Desmoplastic Ameloblastoma: A case report with a brief review
2 Department of Oral Pathology and Microbiology, Yenepoya Dental College, Yenepoya University, Mangalore, Karnataka, India, Email: dentist123@gmail.com
3 Department of Oral Pathology and Microbiology, Mamatha Dental College, Khammam, Telangana, India, Email: dentist123@gmail.com
Received: 26-Dec-2017 Accepted Date: Jan 31, 2018; Published: 07-Feb-2018
Citation: Kulkarni S, Mohtesham I, Karteek D, et al. Desmoplastic Ameloblastoma: A case report with a brief review. J Dent and Oral Res 2018;1(1):5-8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Desmoplastic ameloblastoma (DA) is relatively rare histologic variant of solid multicystic ameloblastoma (SMA) which is characterized by its peculiar histomorphology, that is stromal collagenization which has led to the term ‘ameloblastoma with pronounced desmoplasia’ hereby present a case of desmoplastic ameloblastoma in a 22 year female and have made an attempt to discuss the various molecular and genetic markers of amelobalstoma that could affect the treatment plan and aid for the better prognosis.
Keywords
Desmoplasia; Stromal changes; Immunohistochemical markers
Introduction
Ameloblastoma is the second most common odontogenic tumor ofepithelial origin [1]. It is a slow growing tumor and has a locally invasive behavior with high recurrence rate [2]. Ameloblastoma has three clinical variants namely solid or multicystic, unicystic and extraosseous. Histologically the tumor occurs in different patterns namely follicular, plexiform, basal, granular and acanthomatous [1]. Basal cell, clear cell, keratoameloblastoma, papilliferous and desmoplastic are the other rare histological variants [2].
Desomoplastic ameloblastoma (DA), a rare histologic variant of solid multicystic ameloblastoma (SMA) was first reported in 1981 [3]. A detailed report was given in 1984 by Eversole and his coworkers on this variant of ameloblastoma. They called this variant as ‘ameloblastoma with pronounced desmoplasia’ [4]. The information regarding the clinical, radiological presentation and pathology of DAs has led World Health Organization to include this variant in ‘histopathological Typing of odontogenic Tumors’ in the year 2005 [5]. The origin of the tumor is either from the epithelial rests of malassez in the periodontal membrane or from the oxytalan fibres of the periodontal membrane [2].
DA may be defined as ‘a benign but locally infiltrative variant of solid/ multicystic ameloblastoma comprising of proliferating, irregular, bizarrely shaped islands and cords of odontogenic epithelium which is often “compressed” and supported by collagenized stroma’ [6]. It differs from SMA in the clinical, radiological, and histological features and thus should be considered as a separate entity [1]. The incidence of DA varies from 0.9% to12.1% of all ameloblastomas [7]. This paper highlights the typical histopathological features of DA and also the various molecular markers expressed in Ameloblastoma, DA & Adenomatoid Odontogenic Tumor (AOT). This gives an overview of genes that should be targeted by drugs thereby modifying the therapeutic approach which aids in better prognosis.
Case Report
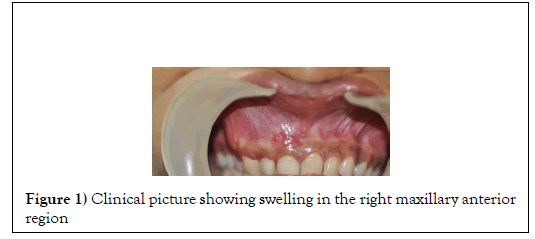
A 22 year old female patient has reported to the dental OPD complaining of pain and swelling in the maxillary anterior region. The duration of the swelling was one month. Extra oral examination did not reveal any asymmetry and the findings were non- contributory. Intra oral examination revealed swelling in the maxillary anterior region. The swelling present on the anterior alveolar gingiva was hard and non-tender on palpation with obliteration of nasolabial fold (Figure 1).
Cervical lymph nodes were not palpable. The patient had no habit history. Orthopantomogram (OPG) showed well defined unilocular radiolucency, extending from the region of 14 to 21. Displacement of the roots of 12 and 13 was also found (Figure 2).
