Denosumab recovers aortic arch calcification in hemodialysis
2 Department of Nephrology, Hemodialysis Center, Edogawabashi Suzuki Clinic, Tokyo, Japan
Received: 19-Apr-2021 Accepted Date: May 03, 2021; Published: 10-May-2021
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Vascular calcification, which is highly prevalent in patients with chronic kidney disease, is relevant to mortality in hemodialysis patients. For more than three decades, loss of renal mass has been regarded as inducing a decrease in active vitamin D3, which leads to hyperparathyroidism with impaired calcium (Ca) and phosphate (P) homeostasis. Hyperparathyroidism causes osteolysis, which releases excess Ca and P and promotes their precipitation.
Keywords
Hemodialysis; Parathyroid hormone; Chondrocytic; Carboxyglutamic
Abbrevations
GLA: Gamma-Carboxyglutamic acid; PTH: Parathyroid Hormone; OPG: Osteoprotegeri; ALP: Aortic alkaline phosphatase
Introduction
CaxP precipitation occurs in an alkali milieu such as the eyeball surface or pancreatic duct. However, vascular calcification in a non-alkali milieu must be explained by other mechanisms. In 1863, Virchow described that the mineral component in calcified arteries “is an ossification, and not a mere calcification” [1]. Molecular biological investigations in 1993 revealed expression of bone morphogenic protein in atherosclerotic lesions [2]. Many researchers have since examined the molecular mechanism of vascular calcification, revealing more complex biological processes. The most important key player in hemodialysis is inorganic P, where hyperphosphatemia is an independent factor in the risk of coronary calcification by careful examination with CT scanning in young patients [3]. Based on these findings, P induced vascular medial layer calcification through ossification of vascular smooth muscle cells. Paradoxically, vascular calcification is associated closely with bone decalcification: osteoporosis. Consequently, the mechanisms of osteoporosis and vascular calcification might share molecular mechanisms. We summarize herein the molecular basis of vascular calcification in hemodialysis and extend the possible but potential candidate of the treatment: denosumab.
High cardiovascular mortality observed in patients with end-stage renal disease is partly attributable to deleterious effects of vascular calcification that develops from dialysis over time. One treatment option was clarified recently [4]. It is reviewed here.
Literature Review
Molecular mechanism of proposed vascular calcification
Vascular calcification is generally found in intimal and medial layers of arterial vessels. The vascular smooth muscle cells on the medial layer are characteristically calcified in hemodialysis patients. Various mechanisms underlying Ca and phosphate deposition in the medial layer of vasculature have been investigated [5]. Several reviews have summarized experimentally obtained results including phosphate transport [6], parathyroid hormone (PTH) [7], matrix Gammacarboxyglutamic Acid (GLA) protein) [8], FGF 23, fetuin [9], soluble klotho [10], and vitamin D3 as a cause. The mechanism includes changes of vascular smooth muscle cells into osteo/chondrocyticlike cells that have capacity to modulate the mineralization process and which begin to express genes specific to osteoblasts by several factors, as described above [11].
This osteoblast-like cell transformation is found in various vessels such as coronary arteries, carotid arteries and aorta, and cardiac valves. It is noteworthy that osteoporosis has been demonstrated consistently with vascular calcification. It is most prevalent in hemodialysis patients [12]. This coexistence is also found as an atherosclerotic feature in postmenopausal women and elderly people. These findings suggest that the existence of common pathways negatively affects bone metabolism and the vasculature. Consequently, a player enhancing calcification of osteoblast-like cells (in vasculature) as well as induction of osteoclasts (in bone) is a key to solving the pathological vascular calcification.
