Detection of PNH red cell population in a cohort of patients by using the gel test in a resource constrained country: a cross-sectional study
Received: 19-Feb-2018 Accepted Date: Apr 02, 2018; Published: 12-Apr-2018
Citation: Fareed NA, Borhany M, Khurram S, et al. Detection of PNH red cell population in a cohort of patients by using the gel test in a resource constrained country: a cross-sectional study. J Blood Disord Treat. 2018;1(2):6-8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal stem cell disorder that is caused due to the absence of certain glycosylphophatidylinositol (GPI)-anchored proteins, such as CD55 and CD59. This accounts for the complement-mediated hemolysis. The gel card test is a useful screening tool to detect red cell antigen-antibody reactions. This is a fairly easy test to perform. The objective of our study was to detect PNH red cell population (CD55-ve & CD59-ve) using the PNH gel card as a quick screening test where flow cytometry test is not available. A cross sectional study in which patients and healthy donors were included at the National Institute of Blood Disease’s blood bank department. Clinico-pathological parameters were entered. All the peripheral blood samples of patients were collected in EDTA and evaluated through ID PNH gel card (BIO RAD) screening test. A total of 41 patients were enrolled, including 26 (63.4%) males & 15 (36.5%) females. Median age was 36.5 years (range 09-64 years). Forty-one healthy blood donors were also tested along with patients for PNH using the PNH gel test. Out of the 41.7 (17%) patients showed significant population of PNH red cells while all the healthy blood donors were negative. In Pakistan, due to the non-availability of flow cytometry for PNH in many centers, the presence of PNH clone could not be confirmed in these patients. The gel test appears to be a useful screening tool for PNH because of its simplicity and the ease with which its results could be interpreted.
Keywords
Paroxysmal nocturnal hemoglobinuria; ID-PNH; Aplastic anemia
Paroxysmal Nocturnal Haemoglobinuria (PNH) is an acquired haematopoietic stem cell disorder characterized by a triad of hemolytic Anemia, bone marrow failure and thrombosis [1]. It is caused by a mutation in the PIG-A genelocated at Xq of a haematopoietic stem cell that results in the disruption of glycosylphophatidylinositol (GPI) synthesis and a deficiency in all GPI-anchored proteins. This leads to deficiency of the GPI linked complement inhibitors CD55 (Decay accelerating factor, DAF) and CD59 (Membrane inhibitor of reactive lysis, MIRL which results in blood cells susceptible to complement mediated lysis [2-4]. Traditionally, the Ham test which was proposed in 1937 [5] and the sucrose hemolysis test [6] were used to diagnose PNH but not adopted widely because methods were complicated and time consuming; moreover, the sensitivities and specificities of examinations by spectrophotometry are quite low. Over the past several years, evaluation by flowcytometry of GPI-anchored proteins (CD55, CD59) of red blood cells (RBC) and granulocytes has become the gold standard for PNH diagnosis [7,8] however, all laboratories have not adopted this approach due to constrained resources and hematology specialists who are required for the appropriate diagnosis. Due to these reasons a screening test is needed which can replace lytic tests (Ham test and sucrose hemolysis test) in a resource constrained country like Pakistan where in many centers flow cytometry for PNH is not available. The gel card test is a rapid useful screening tool to detect red cell antigen-antibody reactions. It allows direct evaluation of red cell abnormalities. It is quick, easy to perform, economical and provides reliable results [9-14]. The objective of this study was the detection of PNH red cell population (CD55-ve & CD59-ve) using the PNH gel card as a screening test where flowcytometry is not available.
Materials and Methods
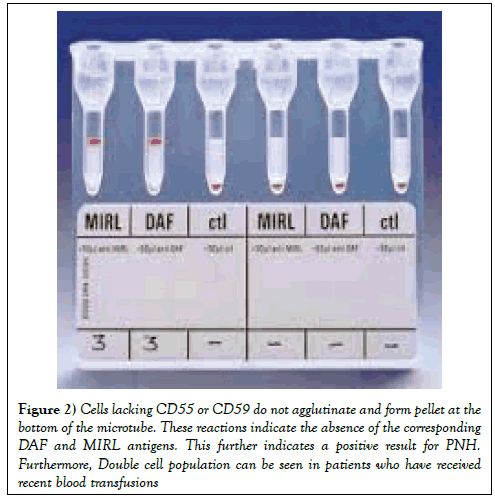
A cross sectional study was done at the National institute of blood disease and bone marrow transplantation Karachi over the period of 2 years from June 2014 to June 2016. Clinico-pathological parameters were retrieved from the patients’ records. All patients’ peripheral blood samples were collected in EDTA and evaluated through ID PNH gel card (BIO RAD, Dia Med, GMBH 1785,Cressier FR, Switzerland) screening test (Figure 1). Forty-one patients with suspected PNH were included in this study from outpatient and inpatient departments. Similarly 41 healthy blood donors were also tested with each test. Blood from patients collected in EDTA tube was used for the detection of CD55 (Decay Accelerating Factor DAF) and CD59 (Membrane inhibitor of Reactive Lysis MIRL) red cell populations. Fifty microliters of RBC suspension in a low ionic strength buffer (ID-diluent 2) was added to the three appropriate micro tubes of the ID-cards for the PNH test consisting of MIRL, DAF and negative control (PNHctl). Then, 25 μL -- each of anti-MIRL, anti-DAF and negative control - were added to the corresponding micro tubes. The cards were incubated at 37°C for 15 min, centrifuged for 10 min in the ID-centrifuge and the reactions were read and interpreted. Positive reactions of 3+ to 4+ (agglutinated cells forming a red line on the surface of the gel) indicate a normal red cell population with the presence of the corresponding DAF and MIRL antigens. This indicates a negative result for PNH and the PNH-control micro tube must show a negative reaction (a compact button of cells on the bottom of the micro tube). Cells lacking CD55 or CD59 do not agglutinate and form pellet at the bottom of the microtube. These reactions indicate the absence of the corresponding DAF and MIRL antigens. This further indicates a positive result for PNH. Furthermore, Double cell population can be seen in patients who have received recent blood transfusions (Figure 2). The sensitivity and specificity of the PNH gel test was calculated. Mean and standard deviation were calculated for quantitative variables and frequency and percentages were calculated for categorical variable by using SPSS version 17.0.