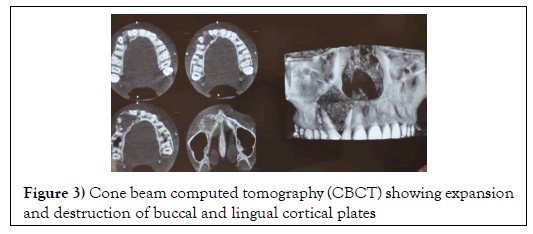
Cone Beam Computed Tomography (CBCT) revealed the expansion and destruction of buccal cortical bone with thinning of the lingual cortical bone (Figure 3).
Figure 3: Cone beam computed tomography (CBCT) showing expansion and destruction of buccal and lingual cortical plates
A provisional diagnosis of Ameloblastoma/ Adenomatoid Odontogenic tumor was given based on clinical and radiographic findings.
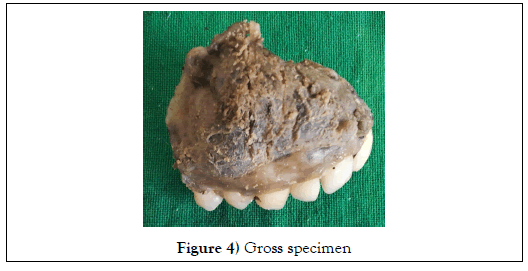
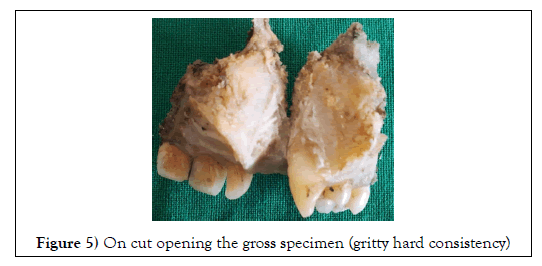
Hemi-maxillectomy was done and the specimen was submitted to the Department of Oral and Maxillofacial Pathology for the histopathological examination. Gross examination revealed that the specimen mass was solid, greyish black in color, measuring approximately 4 x 3cm in size. The cut surface of the specimen has gritty consistency (Figure 4,5).
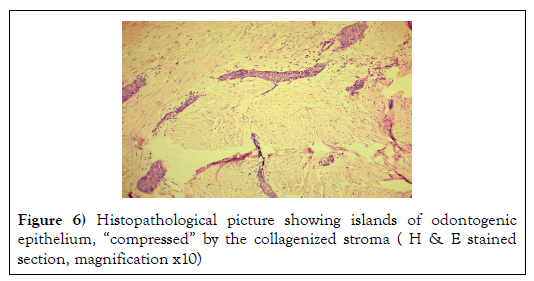
Histological examination of the slides stained with Haematoxylin & Eosin showed numerous islands of odontogenic epithelium in a dense collagenous connective tissue stroma (Figure 6).
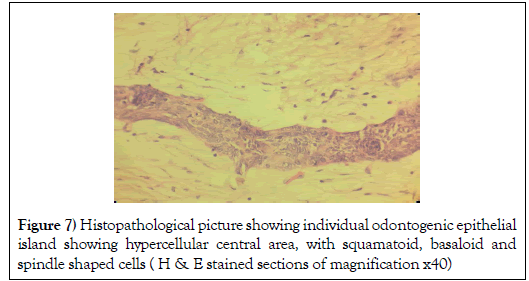
Some odontogenic islands were compressed by the densely collagenous connective tissue stroma and few islands resembled open follicles (Figure 7).
Figure 7: Histopathological picture showing individual odontogenic epithelial island showing hypercellular central area, with squamatoid, basaloid and spindle shaped cells ( H & E stained sections of magnification x40)
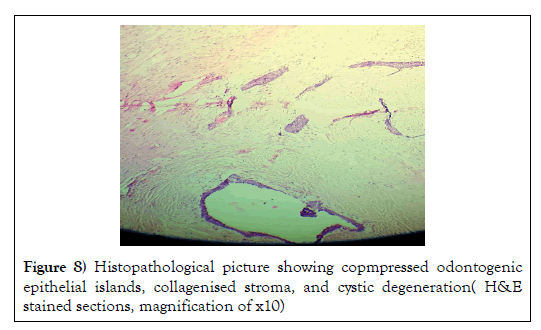
The follicles were occasionally lined by peripheral cuboidal cells and showed hypercellular central area, with squamatoid, basaloid and spindle shaped cells. Some islands exhibited cystic degeneration (Figure 8).
