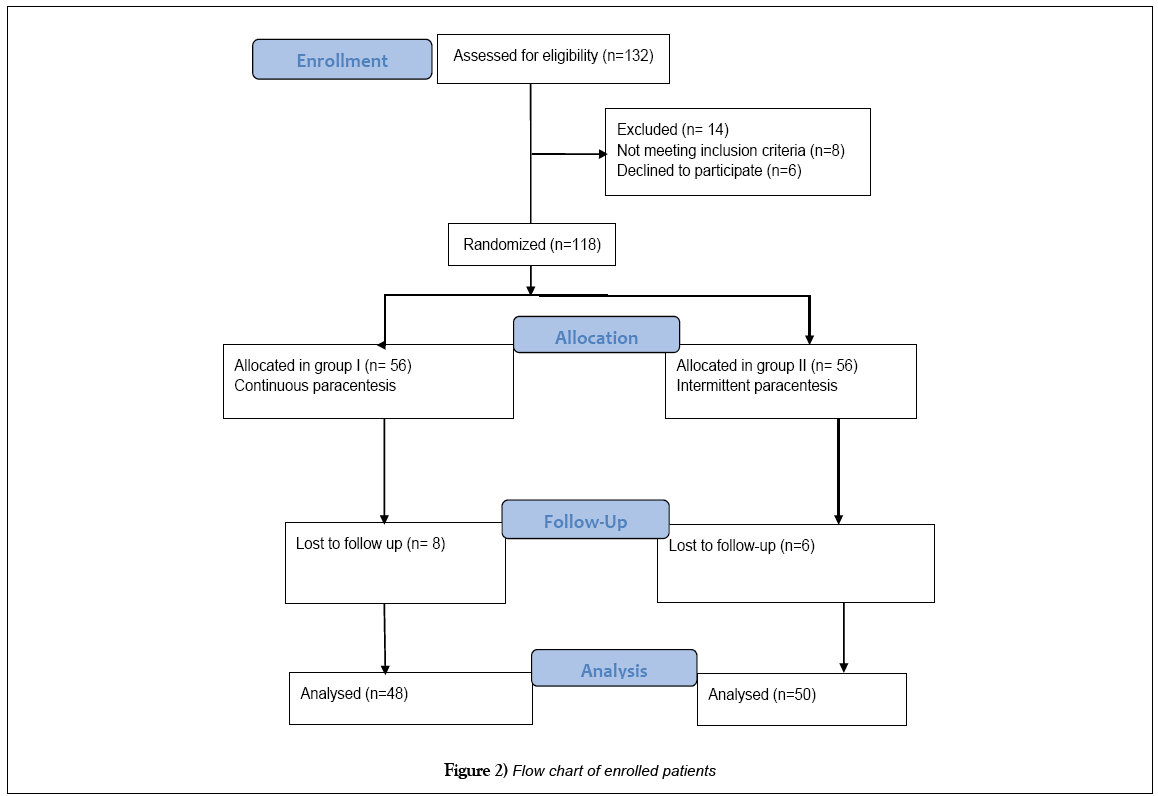
The recruited patients (n=132) were assessed for eligibility and 14 cases were excluded either not meeting inclusion criteria (n=8) or declined to participate (n=6). The eligible patients were randomly allocated into 2 groups with equal allocation of 56 cases in each group. The flow chart of patients is shown in Figure 2.
The demographic characteristics of enrolled patients with severe OHSS were as follow: mean age was 29.78 ± 4.23 years. The mean gravidity and parity were 2 ± 1.70 and 1.4 ± 0.55, respectively. The mean duration of infertility was 5.04 ± 1.20 years and most cases had primary infertility 77/118 (77.12%) while 27/118 (22.88%) were with secondary infertility. The mean BMI was 21.2 ± 1.88 as shown in Table 1.