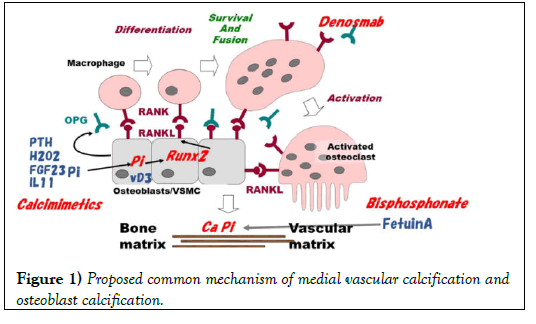
Several experiments conducted with animals or cultured cells suggest a mechanism of osteoblast-like cell activation. The proposed mechanisms of osteoblast activation and smooth muscle cell transformation are presented in (Figure 1). The first key molecule is Runt-related transcription factor 2 (Runx2) isolated by homology cloning of leukemia related molecule, Runx1. This molecule is the key in osteoblast activation. Stein, et al. [13] reviewed the function of mammalian Runx proteins in osteogenesis. They stated that Runx2 is the principal osteogenic master switch, which was upregulated by artificially applied weak stretch on cultured osteoblastic cells, whereas Runx1 and Runx3 are expressed in bone cells and appear to support bone cell development and differentiation. Parathyroid hormone upregulates Runx2 gene through protein kinase A in cultured osteoblast-like cells [14]. A signaling partner of Runx2 is the nuclear Vitamin D Receptor (VDR) that becomes active when bound by its ligand 1,25-dihydroxyvitamin D3. Interleukins also activates Runx2 in osteoblast-like cells.
By contrast, in smooth muscle cells, increased expression of Runx2 was associated with phosphates-induced calcification of bovine calcifying vascular cells [15]. High P might induce the upregulation of Runx2 directly via activation of type III sodium-dependent P co-transporter [16]. Hydrogen peroxide or xanthine/xanthine oxidase-induced oxidative stress associated vascular smooth muscle cell calcification by upregulation of Runx2, via inhibiting Runx2 ubiquitination. Also, PTH and vitamin D3 play independent roles in smooth muscle cell transformation and Runx2 activation. When a nuclear switch is on by Runx2, the next key role is transferred to RANK/RANKL/OPG (Receptor Activation of NF-Kappa B, its ligand, osteoprotegerin) system. Runx2 begins to transcript RANKL protein in these cells. As described below, this system linked osteoblast-like cell activation and macrophage activation. Nevertheless, little is known about how these cells cooperate to induce local mineralization in vascular matrix. In bone tissue, activated macrophage develops osteoclasts that provide a local low pH milieu (ruffled border) by activation of H-ATPase to melt CaxP precipitate in the matrix. Furthermore, osteoblasts develop osteocytes resulting in construction of a new matrix. Whether such cooperation of transformed, vascular cells and probable macrophage in blood actually occurs remains unclarified in the vascular matrix.
Discovery of RANK/RANKL/OPG system and the contribution of calcification
Histological evidence from earlier results of studies suggests that osteoclasts are macrophage-derived and that they are transformed by a factor released from osteoblasts in the bone matrix. The soluble “communicating factor” was presumed as autocrine produced by osteoblasts to introduce osteoclasts. Use of expression cloning with coculture of osteoblasts and macrophage/osteoclasts [17,18] identified the ligand for osteoprotegerin (OPG). The OPG ligand is RANKL that replaces the requirement for stromal cells, vitamin D3, and glucocorticoids in the coculture model of in vitro osteoclastogenesis. Parathyroid hormone stimulates the PTH/PTHrP receptor in osteoblasts, Runx2. Then it transforms the attached stromal cells into osteoclasts via the RANK/RANKL pathway. Runx2 directly increases the expression of RANKL in the osteoblast-like cells. The OPG released from osteoblasts inhibits this pathway by binding to RANKL receptor as a decoy. The local RANK/RANKL and OPG balance is important for osteoclast activation and bone remodeling.