Figure 1: A cross sectional study was done at the National institute of blood disease and bone marrow transplantation Karachi over the period of 2 years from June 2014 to June 2016. Clinico-pathological parameters were retrieved from the patients’ records. All patients’ peripheral blood samples were collected in EDTA and evaluated through ID PNH gel card (BIO RAD, Dia Med, GMBH 1785, Cressier FR, Switzerland) screening test
Figure 2: Cells lacking CD55 or CD59 do not agglutinate and form pellet at the bottom of the microtube. These reactions indicate the absence of the corresponding DAF and MIRL antigens. This further indicates a positive result for PNH. Furthermore, Double cell population can be seen in patients who have received recent blood transfusions
Results
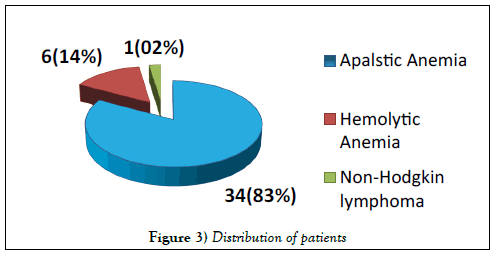
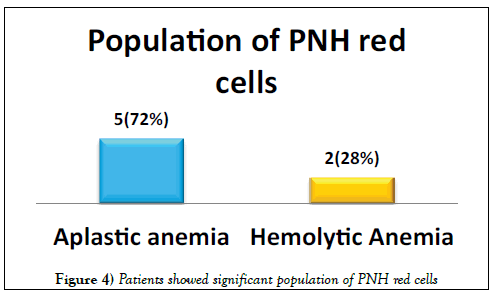
A total of 41 patients were enrolled including 26 (63%) males & 15 (37%) females. Median age was 36.5 years (range 9-64 years). Distribution of patients is shown in Figure 3. Seven (17%) patients showed significant population of PNH red cells as shown in Figure 4. Double cell populations (positive reactions)were demonstrated by the PNH gel test in 3-blood samples. 41 healthy blood donors of 18-60 years of age were tested for PNH and all were negative. The presence of PNH clone could not be confirmed in these patients due to the non-availability of flow cytometry for PNH, which is in turn needed for confirmation of PNH diagnosis [15].
Discussion
Paroxysmal nocturnal haemoglobinuria (PNH) is a clonal stem cell disorder in which there is a deficiency of glycosylphosphatidylinositol (GPI) anchored proteins, which results in an abnormal sensitivity of red cells to complement [2,3]. PNH manifests itself in a many ways, including complement mediated hemolytic anemia, pancytopenia and thrombosis [5]. Commonly, PNH is under diagnosed due to its heterogeneity and atypical symptoms that do not resemble classic cases of PNH. Furthermore, the accessible laboratory methods to detect PNH are time-consuming and complicated. The use of the hemagglutinating gel test as a screening test of PNH seems to open new possibilities [16-18]. Apart from classical PNH, PNH like cells are encountered in various other disorders, mostly seen in Aplastic Anemia and in a few cases of Myelodysplastic syndrome and Myelofibrosis [16]. Moreover, the presence of a PNH clone in severe aplastic anemia is associated with low morbidity and mortality [19]. Our study shows the presence of PNH red cells in 5 patients with Aplastic Anemia and 2 patients with Hemolytic Anemia. The study by Meletis et al. [16] in 1997 16 depicts CD55 and CD59 deficient red cell populations in 73 (29.2%) and 8 (3.1%) out of 255patients respectively by using the gel card. The study by Asimakopoulos et al. [20] in 2014 depicts the frequency of erythrocytes with isolated deficiency of CD55that was higher than that of cells with isolated deficiency of CD59 by using the gel card method. Another study by Meletis et al. [21] showed red cell populations deficient in both CD55 and CD 59 in 9.2% of patients with lymphoproliferative disorders and in all PNH patients by using sephacryl gel micro typing system. On the other hand, a study done in Thailand reported 20% PNH clone in a cohort of 35 patients using PNH gel test [13].