| Range | Mean ± SD | |
|---|---|---|
| Age (years) | 23-35 | 29.78 ± 4.23 |
| Gravidity | 0-5 | 2 ± 1.70 |
| Parity | 0-2 | 1.4 ± 0.55 |
| Type of infertility •Primary | 91 | 77.12% |
| •Secondary | 27 | 22.88% |
| Duration of infertility | 1-7.3 | 5.04 ± 1.20 |
| BMI | 20.4-34.7 | 21.2 ± 1.88 |
| Duration of stimulation (days) | 8-11 | 9.5 ± 1.10 |
| Stimulation protocol (%) •Agonist | 97 | (82.20%) |
| •Antagonist | 21 | (17.80%) |
| Gonadotropins dose (IU/mL) | 1875-3750 | 2762.5 ± 125.35 |
| Triggering factor •Urinary HCG | 89 | 75.42% |
| •Recombinant HCG | 17 | 14.41% |
| •Agonist in antagonist cycles | 12 | 10.17% |
| Size of ovaries (cm3) | 12.4-17.9 | 14.7 ± 1.40 |
| Amount of ascites by Ultrasound (Liters) | 18.7-23.6 | 19.06 ± 1.47 |
| Main presentation (%) •Respiratory symptoms | 51 | 43.22% |
| •Abdominal pains | 27 | 22.88% |
| •Abdominal enlargement | 18 | 15.26% |
| •Nausea/Vomiting | 16 | 13.56% |
| •Oliguria | 6 | 5.08% |
Table 1: Demographic and clinical data of enrolled patients (n=118)
The cycle characteristics for enrolled patients were as follow: Eighty seven cases (82.20%) were down-regulated by agonist long protocol while 21/118 (17.80%) were down-regulated by antagonist protocol, the mean duration of stimulation was 9.5 ± 1.10 days, the total dose of gonadotropin used ranged from 1875 to 3750 IU with mean of 2762.5 ± 125.35 IU. The used triggering agents were urinary HCG (89/118, 75.42%), recombinant HCG (17, 14.41%) and agonist in antagonist cycles (12, 10.17%). The size of ovaries ranged from 12.4 to 17.9 cm3 with mean of (14.7 ± 1.40 cm3). The amount of ascites present was ranged from 18.7 to 23.6 liters with mean of 19.06 ± 1.47 liters as shown in Table 1.
The patients were presenting with multi-symptoms but the main complaint was taken for each patient as follow: respiratory symptoms reported by 51/118 (43.22%), abdominal pains 27/118 (22.88%), abdominal enlargement 18/118 (15.26%), nausea/vomiting 16/118 (13.56%) and oliguria 6/118 (5.08%) as shown in Table 1. The investigations of patients at admission were as follow: the mean serum albumin was 2.00 ± 0.21g/dL, the mean hematocrit was 47.03 ± 1.35%, the mean estrogen level was 3471 ± 312 pg/ ml. Serum electrolytes were as follow: reduced with mean Na+ level of 131.20 ± 0.88 mEq/L and increased mean K+ level of 5.17 ± 0.77 mEq/L as shown in Table 2.
| Range | Mean±SD | |
|---|---|---|
| Serum Albumin (g/dL) | 2.2-2.3 | 2.00 ± 0.21 |
| Serum Na+ (mEq/L) | 129.8-133.4 | 131.20 ± 0.88 |
| Serum K+ (mEq/L) | 5.11-5.25 | 5.17 ± 0.77 |
| Hematocrite (%) | 46.21-48.02 | 47.03 ± 1.35 |
| Estrogen level (pg/ml) | 2945-4239 | 3471 ± 312 |
Table 2: Laboratory investigation prior to treatment
Assessment of both treatment modalities was done 5 days after treatment by clinical and laboratory investigations. As regard symptoms improvement, all patients in group I (continuous paracentesis) showed complete improvement of symptoms except one case (2.08%) of nausea and vomiting persist for 3 days later. In group II (intermittent paracentesis) there were 15 (30.00%) suffering respiratory symptoms, 7 (14%) with nausea/vomiting, 8 (16.00%) with abdominal pains and 5 (10%) with abdominal enlargement. The cure rate was 97.92% in group I compared to 30.00% in group II. There was significant difference between both groups (P-value<0.0001 as shown in Table 3. One case (2.08%) in group I required ICU admission while in group II, 28 cases (56%) required ICU admission.