The lesion also showed few blood vessels lined by endothelial cells, with extravasated red blood corpuscles (RBC) and bony spicules in the connective tissue. The diagnosis of Desmoplastic ameloblastoma was made based on the clinical, radiological and histological features.
Discussion
DA is a locally infiltrative neoplasm marked by stromal desmoplasia. Clinically it is seen more commonly in the 4th and 5th decades of life, with mean age being 42.8 years. Frequency of DA is more in females and the maxillary anterior region is the common site of occurrence. The thin cortical bone of the maxilla favors dissemination of the tumor and also the juxtaposition of the maxillary lesions to the vital structures and the sinus makes the tumor more insidious [8].
Radiographically there are 3 types of DA ( Table 1) [9]. In the present case, DA was seen as a mixed radiolucent-opaque lesion which falls under type I (osteofibrosis), which is the most common type thus mimicking a fibroosseous lesion on radiographic diagnosis. The osseous metaplasia within the dense fibrous septa gives characteristic mixed radiographic appearance to the lesion [10]. The study conducted by Takata et al., states that the peculiar radiographic picture expresses its infiltrative nature [11].
| Type 1:Osteofibrosis | Has radiolucent as well as radiopaque appearance |
| Type 2:Radiolucent type | Completely radiolucent |
| Type 3:Compound type | Radiolucent as well as radiopaque appearance combined with a large radiolucent change |
Table 1 Radiographic types of DA
The histological features of DA includes:
(1) Stromal desmoplasia with abundant collagen in the form of moderately cellular and fibrous connective tissue,
(2) Islands of different shapes (kite-like, animal-like, bizarre) in the epithelial component,
(3) Peripheral layer of cuboidal cells,
(4) Hypercellular central area composed of spindle-shaped or squamous cells,
(5) Osseous metaplasia
(6) Myxoid changes of the juxtaepithelial stroma,
(7) Absence of fibrous capsule corresponding to the radiographically poorly defined tumor margin [12].
The histological findings in our case are in accordance with other case reports of DA. The pressure exerted by the collagenous stroma elicits the changes in the epithelial islands [13]. The more aggressiveness of the DA when compared to other ameloblastomas is due to:
(1) Potential to grow to a larger size,
(2) Maxillary location (common) favouring early invasion of adjacent structures,
(3) Inconspicuous radiographic margins, and
(4) Bony invasion [14].
Immunohistochemical (IHC) studies conducted by Molina et al., showed positive expression of cytokeratin (CK) in the basal & suprabasal cells (Table 2). CK14 was positive in the central cells, CD138 also showed positivity for the epithelial cells. They also found low proliferative index of Ki67 and P53 [15]. These results are in accordance with the study of Lee et al., where they found low index of Proliferating Cell Nuclear Antigen (PCNA) and ki67, which thereby elucidates benign biological behavior of DA [16]. The over-expression of MMP-9 in SMA correlates with its invasive & aggressive behavior, which may also be responsible for its recurrence [17].
| Lesion | Marker | Molecular markers overexpressed and are suggestive of |
|---|---|---|
| SMA | MMP-2,MMP-9, syndecan1,17, perlecan,α-dystroglycan, integrin β1,18 CD10, osteopontin | Local invasiveness, aggressiveness, recurrence |
| RANKL and MMP-9 | Induce osteoclastogenesis, subsequently causing rapid destruction of the bone marrow. | |
| IL-1α,T IL-6 TNF, RANKL | caused bone resorption & related to the tumor size | |
| Ki67, CD10 | Recurrence | |
| p53 and murine double minute 2 (MDM2) | Oncogenesis & pathogenesis of SMA | |
| TGF-Beta | Aggressive biological behavior | |
| WNT proteins | Tumor differentiation & invasion. | |
| Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) | neoplastic transformation of odontogenic epithelium | |
| FAS (CD95), FAS ligand, caspase-3 | Responsible for intrinsic regulation of neoplastic cell proliferation and death, thus elucidating their slow growth and inability to metastasize | |
| Podoplanin | Cell migration | |
| p21,p27 | Cell proliferation | |
| AOT | Hepatocyte growth factor (HGF) and c-met | Effect on squamous differentiation of the odontogenic epithelium of AOT |
| Amelogenin, Ameloblastin, and Amelotin, TGF-β are more intensely expressed in AOTs | less aggressive behavior, cytodifferentiation and apoptosis than ameloblastomas. | |
| Desmoplastic ameloblastoma | TGF-beta, Type VI collagen, desmin, S-100 | Suggest extracellular matrix. |
Table 2 Time of day of NHSIII PEWS scores that would trigger a response
Higher expression of syndecan 1 in DA suggests better cell to cell adhesion and explains its lesser amount of invasiveness. The positive expression of laminin V & IV suggests an inductive effect of the epithelium over the fibrous stroma, which may result in a duplicated basal lamina. These findings were supported by the study of Lam et al., in which inductive effects were expressed in the form of deposits of an acellular eosinophilic matrix associated to epithelial islands [17].
