Effect of rosuvastatin on serum levels of vaspin and visfatin in patients with CAD
2 Cardiology Lecturer,Cardiology Department, Faculty of Medicine, Tanta University, Egypt, Email: rehab_werida@hotmail.com
3 Professor of Pharmacology & Toxicology,Pharmacology & Toxicology Department, Faculty of Pharmacy, Tanta University, Egypt, Email: rehab_werida@hotmail.com
4 Professor of Cardiology, Cardiology Department,Faculty of Medicine, Tanta University, Egypt, Email: rehab_werida@hotmail.com
Received: 19-Jul-2018 Accepted Date: Jul 27, 2018; Published: 31-Jul-2018, DOI: 10.4172/2368-0512.1000107
Citation: Werida R, Khedr L, El-Sisi AE, et al. Effect of rosuvastatin on serum levels of vaspin and visfatin in patients with CAD. Curr Res Cardiol 2018;5(2):26-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
AIM OF THE STUDY: Investigate the effect of Rosuvastatin on serum level of Vaspin and Visfatin in patients with CAD and determine the association between those adipokines and the severity of CAD.
BACKGROUND: Statin treatment is considered one of the most effective strategies for the stabilization of atherosclerotic plaques and is related with enhancements in result in patients with coronary course diseases (CAD). Vaspin and Visfatin are adipokines involved in atherosclerosis progression. Objective: assess the impact of Rosuvastatin on serum level of Vaspin and Visfatin in patients with CAD and the relationship of those adipokines with seriousness of CAD.
SETTING: Cardiology department, Tanta University Hospital, Egypt. METHODS: 80 patients who underwent coronary angiography due to symptoms of stable angina were enrolled in the study. 40 patients received Rosuvastatin 20 mg/day was compared to another 40 patients, as a control group, who refused statin treatment and preferred lifestyle modifications intervention. Main outcome measure: Clinical parameters, lipid profile, troponin I, high-sensitivity C-reactive protein (hs-CRP), Vaspin and Visfatin levels were assayed at the beginning and after 3 months. CAD severity was assessed by the Gensini score.
RESULTS: Rosuvastatin administration considerably ameliorated most lipid parameters. Moreover, a significant increase of Vaspin and decrease of Visfatin concentrations (p<0.001) after Rosuvastatin treatment were observed (from 2.81 ± 0.74 ng/ml to 4.26 ± 0.76 ng/ml, from 4.62 ± 0.76 to 3.13 ± 0.98 respectively). There was a negative correlation between Vaspin and the Gensini score and positive correlation between Visfatin and Gensini score (r=-0.18 and r=0.19, p=0.05, respectively).
CONCLUSION: Rousvastatin treatment reduces serum visfatin and increase serum vaspin levels in CAD patients. Those effects of Rousvastatin are of clinical importance.
Keywords
Vaspin; Visfatin; Rosuvastatin; Gensini score; Coronary Artery Disease
Abbreviations
CAD: Coronary Artery Disease; ELISA: Enzyme-Linked Immunosorbent Assay; TCH: Total Cholesterol; LDL-C: Low Density Lipoprotein-Cholesterol; HDL-C: High Density Lipoprotein-Cholesterol; HMG-CoA reductase: 3-Hydroxy-3-methylglutaryl coenzyme A reductase; TGs: Triglycerides
Coronary heart disease (CHD) is the prominent cause of death worldwide and the main reason for coronary artery disease, cardiovascular diseases, and stroke is hypercholesterolemia [1]. Statins are the most broadly utilized cholesterol-lowering agents. They reduce lipid levels in patients with dyslipidemia, cardiovascular disease and atherosclerosis, furthermore decrease morbidity and mortality [2,3]. Over the previous few years’ lipidindependent actions are attributed to this class of drugs progressing their antiatherosclerotic role further than lipid lowering i.e., pleiotropic – mechanisms [4]. The question is whether or not the pleiotropic anti-inflammatory effects of statins are due to the modification of serum adipokines or not. In this perspective, contradictory results have been shown by previous studies that tested the effects of statins on adipokines [5]. Visfatin is an adipokine which mostly found in visceral adipose tissue [6]. Visfatin, has role in plaque destabilization, the promotion of angiogenesis, and glucose homeostasis, so it may have a role in atherosclerosis and coronary artery disease [7,8]. Visfatin seems to be a direct contributor to vascular inflammation and a distinctive attribute of atherothrombotic diseases linked to metabolic disorders [7]. Vaspin is another member of adipokines which eventually identified in visceral white adipose tissues [9]. It has anti-inflammatory and anti-apoptotic effects on vascular cells as well as improving insulin resistance. Vaspin could impede inflammatory factor secretion from vascular smooth muscle cells and counteract endothelial cell apoptosis induced by free fatty acid [10]. Rosuvastatin is a commonly used HMGCoA reductase inhibitor (statin) and is used to improve lipid profiles in patients with coronary artery diseases [11].