In a mouse model, deficiency of OPG (OPG −/−) caused severe osteoporosis but also caused an unexpected phenotype of vascular calcification [19]. Furthermore, RANK receptor mutation that is unresponsive to OPG is found in juvenile Paget disease, which includes hearing loss, retinopathy, vascular calcification, and internal carotid artery aneurysm formation in addition to skeletal deformity [20]. Because this combination of osteoporotic bone loss and arterial mineral accumulation mirrors similar associations found in patients, OPG/RANK/RANKL pathway has been inferred as a key link between bone and vascular disease.
Although results of most animal studies support a protective role for OPG in the vasculature [21, 22], results of observational studies of patients have paradoxically shown a positive association between serum OPG levels and clinical cardiovascular disease. Serum higher OPG levels in crosssectional studies of CAD patients undergoing coronary angiography have been associated with the presence and the severity of coronary atherosclerosis [23,24]. More over polymorphisms in the OPG gene have been linked to CAD [25]. Although the local level of OPG was correlated with serum OPG, local balance between RANK/RANKL and OPG might be more important in this mechanism.
In animal models, vascular calcification has been recovered by exogenous administration of OPG. Aortic Alkaline Phosphatase (ALP) activity, a crucially important initiator of mineralization, and the aortic calcification area in OPG−/− mice suggest an anti-calcification role of OPG with decreased ALP activity. Recovery of calcification requires a longer treatment period, suggesting that cell-mediated biological procedures are involved rather than simple deposition of Ca, and suggesting that phosphate is resolved. A rat model demonstrated the ability of OPG administration to prevent vascular calcification induced by warfarin or high vitamin D doses [26]. These factors also stimulated RANK/RANKL pathway to vascular calcification. RANKL promotes smooth muscle cell calcification indirectly by enhancing macrophage paracrine pro-calcific activity through the release of IL-6 and TNFα [27]. Inflammation induces IL6, which suppresses OPG expression, thereby enhancing RANKL pathway.
Treatment options and use of denosumab
Figure 1 shows possible treatment options aimed at the target molecules. P binders and inhibition of P transporter are crucial treatments targeted at P itself. Calcimimetics inhibit stimulation by PTH in this pathway. Chelation of Ca by pyrophosphate analogue, bisphosphonate might prevent the precipitation of CaxP. Intrinsic chelators such as fetuin A, GLA and analogues vitamin K might also protect vascular calcification in metastatic locus. Free radical scavengers might ameliorate oxidative stress produced by activated leukocytes when pass through dialyzer during the hemodialysis treatment. A possible but promised target is RANK/RANKL/ OPG pathway as discussed below.
Denosumab, a fully human monoclonal RANKL directed antibody, prevents RANK receptor binding, thereby decreasing osteoclast-induced bone resorption. Denosumab is used to treat osteoporosis and bone metastasis of tumor cells. Denosumab reduced 50% of aortic calcification as well as ameliorated osteoporosis in RAKL knock out mice [28]. The findings explained above prompted us to treat vascular calcification in hemodialysis patients with denosumab [4]. Earlier treatments of vascular calcification included phosphate restriction, bisphosphonate, and calcimimetics, which led to prevention of the progress of vascular calcification. Surprisingly, aortic calcification was recovered after two years of treatment with denosumab. Denosumab is expected to be involved as an option to treat vascular calcification in hemodialysis patients.
Early case reports describe a study conducted using denosumab [29]; hypocalcemia was prominent. Calcium and phosphate deposition on bone was presumably enhanced using denosumab so that serum Ca and phosphate were decreased. Similarly to hypocalcemia immediately after parathyroidectomy in secondary hyperparathyroidism with patients of hemodialysis, bone tissues were hungry for minerals. However, it remains unknown whether more severe “hungry bone” by denosumab was induced as osteoporosis advanced. Discontinuation of denosumab can be considered when no hungry bone is observed. This not-hungry, lowturnover bone should be prevented when induced by denosumab because low turnover bone is one cause of vascular calcification [30].
