Detecting sensitivity of S. typhimurium on fresh produce by phage-based magnetostrictive biosensors
2 Materials Research and Education Center, Auburn University, Auburn, AL36849, USA, Email: huj3@upmc.edu
3 Department of Mechanical Engineering, Changzhou Institute of Light Industry Technology, Changzhou, 213164, China, Email: huj3@upmc.edu
Received: 03-Jan-2018 Accepted Date: Jan 07, 2018; Published: 25-Jan-2018
Citation: Xiaoji Miao, Byran A Chin, Jing Hu, et al. Detecting sensitivity of S. typhimurium on fresh produce by phage-based magnetostrictive biosensors . Nanotechnology Letters. 2018;2(1):1.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The magnetostrictive (ME) biosensors have been developed to detect pathogen rapidly and sensitively in fresh produce. The effects of concentration and incubated time of S. typhimurium enrichment on the detection sensitivity were investigated in this study. The concentrations ranging from 5 × 102 to 5 × 108 CFU/mL with incubated time of 22 h, and incubated time ranging from 5 h to 22h with the concentration of 5 × 102 CFU/mL were tested. The results showed that the higher concentration and longer incubated time, the more frequency changes due to the more bacteria binding on the ME biosensors. The micrographs by optical microscope were used to verify the bacteria binding on ME biosensors. The student t-test calculation was also used to analysis the difference between measurement and control sensors. The limit of detection (LOD) for phage-based magnetostrictive biosensors on fresh tomatoes was statistically determined to be lower than 5 × 102 CFU/mL for 5 h enrichment with a confidence level of difference higher than 98% (p<0.05).
Keywords
Magnetostrictive; biosensors; S. typhimurium; sensitivity; detectionIntroduction
Fresh produce safety is concerned by global people with the foodborne disease outbreaks, especially salmonellas [1]. Salmonella has been linked to the outbreaks of lettuce, tomatoes, jalapeno peppers and other fresh produce [2]. In the United States, there are 1.2 million cases of salmonellas every year, resulting in over 23,000 hospitalizations and 450 deaths [3]. Recently, a cucumber-associated outbreak of salmonella resulted in 418 cases and 2 deaths in 31 states in September, 2015.
Salmonella can be effectively detected and identified by the traditional methods, such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA) and some other methods [4]. However, these methods usually require approximately 5 days to complete the analysis [5]. Hence, a rapid and reliable detection method should be developed to prevent the salmonellas.
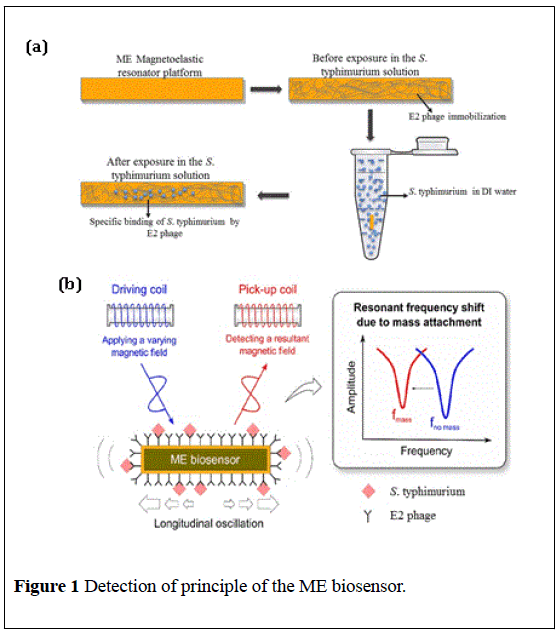
Wireless phage-based magnetostrictive (ME) biosensors have been developed to be a rapid detection method of salmonella in fresh produce. A ME biosensor consists of a magnetostricitive resonator platform as a transducer and phage coated as a bio-recognition element [6]. In this investigation, the resonator platform was coated with E2 phage for the specific binding of S. typhimurium [7]. In this investigation, according to the method described in the Bacteriological Analytical Manual (BAM) by the U.S. Food and Drug Administration (FDA), S. typhimurium was enriched to different concentrations in broth with different incubated time and rapidly detected by ME biosensors.