Research Methodology
Patient population and study design
A non-randomized controlled, prospective study conducted on 80 Patients (Figure 1), who underwent coronary angiography due to symptoms of stable angina at Cardiology department, Faculty of Medicine, Tanta University Hospital from January 2017 to January 2018. Stable angina defined as the presence of chest pain that did not change its pattern during the preceding 2 months.
For all patients, cardiovascular history was taken, complete physical examination was performed. Electrocardiogram and echocardiography were done then any abnormalities were recorded. Patients with a history of acute coronary syndrome within the past 6 months, severe chronic heart failure (NYHA class III–IV), cardiomyopathy, diabetes mellitus, morbid obesity (BMI >35), history of revascularization, malignant disease, major trauma or surgery, renal insufficiency (creatinine >2 mg/dl), liver impairment (ALT >2 times upper normal limit), acute or chronic infectious disease, or any kind of immune-mediated disease were excluded from the study. According to the study design 61 patients completed the study. 31 patients received Rosuvastatin 20 mg/d for three months was compared to 30 patients who refused to take statin medications, and preferred lifestyle modifications intervention (i.e., adherence to a heart-healthy diet, regular exercise habits, avoidance of tobacco products, and maintenance of a healthy weight). Both patient groups were homogeneous and followed up for three months.
Blood assays
After an overnight fasting, 10 ml venous blood samples were withdrawn from the antecubital vein under complete aseptic condition before coronary angiography. The collected blood samples were centrifuged then the separated serums were stored at -80º C until measurement of biochemical parameters. Serum Vaspin and Visfatin were measured by an ELISA kit for quantitative determination (GLORY Science, USA). Total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were assessed by the enzymatic colorimetric method using Roche/Hitachi/911 automated Clinical Chemistry Analyzer. The Kit manufactured by Roche Diagnostics gmbh, D-68298 Mannheim, USA. The serum Troponin-I was assayed using ELISA kit (Biocheck, IncFoster CityCalifornia). Highly Sensitive-C-Reactive Protein was determined by ELIZA method [Labor Diagnostika Nord-Nordhorn-Germany] kits. Routine determination of random blood glucose and creatinine level was performed.
Coronary angiography
Diagnostic coronary angiography was done to all patients through either femoral or radial approach. Gensini score was used to assess the severity of CAD by an experienced cardiologist, unaware of the biochemical results of the patients. Gensini score was calculated through multiplication of score used for grading the luminal narrowing of the main coronary artery by a factor which was take into account the site and importance of the lesion. The score of luminal narrowing was 1 for ≤ 25% stenosis, 2 for 26-50% stenosis, 4 for 51-75% stenosis, and 8 for 76-90% stenosis, 16 for 91-99% stenosis and 32 for total occlusion. The factor of location was 5 for left main, 2.5 for the proximal lesion of either LAD (left anterior descending) or LCX (left circus flex), 1.5 for mid lesion, 1 for distal LAD, mid-distal LCX or RCA (right coronary artery). Then the sum of scores of all coronary arteries was used to express the total Gensini score [12].
Statistical Analysis
Variables were presented as mean ± SD. Comparisons between two groups were carried out using an independent sample t-test. Correlation analysis using the Pearson coefficient of correlation was performed. p-value< 0.05 will consider significant. SPSS for windows software was used for statistical analysis (Version 22, SPSS Inc., Chicago, IL, USA).
Results
The baseline clinical characteristics and biochemical parameters of the studied groups were presented in Table 1.