| Group I Continuous paracentesis (n=48) |
Group II Intermittent paracentesis (n=50) |
Chi-square | P value | |
|---|---|---|---|---|
| Respiratory symptoms (n, %) | 0 (0.00%) | 15 (30.00%) | 16.829 | <0.0001* |
| Nausea/Vomiting (n, %) | 1 (2.08 %) | 7 (14.00%) | 5.811 | 0.0159* |
| Abdominal enlargement (n, %) | 0 (0.00%) | 5 (10.00%) | 5.006 | 0.0253* |
| Abdominal pains (n, %) | 0 (0.00%) | 8 (16.00%) | 8.277 | <0.0040* |
| Total no. still symptomatic | 1 (2.08 %) | 35 (70.00%) | 48.116 | <0.0001* |
| Cure rate | 47 (97.92%) | 15 (30.00%) | 48.116 | <0.0001* |
| Need for ICU admission (n, %) | 1 (2.08 %) | 28 (56.00%) | 33.826 | <0.0001* |
| Units of FFP Range Mean±SD |
1-3 1.3 ± 1.414 |
6-10 8.1 ± 2.828 |
14.958 | <0.0001* |
| Units of Human Albumin Range Mean ± SD |
2-4 1.7 ± 1.414 |
8-10 9.04 ± 1.541 |
25.689 | <0.0001* |
| Duration of hospitalization (Days)Range Mean ± SD |
1-4 3.1 ± 2.121 |
8-12 7.34 ± 1.123 |
11.264 | <0.0001* |
| Time to complete clearance of ascites (Days) Range Mean ± SD |
3-5 3.789 ± 0.854 |
7-14 9.475 ± 3.154 |
12.070 | <0.0001* |
| Complications | 0 (0.00%) | 7/50 (14%) (3 cases of cystitis, 4 cases of lower limb cellulitis) |
7.163 | 0.0074* |
| Patient satisfaction | 90.91% | 45.45% | 22.915 | <0.0001* |
Table 3: Efficacy of both procedures after 5 days of treatment
There was a significant difference between both groups as regard duration of hospitalization (3.1 ± 2.121 days in group I versus 7.34 ± 1.123 days in group II), total amount of albumin infused (1.7 ± 1.414 units versus 9.04 ± 1.541 units), and total amounts of fresh frozen plasma (FFP) (1.3 ± 1.414 units versus 8.1 ± 2.828 units). The time required for complete clearance of ascites was significantly lower in group I compared to group II (3.789 ± 0.854 versus 9.475 ± 3.154 days). No complications were recorded in group I versus 7/50 (14%) of complications in group II. The reported complications were cystitis (3 cases of cystitis, 4 cases of lower limb cellulitis). Patients were more satisfied by the continuous method rather than the intermittent method (90.91% versus 45.45%) as shown in Table 3. The investigations also were rapidly changed after treatment, serum albumin was increased in group I more than group II (3.65 ± 0.636 versus 2.8 ± 0.141g/dL, respectively), serum Na+ level increased (140.305 ± 6.512 versus 136.495 ± 4.772 mEq/L, respectively), serum K+ level decreased (4.205 ± 0.926 versus 4.465 ± 0.049 mEq/L respectively), hematocrit value was decreased in both groups (42.335 ± 4.532 versus 45.615 ± 1.576%, respectively) and estrogen was decreased (2242.5 ± 306.177 versus 2894 ± 291.327 IU, respectively) as shown in Table 4.
| Group I Continuous paracentesis (n=48) |
Group II Intermittent paracentesis (n=50) |
t-test | P value | |
|---|---|---|---|---|
| Serum Albumin (g/dL) Range Mean ± SD |
3.2-4.1 3.65 ± 0.636 |
2.7-2.9 2.8 ± 0.141 |
9.219 | <0.0001* |
| Serum Na+ (mEq/L) Range Mean ± SD |
135.7-144.91 140.305 ± 6.512 |
133.12-139.87 136.495 ± 4.772 |
3.313 | 0.0013* |
| Serum K+ (mEq/L) Range Mean ± SD |
3.55-4.86 4.205±0.926 |
4.73-4.88 4.465 ± 0.049 |
1.983 | 0.0502* |
| Hematocrite (%) Range Mean ± SD |
39.13-45.54 42.335 ± 4.532 |
45.5-47.73 45.615 ± 1.576 |
4.824 | <0.0001* |
| Estrogen level (pg/ml) Range Mean ± SD |
2026-2459 2242.5 ± 306.177 |
2688-3100 2894 ± 291.327 |
10.794 | <0.0001* |
*= significant P value
Table 4: Laboratory investigation after 5 days treatment
Discussion
Ovarian hyperstimulation syndrome (OHSS) is one of common iatrogenic complications during controlled ovarian hyperstimulation in IVF/ICSI protocols. Although occurring in a minority of patients, the results and sequalae of the syndrome are deleterious and may cause significant morbidity and even mortality [9].
Most conditions are mild to moderate and may be treated in outpatient clinics, but women with severe forms of OHSS may require inpatient treatment or sometimes ICU admission to decrease the risk of further complications. The corner stone of OHSS management is directed towards prevention, early diagnosis and treatment of mild and moderate cases to avoid progression to severe OHSS [10]. The severe forms of OHSS are usually complicated by tense ascites and pleural effusion causing nausea, vomiting, abdominal, discomfort, abdominal pains, respiratory distress, renal compression and oliguria. In addition, the marked ovarian enlargement stretch peritoneum and stimulate the vagus nerve leading to palpitation (bradycardia), sweating, diarrhea, and vomiting [11].