Material and Methods
Fabrication of ME resonators and metal deposition
Magnetostrictive resonator platforms were fabricated by METGLAS® 2826MB alloy (30-μm thick, Honeywell International, Inc., NJ, USA). The ribbon was diced into 1 mm × 0.2 mm × 0.028 mm rectangular stripshaped platforms using a computer controlled automatic micro-dicing saw (DAD 3220, Disco Crop, Tokyo, Japan), followed by cleaning in acetone and ethanol by ultrasonic cleaner, and drying in air. Afterwards, annealing was carried out at 220°C for 2 h in vacuum (≤ 10-3 Torr) to relieve the remained stress and correct defects from polishing and dicing. Finally, chromium (90 nm thick) and gold (150 nm thick) layers were deposited onto both sides of the platform surfaces at 2.5 × 10−6 Torr (Denton Vacuum, NJ, USA). The Cr coated layer used to protect the ME biosensors from corrosion, as well as an adhesive interface between the resonator and the gold layer. The Au layer acts as a protection of corrosion and immobilization of the phage bio-probe [8].
Immobilization of E2 phage and bovine serum albumin (BSA) surface blocking
The filamentous E2 phage (1.01 × 1012 vir/mL) was prepared and provide by Dr. James M. Barbaree’s lab in the Department of Biological Science at Auburn University. The E2 phage is specific for S. typhimurium [9]. The bare ME resonators were immersed in a 330 μL tube with the phage suspension that was diluted to 5 × 1011 vir/mL with 1 × Tris-Buffered Saline (TBS) solution and rotated for 1h at room temperature. After washing with distilled water twice to remove the unbound bio-probe, the resonators surface was adsorbed physical phagesuniformly. In order to prevent the nonspecific binding [10], the resonators were immersed in the tube (330 μL) with 1 mg/mL bovine serum albumin (BSA) and rotated for 45 min, followed by dipping in DI water for 5 seconds to remove the debris.
S. typhimurium cultures and contamination on tomatoes
The S. typhimurium was also provided by Dr. James M. Barbaree’s labin the Department of Biological Science at Auburn University. Two tomatoes (purchased in a local grocery store) were placed in a sterile plastic bag, and the preparation of S. typhimurium in this work is conducted basically according to the general rules of BAM (FDA), though with few modifications. S. typhimurium solution of 10 ml at concentration of 5 × 108, 5 × 106, 5 × 104, and 5 × 102 CFU was applied on the tomato surfaces with a sterile adjustable Sprayer (Spray Anywhere, Fisher Scientific, Pittsburgh, PA) inside a sterile tote and incubated in LB broth (the weight ratio of tomato to broth is 1 to 2). The tomatoes with the broth were incubated at different time (5 h, 6 h, 7 h, 22 h) at 37°C after 1 h preenrichment at room temperature. The incubated broth was concentrated to 2 mL with filtered water washing. The measurement sensors (immobilized with phage) and control sensors were immersed in the S. typhimurium solutions in the PCR tubes with the volume of 330 mL for 30 min rotation.
The principle of detection and resonance frequency measurements
ME biosensors made by METGLAS® 2826MB alloy are ferromagnetic materials with the property that the shape or the dimensions would be changed due to magnetization [11]. When subjected to an applied timevarying magnetic field, the ME biosensors can be placed into mechanical resonance [12]. The principle of the detection is based on the resonant frequency shifts of ME biosensors when the mass is changed after binding the pathogenic bacteria. The fundamental resonant frequency of a thin, freestanding, strip-shaped resonator under longitudinal vibration is given by Guntupalli, Ye, Lakshmanan, et al. [13-15]:

Where f is the initial resonant frequency, L is the length of the resonator, E is Young's modulus of elasticity, ρ is the density of the resonator material and ν is the Poisson ratio. When a small mass of the pathogenic bacteria attached to the ME biosensor, the increased mass causes a decrease of resonant frequency. The resonant frequency shift can be given by Chai Y, Park MK, et al. [16,17]:

Where Δm, M (>>Δm), and Δf denote the mass change, the initial mass of the resonator, and the resonant frequency shift, respectively.