Molecular studies done by Ng and Siar., found the expression of desmin & S-100 protein and also a strong positive reaction to collagen VI (ruling out scar tissue), which gave the impression of exrtracellular matrix [18].
TGF-β has an important role in epithelial-mesenchymal transition induction and extracellular matrix formation enhancement by stimulating fibroblasts. DA shows higher expression of TGF- β which results in induction of desmoplastic stroma. Vimentin, a type of intermediate filament, is found in mesenchymal cells. DA has high vimentin expression indicating collagen formation [19].
DA shows variable expression of S-100 and desmin, marked immunoexpression of TGF-α, higher expression of p63, caspase-3 and Fas, decreased expression of CK19. The desmoplastic stroma shows a strong positive reaction for collagen type VI [20]. Other molecular markers expressed in ameloblastoma, DA and AOT have been listed in the Tables 2 & 3. These markers help to modify therapeutic approach by targeting a particular gene and thereby help to improve the treatment with better prognosis.
| Lesion | Markers | Suggestive of |
|---|---|---|
| Ameloblastoma | Syndecan 1, 32,33WNT-related bone-forming genes of WDR5 and runt-related transcription factor 2 (RUNX2) factor 2 (RUNX2) | More invasinessImpedes boneformation |
| DA | PCNA, Ki67 | Low proliferative index of the tumor tumor |
| AOT | p53 and MDM2 | Less aggressive behavior than SMA |
Table 3 Molecular markers downregulat
The histopathological differential diagnosis for DA includes ameloblastic fibroma (AF), odontogenic fibroma (OF), and squamous odontogenic tumor (SOT) (Table 3).
AF shows interlacing scattered thin island of odontogenic epithelium with peripheral columnar cells and central stellate cells. These islands are scattered in primitive mesenchyme. OF is a non-infiltrating lesion composed of mature collagen fiber interspersed with plump fibroblasts and small nests or island of inactive odontogenic epithelium. SOT is composed of the round or broad based island of mature squamous epithelium with peripheral flat or cuboidal cells. The fibrous stroma consists of mature collagen fiber bundles without inductive effect. But DA shows dense collagen stroma that appears hyalinized and hypocellular compressing odontogenic epithelium into bizarre, irregular shaped islands [12-14].
SMA and DA have high recurrence rate therefore the management is controversial [18]. Conservative surgical treatment such as enucleation and curettage resulted in recurrence [21]. Thus, SMA requires radical surgical intervention. Recurrence is seen even after 5-10 years of surgery. Therefore thorough examination of surgical sites is needed for at least 10 years.
The reason for recurrence of DA may be:
(1) Inconspicuous margin making the demarcation between lesion and normal bone difficult .
(2) The more frequent occurrence in the maxilla which results in an early encroachment of the nearby structures.
DA lacks capsule, resulting in the infiltration of tumor cells into trabeculae of the cancellous bone. This results in the inconspicuous radiographic margins and leading to recurrence. Therefore, the recommended technique includes resection with safety margin of 1 cm of bone beyond the radiographic margin [12].
In the present case the mode of treatment done was hemi-maxillectomy . There was no recurrence and the patient is under regular follow up.
Conclusion
Desmoplastic ameloblastoma is a rare histological variant of ameloblastoma. It is known for its different biological behavior and histological architecture. Radiographic picture suggests its infiltrative nature especially in the anterior maxilla. The previous studies give an conflicting results regarding the recurrence following surgical intervention. The definitive diagnosis should be based on histopathological examination and a proper post-surgical follow up is mandatory.