Drainage of excessive ascites (paracentesis) was employed in these cases either by transvaginal or transabdominal routes and was associated with immediate symptomatic relief due to alleviation of intra-abdominal pressure which decompresses major blood vessels allowing for better circulation and function of major organs as liver, kidney and intestine. It could be repeated as needed until the fluid ceases and condition improves [12,13].
In this study, the continuous versus intermittent paracentesis methods were assessed by clinical and laboratory parameters of the designed outcomes. The continuous drainage via abdominal route was found to be superior to the intermittent method in both clinical symptoms relief and laboratory adjustment of hematocrit, electrolytes and serum albumin. The intermittent route was advised by many researchers as they reported no complications and succeeded to withdraw large amounts of ascetic fluid [14-16].
The drained fluid was ultra-filtered and re-infused by Koike et al. [17] and Zhang et al. [18] who described auto transfusion of concentrated ultrafiltered ascetic fluid protein, aiming to replenish the woman’s albumin levels using her own protein, reducing the risk of infection and allergic reaction to exogenous albumin.
There was a marvelous improvement in the continuous paracentesis group earlier than intermittent paracentesis group as evidenced by duration of hospitalization (1-4 days versus 8-12 days in continuous versus intermittent group respectively) and time to complete recovery (3-5 days versus 7-14 days, respectively), total amount of infused albumin (2-4 units versus 8-10 units, respectively) and fresh frozen plasma(FFP) (1-3 units versus 6-10 units, respectively) as shown in table 3.
Abuzeid et al. [19] conducted a similar study using Pigtail catheter in 26 patients with severe OHSS. They divided patients into inpatient and outpatient groups. They found that improvement of symptoms and signs were noted 24–48 h after catheter placement in all patients and complete clearance of ascites required a relatively long period (Range 7–24 with mean of 12.9 ± 4.3 days) compared to the current study results [19]. The difference arises from pregnancy which worsens OHSS with more production of ascetic fluid. The results of Abuzeid study advocates outpatient management of severe OHSS cases [19].
Eleven years later, Abuzeid et al. [20] reviewed all cases of OHSS in whom Pigtail was used in the period between 2004 and 2009. They reached to 2 important conclusions; the first conclusion was that OHSS remains a serious disorder with the potential for rapid deterioration, requiring hospitalization and intensive treatment of a critically ill patient. The second conclusion was that their technique was not suitable for obese patients as the metal introducer provided in the pigtail catheter kit was difficult to use and they instead used the non-disposable gamete intra-fallopian treatment trocar and cannula to facilitate the introduction of the catheter in all cases [20].
Raziel et al. [21] described an alternative technique of transvaginal drainage of ascites in a case of severe ovarian hyperstimulation syndrome (OHSS), generalized edema, and obesity. The indwelling catheter was fixed to the woman’s thigh. They reported that the ascetic fluid was drained efficiently, leading to improvement of the patient’s condition. Unfortunately, that technique was applied in only one case [21].
Similarly, Chan et al. [22] described a case report of severe OHSS treated by continuous abdominal paracentesis. They concluded that earlier continuous aspiration of the ascetic fluid improved the patients’ condition as soon as euvolemia was reached. They advocated abdominal paracentesis with continuous drainage to be performed earlier in such patients [22].
Form the results of Abuzeid [19,20] studies we used trocar and cannula of laparoscope to insert a cheaper Nelaton’s catheter instead of the expensive Pigtail catheter. The advantages of continuous drainage in this study were the following items: (a) Rapid improvement of symptoms and signs of the condition, (b) Rapid return of oral feeding with rapid correction of electrolytes and albumin, (c) Rapid return to oral fluids alleviating hemoconcentration (d) Immediate mobilization of patients avoiding DVT (e) Improvement of renal functions after relief of compression (f) Easy drainage as Nelaton’s catheter is of wide pore than the pigtail catheter (g) Avoiding repeated aspirations which are annoying to the patients and physician as well and (h) Greater safety of the technique (no complication reported).
Currently, the use of this technique is still limited being new and not popular from one side, on the other side the incidence of severe OHSS was decreased owing to the effective methods for prevention, such as the use of lower doses of gonadotropin, frequent monitoring, coasting and avoidance of luteal phase supplementation with HCG, the use of vascular endothelial growth factor (VEGF) inhibitors such as Cabergoline and the use of the more safe triggering recombinant luteinizing hormone (rLH) [23].