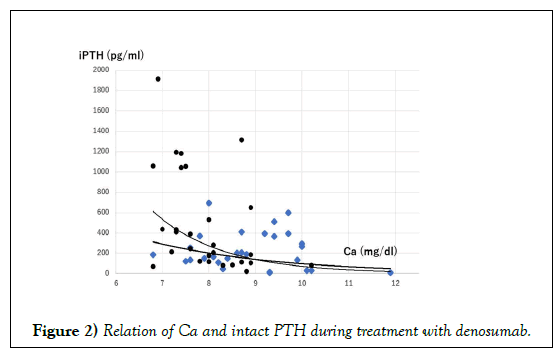
Another of our unpublished observations is that denosumab might regulate PTH secretion. No direct effect of OPG on PTH has been considered because human tissues lacking significant immunoreactivity for RANK include the parathyroid, heart, and esophagus [31]. Actually, serum Ca concentration is a determinant of PTH secretion via Ca sensing receptor where the sigmoidal curve was fitted in Ca vs. PTH relation. Before administration of denosumab, sigmoidal relation of serum Ca and PTH was observed, although no relation was found, and although PTH was unexpectedly high at 2 weeks after administration of denosumab (Figure 2). A similar observation was reported as a case study [32,33]. This hyperparathyroidism might not be treated by calcimimetics because denosumab blocked PTH signal transduction before osteoclast activation. This blockade might induce feedback to PTH secretion by an unknown mechanism, resulting in increased PTH.
PTH stimulates the expression of Runx2 in osteoblast. PTH, oxidative stress such as H2O2, inorganic P (Pi), interleukin 11, and FGF23 stimulates Runx2 in nucleus with vitamin D3 (D3) receptor in Vascular Smooth Muscle Cells (VSMC). RANKL is then upregulated by Runx2. Macrophage expressing RANK is fused with RANKL, surviving and developing osteoclast-like cell. The resulting VSMC/osteoblast/ osteoclast (-like) cells enhance CaxP mineralization in bone or in the vascular matrix. OPG produced by osteoblast-like cells inhibits the binding of RANK and RANKL. Treatment options involve calcimimetics to suppress PTH, and denosumab, which is a mimic of OPG, and bisphosphonate, which advances Ca chelation similarly to fetuin A.
Ca and intact PTH were sampled in our study before administration (square) and 2–4 weeks after administration (filled circle) of denosumab [4]. The relation of Ca vs. PTH was sigmoidal fitting. Denosumab shifts the relation of PTH and Ca toward higher and steeper suggesting the possibility that not only Ca but also an unknown mechanism stimulates PTH secretion by denosumab.
Conclusion
In summary, recent experimentally obtained evidence suggests that the contribution of OPG/RANK/RANKL signal to vascular calcification and osteoporosis is accumulated in animal models. Denosumab is used widely to treat osteoporosis in postmenopausal women and in elderly patients. Over half the population undergoing hemodialysis shows osteoporosis histologically, even though densitometrical analysis of bone does not indicate osteoporosis. In fact, vascular calcification in the aorta, carotid artery, coronary artery or in cardiac valve is observed frequently, even without apparent osteoporosis. Clinical use of denosumab should be regarded as one option to treat or to mitigate the progress of vascular calcification, irrespective of osteoporosis. It might be beneficial for the prognosis of hemodialysis patients.
REFERENCES
- Virchow R. Cellular pathology: As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989; 47 (1): 23-25.
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000; 342 (20): 1478-1483.
- Bostrom K, Watson KE, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993; 91 (4): 1800-1809.
- Suzuki S, Suzuki M, Hanafusa N, et al. Denosumab recovers aortic arch calcification during long-term hemodialysis. Kidney Int Rep. 2021; 6: 605-612.
- Eddington H, Sinha S, Kalra PA, et al. Vascular calcification in chronic kidney disease: A clinical review. J Ren Care. 2009; 35 Suppl 1: 45-50.
- Hutchison AJ, Smith CP, Brenchley PE, et al. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol. 2011; 7: 578-589.