Details of Contributors
All authors contributed to the conception of the work; EDE and AO to the acquisition of data, BWM to the analysis of data, CVEP to the interpretation of data for the work.
BWM and EDE drafted the manuscript; CVEP and AO revised it critically for important intellectual content. All authors approved the final version of the manuscript submitted for publication.
Funding
None.
Ethical Approval
Ethical approval for the original data collection was granted by the South East Wales Local Research Ethics Committee
REFERENCES
- Sheikh S, Pallagatti S, Singla I, et al. Desmoplastic ameloblastoma: A case report. JODDD 2011;5:27-32.
- Reichart PA, Philipsen HP. Odontogenic tumors and allied lesions. Quintessence, London 2004.
- Takigawa T, Matsumoto M, Sekine Y. A case report of ameloblastoma proliferated like epulis of maxilla [in Japanese]. Nihon Univ Dent 1981;55:920-924.
- Eversole LR, Leider AS, Hansen LS. Ameloblastomas with pronounced desmoplasia. J Oral Maxillofac Surg1984;42:735-740.
- Kramer IR, Pindborg JJ, Shear M. The WHO histological typing of odontogenic tumours. A commentary on the second edition. Cancer 1992;70:2988–2994.
- Savithri V, Janardhanan M, Suresh R, et al. Desmoplastic ameloblastoma with osteoplasia: Review of literature with a case report. J Oral Maxillofac Pathol 2013;17:298-301
- Pillai R, Ongole R, Ahsan A, et al. Recurrent desmoplastic ameloblastoma of the maxilla: A Case Report. J Can Dent Assoc 2004;70:100–104.
- Rawson K, Bastian TS, Shiva B, et al. A case of desmoplastic ameloblastoma of the anterior maxilla. JIAOMR 2010;22:60-62.
- Lamichhane NS, Liu Q, Sun H, et al. A case report on desmoplastic ameloblastoma of anterior mandible. BMC Res Notes 2016;9:171.
- Neville BW, Damm DD, Allen CM, et al. Odontogenic cysts and tumors. In oral and maxillofacial pathology. 3rd ed. St.Louis: Saunders 2009;678-732.
- Takata T, Miyauchi M, Ito H, et al. Clinical and histopathological analyses of desmoplastic ameloblastoma. Pathol Res Pract 1999;195:669-675.
- Lamichhane NS, Liu Q, Sun H, et al. A case report on desmoplastic ameloblastoma of anterior mandible. BMC Res Notes 2016;9:171.
- Sivapathasundharam B, Einstein A, Syed RI. Desmoplastic ameloblastoma in Indians: Report of five cases and review of literature. Indian J Dent Res 2007;18:218-221.
- Doddawad VG, Shivananda S, Subbaiah P, et al. Desmoplastic ameloblastoma in maxilla: A Report of rare case with review of literature and classic histopathology annual research & review in biology 2017;21:1-7.
- Molina RB, Taylor AM, Oslei P de A, et al. Peripheral desmoplastic ameloblastoma: Histopathological and immunohistochemical profile of a case. Med Oral Patol Oral Cir Bucal. 2010;15:e846-849.
- Lee SK, Kim SY. Current concepts and occurrence of epithelial odontogenic tumors: Ameloblastoma and adenomatoid odontogenic tumor. Korean J Pathol 2013;47:191-202.
- Lam KY, Chan AC, Wu PC, et al. Desmoplastic variant of ameloblastoma in chinese patients. Br J Oral Maxillofac Surg. 1998;36:129-134.
- Siar CH, Lau SH, Ng KH. Ameloblastoma of the jaws: A retrospective analysis of 340 cases in a Malaysian population. J Oral Maxillofac Surg. 2012;70:608-615.
- Liang P, Zheng G, Liu X, et al. Clinical and pathological features of desmoplastic ameloblastoma: A literature review with three case reports. Int J Clin Exp Med 2017;10:7204-7212.
- Surbhi, Metgud R, Naik S, et al. Spindle cell lesions: A review on immunohistochemical markers. J Can Res Ther 2017;13:412-418.
- Sun ZJ, Wu YR, Cheng N, et al. Desmoplastic ameloblastoma–A review. Oral Oncol 2009;45:752-759.