| Characteristics | Control Group n=30 | Rosuvastatin Group n=31 | p-value |
|---|---|---|---|
| Age (years) | 44.67 ± 5.86 | 44.32 ± 4.70 | 0.4 |
| Gender (F/M) | 10/20 (50%) | 10/21 (47.62%) | NS |
| BMI (kg/m2) | 31.05 ± 1.54 | 31.53 ± 1.40 | 0.1 |
| Systolic BP (mmHg) | 133.23 ± 7.73 | 134.87 + 6.67 | 0.19 |
| Diastolic BP (mmHg) | 81.37 ± 3.19 | 82.10 ± 4.04 | 0.22 |
| Total Cholesterol (mg\ dl) | 252.15 +15.14 | 254.16 +12.70 | 0.58 |
| LDL-C (mg/dl) | 180.18 ± 23.95 | 182.72 + 22.77 | 0.67 |
| HDL-C (mg/dl) | 33.89 + 1.49 | 33.55 ± 1.32 | 0.35 |
| Triglyceride | 171.35 + 12.55 | 172.04 + 13.74 | 0.84 |
| Troponin I (ng/ml) | 1.46 ± 0.21 | 1.41 ± 0.12 | 0.3 |
| hs-CRP (mg/ml) | 10.33 ± 1.36 | 10.12 ± 1.64 | 0.59 |
| Gensini Score | 12.93 ± 1.34 | 12.81 ± 2.01 | 0.78 |
| Visfatin | 4.77 ± 0.59 | 4.62 + 0.76 | 0.38 |
| Vaspin | 2.87 ± 0.60 | 2.81 ± 0.74 | 0.73 |
TABLE 1: Baseline clinical characteristics and biochemical parameters of patients in the control and Rosuvastatin Group
The two groups had similar baseline characteristics, including gender, age, body mass index, systolic and diastolic blood pressure, lipid profiles, Troponin I, hs-CRP, Vaspin levels, Visfatin level and Gensini score (p>0.05). Results revealed that serum Vaspin level increased significantly after 3 months of treatment with a significant difference when comparing control group to Rosuvastatin group. On the other hand, serum Visfatin concentrations significantly decreased after 3 months of treatment when comparing the control patients to the patients received Rosuvastatin treatment.
Serum hs-CRP, Troponin I levels, TCH, LDL and TG levels were significantly decreased in patients who received Rosuvastatin treatment compared with control group, with a significant difference when comparing control and treated groups. Serum HDL level exhibited insignificant increase in both groups. The mean angiographic Gensini scores significantly decreased in patients received Rosuvastatin treatment compared with control group (Table 2).
| Group/Parameters | Control Group n=30 | Rosuvastatin Group n=31 | p-value |
|---|---|---|---|
| Total Cholesterol (mg\ dl) | 251.98 ± 15.54 | 226.84 ± 20.27*# | <0.001 |
| LDL-C (mg/dl) | 184.36 ± 15.42 | 163.50 ± 21.76*# | <0.001 |
| HDL-C (mg/dl) | 33.79 ± 1.38 | 34.08 ± 1.21 | 0.4 |
| Triglyceride | 169.16 ± 14.44 | 146.29 ± 19.82*# | <0.001 |
| Troponin I (ng/ml) | 1.43 ± 0.18 | 1.34 ± 0.18 | 0.07 |
| Hs-CRP (mg/ml) | 10.10 ± 1.06 | 9.31 ± 1.32*# | 0.012 |
| Gensini Score | 12.90 ± 1.31 | 11.79 ± 1.93*# | 0.011 |
| Visfatin | 4.74 ± 0.57 | 3.13 ± 0.98*# | <0.001 |
| Vaspin | 2.84 ± 0.62 | 4.26 ± 0.76*# | <0.001 |
TABLE 2: Patient’s clinical characteristics and biochemical parameters after 12 weeks of treatment
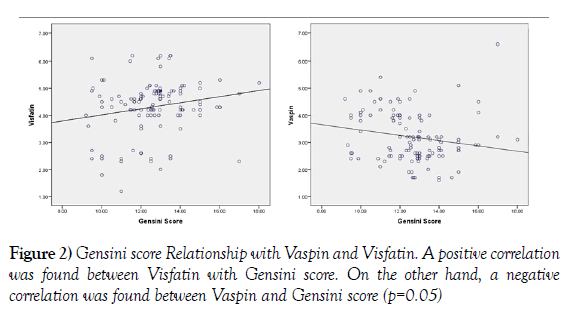
A positive correlation was found between Visfatin with Gensini scores (Figure 2), hs-CRP, Troponin I, TC, LDL and TGs by Pearson’s correlation (2-tailed).
On the other hand, a negative correlation was found between Visfatin and Vaspin. A negative correlation was found between Vaspin and Gensini scores, hs-CRP, TC, LDL and TGs. A significant positive correlation was observed between Vaspin with HDL (Table 3).