As shown in Figure 1, the initial and final resonant frequency (before and after immersing in the S. typhimurium solution) of measurement sensors and control sensors were measured by a single-layer solenoid coil [18,19] connected with a network analyzer (HP/Agilent 8751A, Agilent Technologies, Inc., Santa Clara, CA, USA). The solenoid coil was made by glass capillary (1 mm inner-diameter) with copper wire (0.1 mm thick). The frequency shifts presented the mass change because of specific bindings on the ME biosensors.
Optical microscope
The specific binding was observed and verified by optical microscope (50 ×, Olympus BX51, Tokyo, Japan). In order to clearly observe the cells, the ME biosensors were exposed in the crystal violet dye (5% in DI water) to absorb the color for 10 min, and washed by distilled water to remove the remained dye on the surface prior to observing.
Statistical analysis
Statistical analysis was conducted on the responsesof ME biosensors exposed to tomato surfaces spiked with differentconcentrations of S. typhimurium. For each concentration, a Student’s t-test was performed to compare the difference between measurement and control sensors to get the confidence level of the experiment.
Result and Discussion
The effects of S. typhimurium concentrations
S. typhimurium with four different concentrations ranging from 5 × 102 to 5 × 108 CFU/mL was spiked on tomatoes and incubated in LB broth for 22 hours. The incubated broth with the volume of 3 mL was taken to concentrate to 1 mL. Figure 2 shows the frequency changes of the measurement sensors and control sensors after exposure in bacteria with the different concentrations. As can be seen, the frequency shifts of measurement sensors have big difference with those of control sensors, which illustrates that the measurement sensors have much more mass changes because of the bacteria cells binding. And it also shows that at the same incubated time, the higher bacteria concentration spiked on tomatoes, the more resonant frequency shifts due to more bacteria capturing. Meanwhile, the student t-test calculation was used to analyze the difference between the measurement and control sensors, and it is found that the confidence level of the difference is higher than 99% (p<0.05), thus it can be concluded that the detection limit is lower than 5 × 102 CFU/mL for 22 h enrichment.
Figure 2: The frequency shifts of ME biosensors immersed in different concentration of S. typhimurium incubated in broth for 22 hours with the contaminated tomatoes.
The micrographs shown in Figure 3 visually confirmed that a large amount of bacteria were captured on measurement biosensors, while few bacteria on control sensors. Meanwhile, it can be obviously seen that the amount of bacteria captured by ME biosensor increases with the concentration of S. typhimurium spiked on the tomatoes.
The effects of incubated time
10 mL of S. typhimurium solution with the concentration of 102 CFU was spiked on tomatoes, and then incubated in LB broth for different time ranging from 5 h to 22 h. All the incubated broth was concentrated to 2 mL, and washed by DI water. Then the salmonella in DI water was concentrated from 2 mL to 1 mL. Figure 4 shows the frequency shifts of ME biosensors immersed in S. typhimurium with the concentration of 102 CFU incubated in broth for different time. It presents that the frequency shifts of measurement sensors are much bigger than those of control sensors, and it also shows the tendency that the frequency shifts increase with the incubating time, due to more S. typhimurium captured by ME biosensor, which was confirmed by micrographs shown in Figure 5.
Figure 4: The frequency shifts of ME biosensors immersed in S. typhimurium incubated in broth with the contaminated tomatoes for different time.
The student t-test calculation was also used to analysis the difference between measurement and control sensors. And the confidence level of difference higher than 98% (p<0.05) was obtained. Therefore, it can be concluded that the detection limit is lower than 5 × 102 CFU/mL for 5 h enrichment as well.
Conclusion
The effects of S. typhimurium concentrations and incubated time on the detection sensitivity of ME biosensors were studied. Phage based biosensors were used to specifically bind S. typhimurium and the bound bacteria were verified by optical microscope. The student t-test calculation was also used to analysis the difference between measurement and control sensors. Both the experiments and statistical analysis results illustrated that the limit of detection (LOD) was determined to be lower than 5 × 102 CFU/mL for 5 h enrichment with a confidence level of difference higher than 98% (p<0.05).
Acknowledgements
This project was supported by National Natural Science Foundation of China (51271039), USDA Grant 2011-51181-30642A and PAPD.