The expression of CD55 &CD59 proteins in neutrophils and lymphocytes should be further investigated in such patients because the gel test system detects only red cell abnormal population, while the PNH defect also involves white cells. In this context, studies comparing the sensitivity of this semi quantitative method with a quantitative test such as flow cytometry in the same sample are also needed. In this way, the gel test could become accepted as a screening method, and, depending on the extent of correlation with flow cytometry, its specificity could be regarded as having been adequately tested. In a developing country like Pakistan, clinical profile, laboratory diagnosis and follow-up of patients with PNH clearly indicates that many patients with a smaller clone of PNH cells may go undiagnosed due to lack of flow cytometry facilities in many centers. The application of this technique would be especially easy to introduce in laboratories that are already using this system for blood grouping and cross matching but we miss the PNH patients by gel card due to prior blood transfusions. To further validate the usefulness of the PNH gel test, larger sample size before to blood transfusion is needed.
Conclusion
In conclusion, the gel test appears to be a useful screening test for PNH in resource constrained countries because of its simplicity and increased sensitivity to diagnose PNH. This test would be especially easy to introduce in laboratories that are already using this system for blood grouping and antibody screening, identification and cross matching.
Acknowledgement
We acknowledge all the patients and technical staff for their participation and contribution.
REFERENCES
- Parker CJ. Paroxysmal nocturnal hemoglobinuria: An historical overview. Hematology American Society of Hematology Education Program. 2008;1:93-103.
- Rosse WF, Ware RE. The molecular basis of paroxysmal nocturnal hemoglobinuria. Blood. 1995; 86:3277-86.
- Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703-11.
- Galassi N, Canalejo K, Riera N, et al. Flow cytometric analysis of an expansion paroxysmal nocturnal hemoglobinuria (PNH) clone in a patient with bone marrow failure. Am J Hematol. 2001;67:277-8.
- Ham TH, Dingle JH. Studies on destruction of red blood cells. II. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria: Certain immunological aspects of the hemolytic mechanism with special reference to serum complement. J Clin Investig. 1939; 18:657-72.
- Hartmann RC, Jenkins DE. The “sugar-water” test for paroxysmal nocturnal hemoglobinuria. NEJM. 1966;275:155-7.
- Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113: 6522-7.
- Borowitz MJ, Craig FE, DiGiuseppe JA, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytomet B Clin Cytomet. 2010;78:211-30.
- Höchsmann B, Rojewski M, Schrezenmeier H. Paroxysmal nocturnal hemoglobinuria (PNH): Higher sensitivity and validity in diagnosis and serial monitoring by flow cytometric analysis of reticulocytes. Ann Hematol. 2011;90:887-99.
- Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-709.
- Nathalang O, Kuvanont S, Suwanasit T, et al. Preliminary study of the gel test for cross-matching in Thailand. J Med Tech Assoc Thailand 1993;21:101-6.
- Gupta R, Pandey P, Choudhry R, et al. Prospective comparison of four techniques for diagnosis of paroxysmal nocturnal hemoglobinuria. Int J Lab Hematol. 2007;29:119-26.
- Nathalang O, Chuansumrit A, Prayoonwiwat W. Rapid screening of PNH red cell populations using the gel test. South Asia J Trop Med Pub Health. 2003;34:887-90.
- Alfinito F, Del Vecchio L, Rocco S, et al. Blood cell flowcytometry in paroxysmal nocturnal hemoglobinuria: A tool for measuring the extent of the PNH clone. Leukemia. 1996;10:1326-30.
- Hall SE, Rosse WF. The use of monoclonal antibodies and flowcytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-40.
- Meletis J, Michali E, Samarkos M, et al. Detection of “PNH red cell” populations in hematological disorders using the sephacryl gel test microtyping system. Leukemia Lymphoma. 1997;28:177-82.
- Zupanska B, Bogdanik B, Pyl H. A gel microtyping system for diagnosis of paroxysmal nocturnal hemoglobinuria. Immunohematol 2002;18:9-12.
- Madkaikar M, Gupta M, Jijina F, et al. Paroxysmal nocturnal haemoglobinuria: diagnostic tests, advantages & limitations. 2009. Eur J Haematol. 2009;83:503-11.
- Scheinberg P, Marte M, Nunez O, et al. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-80.
- Asimakopoulos JV, Terpos E, Papageorgiou L, et al. The presence of CD55- and/or CD59-deficient erythrocytic populations in patients with rheumatic diseases reflects an immune-mediated bone marrow derived phenomenon. Medical science monitor. Int Med J Exp Clin Res. 2014;20:123-39.
- Meletis J, Terpos E, Samarkos M, et al. Detection of CD55- and/or CD59-deficient red cell populations in patients with lymphoproliferative syndromes. Hematol J. 2001;2:33-7.