- Walker MD. Effect of parathyroidectomy on subclinical cardiovascular disease in mild primary hyperparathyroidism. Eur J Endocrinol. 2012; 167: 277-285.
- Holden RM, Booth SL. Vascular calcification in chronic kidney disease: The role of vitamin K. Nat Clin Pract Nephrol. 2007; 3: 522-3.
- Westenfeld R, Schafer C, Kruger T, et al. Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol. 2009; 20 (6): 1264-1274.
- Nguyen-Yamamoto L, Karaplis AC, St-Arnaud R, et al. Fibroblast growth factor 23 regulation by systemic and local osteoblast-synthesized 1,25-dihydroxyvitamin D. J Am Soc Nephrol. 2017; 28 (2): 586-597.
- Mizobuchi M, Towler D, Slatopolsky E, et al. Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009; 20 (7): 1453-1464.
- Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000; 58: 396-399.
- Stein GS, Lian JB, van Wijnen AJ, et al. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004; 23 (24): 4315-4329.
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93 (2): 165-176.
- Tonelli M, Sacks F, Pfeffer M, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005; 2627–2633.
- Turtle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clinical Journal of the American Society of Nephrology : CJASN. 2009; 1968–1973.
- Maziere C, Savitsky V, Galmiche A, et al. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim Biophys Acta. 2010; 1802: 1013–1019.
- Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med (Berl). 2001; 79: 243-253.
- Bucay N, Sarosi I, Dunstan CR, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998; 12: 1260-1268.
- Polyzos SA, Cundy T, Mantzoros CS, et al. Juvenile Paget disease. Metabolism. 2018; 80: 15-26.
- Min H, Morony S, Sarosi I, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000; 192 (4): 463-474.
- Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004; 95: 1046-1057.
- Huang QX, Li JB, Huang XW, et al. Circulating Osteoprotegerin Levels Independently Predict All-cause Mortality in Patients with Chronic Kidney Disease: A Meta-analysis. Int J Med Sci. 2019; 16 (10): 1328-1337.
- Nugroho J, Widorini W. Correlation between osteoprotegerin serum level and coronary calcification using coronary artery calcium score in patient with moderate-severe cardiovascular risk factor. Int J Angiol. 2017; 26: 234-237.
- Rhee EJ, Oh KW, Jung CH, et al. The relationship between four single nucleotide polymorphisms in the promoter region of the osteoprotegerin gene and aortic calcification or coronary artery disease in Koreans. Clin Endocrinol (Oxf). 2006; 64 (6): 689-697.
- Morony S, Tintut Y, Zhang Z, et al. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008; 117 (3): 411-420.
- Deuell KA, Callegari A, Giachelli CM, et al. RANKL enhances macrophage paracrine pro-calcific activity in high phosphate-treated smooth muscle cells: Dependence on IL-6 and TNF-alpha. J Vasc Res. 2012; 49 (6): 510-521.
- Helas S, Goettsch C, Schoppet M, et al. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009; 175 (2): 473-478.
- Jalleh R, Basu G, Le Leu R, et al. Denosumab-induced severe hypocalcaemia in chronic kidney disease. Case Rep Nephrol. 2018; 2018 (2): 7384763.
- Davies MR, Lund RJ, Mathew S, et al. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005; 16 (4): 917-928.
- Taylor CR, Branstetter D, Manna E, et al. Distribution of rank and rank ligand in normal human tissues as determined by an optimized immunohistochemical method. Appl Immunohistochem Mol Morphol. 2017; 25 (5): 299-307.
- Bhanot RD, Kaur J, Bhat Z, et al. Severe hypocalcemia and dramatic increase in parathyroid hormone after denosumab in a dialysis patient: A case report and review of the literature. Case Rep Nephrol. 2019; 2019 (4): 3027419.
- Samelson EJ, Miller PD, Christiansen C, et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014; 29 (2): 450-457.