REFERENCES
- Yoshitomi KJ, Jinneman KC, Orlandi PA, et al. Evaluation of rapid screening techniques for detection of Salmonella spp. from produce samples after pre-enrichment according to FDA BAM and a short secondary enrichment. Lett Appl Microbiol. 2015;61:7-12.
- Miller ND, Davidson PM, D’Souza DH. Real-time reverse-transcriptase PCR for Salmonella Typhimurium detection from lettuce and tomatoes. LWT Food Sci Technol. 2011;44:1088-97.
- Yang Q, Wang F, Jones KL, et al. Evaluation of loop-mediated isothermal amplification for the rapid, reliable, and robust detection of Salmonella in produce Food Microbiol. 2015;46:485-93.
- Park MK, Wikle HC, Chai Y, et al. The effect of incubation time for Salmonella Typhimurium binding to phage-based magnetostrictive biosensors. Food Control. 2012;26:539-45.
- Zhang G, Brown EW, González EN. Comparison of Real-Time PCR, Reverse Transcriptase Real-Time PCR, Loop-Mediated Isothermal Amplification, and the FDA Conventional Microbiological Method for the Detection of Salmonella spp. in Produce. Appl Environ Microbiol2011;77:6495-501.
- Hu J, Guntupalli R, Lakshmanan RS, et al. The Longevity of Polyclonal Antibody to Salmonella typhimurium at Different Temperatures on a Magnetostrictive Sensor Platform. Nanosci Nanotechnol Asia. 2011;1:25-30.
- Ye XM, Guntupalli R, Chin BA, et al. Comparative study of thermal stability of magnetostrictive biosensors between two kinds of biorecognition elements Mater Sci Eng. 2014;41:78-82.
- Horikawa S, Bedi D, Li S, et al. Effects of surface functionalization on the surface phage coverage and the subsequent performance of phage-immobilized magnetostrictive biosensors. Biosens Bioelectron. 2011;26:2361-367.
- Hu JJ, Chai YT, Horikawa S, et al. The blocking reagent optimization for the magnetostrictive biosensor. Pro of SPIE. 2015;9488:1-8.
- Shen W, Li S, Park MK, et al. Blocking Agent Optimization for Nonspecific Binding on Phage Based Magnetostrictive Biosensors J Electrochem Soc. 2012;159:818-23.
- Chai Y, Horikawa S, Park MK, et al. Rapid and Sensitive Detection of Salmonella Typhimurium on Eggshells by Using Wireless Biosensors. J Food Prot. 2012;75:631-36.
- Nitilaksha H, Rajesh G, Vitaly V, et al. Detection of methicillin-resistant Staphylococcus aureus using novel lytic phage-based magnetoelastic biosensors Sensors and Actuators B: Chemical. 2015;210:129-36.
- Guntupalli R, Hu J, Lakshmanan RS, et al. A magnetostrictive resonance biosensor immobilized with polyclonal antibody for the detection of Salmonella typhimurium. Biosens Bioelectron. 2007;22:1474-79.
- Ye X, Guntupalli R, Lakshmanan RS, et al. Comparative study of thermal stability of magnetostrictive biosensor between two kinds of biorecognition elements Mater Sci Eng. 2014;41:78-82.
- Lakshmanan RS, Guntupalli R, Petrenko VA, et al. Detection of Salmonella typhimurium in fat free milk using a phage immobilized magnetostrictive sensor Sens Actuators B Chem. 2007;126:544-50.
- Chai Y, Horikawa S, Li S, et al. surface-scanning coil detector for real-time, in-situ detection of bacteria on fresh food surfaces. Biosens Bioelectron. 2013;50:311-17.
- Park MK, Weerakoon KA, Oh JH, et al. The analytical comparison of phage-based magnetostrictive biosensor with TaqMan-based quantitative PCR method to detect Salmonella Typhimurium on cantaloupes. Food Control. 2013;33:330-36.
- Chai Y, Wikle HC, Wang Z, et al. Design of a surface-scanning coil detector for direct bacteria detection on food surfaces using a magnetostrictive biosensor. J Appl Phys. 2013;14:104-504.
- Chai Y, Horikawa S, Wikle HC, et al. Surface-scanning coil detectors for magnetostrictive biosensors: A comparison of planar-spiral and solenoid coils. Appl Phys Lett. 2013;103:173-510.