Conclusion
Percutaneous placement of a Nelaton’s catheter is a safe and effective treatment modality for the management of ascites in severe OHSS. It may represent an attractive alternative to multiple paracentesis. Application of this technique in the current study reduced ICU admissions. We recommend its popular use as it is simple, cheap, safe and effective procedure in management of severe OHSS.
REFERENCES
- Homburg R. Ovulation induction and controlled ovarian stimulation. A practical guide. 2014;185-97.
- Humaidan P, Bredkjaer HE, Westergaard LG, et al. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: A prospective, randomized, controlled study. Fertil Steril. 2010;93:847-54.
- Griesinger G, Schultz L, Bauer T, et al. Ovarian hyperstimulation syndrome prevention by gonadotropin- releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a ‘freeze-all’ strategy: A prospective multicentric study. Fertil Steril. 2011;95:2029-33.
- Leitao VM, Moroni RM, Seko LM, et al. Carbergoline for the prevention of ovarian hyperstimulation syndrome: systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2014;101:664-75.
- Davenport MJ, Vollenhoven B, Talmor AJ. Gonadotropin-releasing hormone–agonist triggering and a freeze-all approach: The final step in eliminating ovarian hyperstimulation syndrome? Obstetrical & Gynecological Survey. 2017;72(5):296-308.
- Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634-47.
- Joint Society of Obstetricians and Gynaecologists (SOGC) and Canadian Fertility and Andrology Society (CFAS). The diagnosis and management of ovarian hyperstimulation syndrome. 2011.
- Royal College of Obstetrician and Gynecologists. Green-top guidelines, No.5. 2016.
- Toftager M, Bogstad J, Bryndorf T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31(6):1253-64.
- Grossman LC, Michalakis KG, Browne H, et al. The pathophysiology of ovarian hyperstimulation syndrome: An unrecognized compartment syndrome. Fertil Steril. 2010;94(4):1392-8.
- Kwik M, Maxwell E. Pathophysiology, treatment and prevention of ovarian hyperstimulation syndrome. Curr Opin Obstetr Gynecol. 2016;28(4):236-41.
- Kumar P, Sait SF, Sharma A, et al. Ovarian hyperstimulation syndrome. J Hum Reprod Sci 2011;4:70-5.
- Maslovitz S, Jaffa A, Eytan O, et al. Renal blood flow alteration after paracentesis in women with ovarian hyperstimulation. Obstetr Gynecol. 2004; 104(2):321-6.
- Smith LP, Hacker MR, Alper MM. Patients with severe ovarian hyperstimulation syndrome can be managed safely with aggressive outpatient transvaginal paracentesis. Fertil Steril. 2009;92(6):1953-9.
- Smith LP. Ultrasound and ovarian hyperstimulation syndrome. In Ultrasound Imaging in Reproductive Medicine. Springer New York. 2014;303-13.
- Ozgun MT, Batukan C, Oner G, et al. Removal of ascites up to 7.5 l on one occasion and 45 l in total may be safe in patients with severe ovarian hyperstimulation syndrome. Gynecol Endocrinol. 2008;24(11):656-8.
- Koike T, Araki S, Minakami H, et al. Clinical efficacy of peritoneovenous shunting for the treatment of severe ovarian hyperstimulation syndrome. Hum Reprod. 2000;15(1):113-7.
- Zhang Q, Xia L, Gao G. A new effective method in the treatment of severe ovarian hyperstimulation syndrome. Iran J Reprod Med. 2012;10(6):589.
- Abuzeid MI, Nassar Z, Massaad Z, et al. Pigtail catheter for the treatment of ascites associated with ovarian hyperstimulation syndrome. Hum Reprod. 2003;18(2):370-3.
- Abuzeid M, Warda H, Joseph S, et al. Outpatient management of severe ovarian hyperstimulation syndrome (OHSS) with placement of pigtail catheter. Facts, views & vision in ObGyn. 2014;6(1):31-7.
- Raziel A, Friedler S, Schachter M, et al. Transvaginal drainage of ascites as an alternative to abdominal paracentesis in patients with severe ovarian hyperstimulation syndrome, obesity and generalized edema. Fertil Steril. 1998;69(4):780-3.
- Chan CC, Yin CS, Lan SC, et al. Continuous abdominal paracentesis for management of late type severe ovarian hyperstimulation syndrome. Chin Med Assoc. 2004;67(4):197-9.
- Nastri CO, Teixeira DM, Moroni RM, et al. Ovarian hyperstimulation syndrome: Pathophysiology, staging, prediction and prevention. Ultrasound Obstetr Gynecol. 2015;45(4):377-93.