| Variables | LDL | HDL | Triglyceride | Troponin I | hs-CRP | Gensini Score | Visfatin | Vaspin |
|---|---|---|---|---|---|---|---|---|
| Total Cholesterol | 0.76** | 0.04 | 0.29** | 0.29** | 0.20* | 0.31** | 0.50** | -0.46** |
| LDL-C | -0.001 | 0.06 | 0.29** | 0.15 | 0.25** | 0.29** | -0.31** | |
| HDL-C | -0.20* | -0.10 | 0.17 | -0.06 | -0.03 | 0.25** | ||
| Triglyceride | 0.18* | 0.19* | 0.04 | 0.42** | -0.36** | |||
| Troponin I | 0.03 | 0.14 | 0.21* | -0.12 | ||||
| hs-CRP | 0.16 | 0.18* | -0.24** | |||||
| Gensini Score | 0.19* | -0.18* | ||||||
| Visfatin | -0.45** | |||||||
TABLE 3: Correlation between measured Vaspin, Visfatin, lipid profile, Troponin I, hs-CRP and Gensini score parameters in Rosuvastatin group (n=31) and control group (n=30)
Discussion
The present study showed that treatment with Rosuvastatin increased Vaspin serum levels in patients compared to control groups. In agreement with a recent study stated that Rosuvastatin significantly increases vaspin serum levels in acute coronary syndrome [13]. On the other hand, a reduced Visfatin serum levels were observed in patients treated with Rosuvastatin compared to control groups. Consistence with a meta-analysis found that visfatin levels slightly reduced after statin therapy [14].
Furthermore, the present results showed a negative correlation of Vaspin with severity of CAD which assessed using Gensini score. In agreement with the present results, Kadoglou et al. [15] revealed a decreased Vaspin serum levels in asymptomatic patients with CAD compared with healthy control subjects. Moreover, they observed that low circulating vaspin concentrations were significantly correlated with CAD severity. In addition, Kobat et al. [16] found that, serum Vaspin levels significantly lower in patients with CAD consistently with our result. So, it may be used as a prognosticator of this disease However, they did not define the correlation of Vaspin and its relation to the severity of CAD. Moreover, Li et al. [17] studied the association of Vaspin gene polymorphisms with CAD in Chinese population. Their results showed that the serum Vaspin levels and risk for CAD. On the other hand, Aust et al. [18] could not found any relation between serum Vaspin levels and severity of atherosclerosis but they demonstrated that lower Vaspin serum levels had correlation with recent ischemic events in patients with carotid artery stenosis. Accordingly, they assumed that this biomarker may have a protecting role in patients with CAD. On the other hand, our results revealed a significant positive correlation between Visfatin serum levels with severity of CAD which assessed using Gensini score. In agreement with our result, Fu et al. [19] found a significant positive correlation between plasma Visfatin level and coronary lesion severity score. They concluded that there is a link between Visfatin level and the pathogenesis of CAD, so the detection of this adipokine might be useful for early diagnosis of CAD. In addition, Liu et al. [20] found that, plasma Visfatin levels were significantly higher in chronic CAD and acute coronary syndromes compared with control patients. The present study also found a significance decrease in total cholesterol and triglyceride LDL-cholesterol levels in patients received Rosuvastatin for three months compared to control group. In agreement with a recent study stated that treatment with Rosuvastatin displays a high efficacy in the improvement of lipid profile, and its pleiotropic effects (anti-inflammatory, antioxidant and antithrombotic), represents a fundamental role for cardiovascular primary and secondary prevention [21].
The present study revealed that Rosuvastatin therapy significantly decrease the serum level of hs-CPR level compared with control. This effect could be associated with the ability of statins to block pro-inflammatory transduction pathways [22]. Pervious study assessed the effect of statin on inflammatory markers in chronic heart failure patients demonstrated that significant decrease in hsCPR level was associated with statin therapy [13,23]. The present study found a significant negative correlation between hsCPR serum levels with Vaspin serum levels. Consistently, Seeger et al. [24] found a negative relationship between Vaspin and CRP in patients on chronic hemodialysis and Kadoglou et al. [15] found an independent association of reduced Vaspin with increased hs-CRP and Visfatin levels suggesting a protective mechanism. Previous studies also, have indicated that CRP is an independent risk factor for atherosclerotic cardiovascular disease [25].
The present study also revealed that serum TroponinI levels, as indicator of myocyte injury, were decreased after Rosuvastatin treatment but insignificant difference was found when compared Rosuvastatin group with control group (p=0.07). Consistently, Everett et al., found no difference in high-sensitivity cardiac troponin I concentrations after Rosuvastatin 20 mg/d or control [26]. Conversely, with the present results a recent study conducted on rats by Zhou et al. [27] showed that Rosuvastatin (5 mg/kg) significantly reduced the increased serum content of cardiac troponin I. The present study showed that Rosuvastatin treatment reduce serum Visfatin levels and increase serum Vaspin level, that may represent an anti-atherogenic property of the drug, which is independent of its lipid-lowering capability.
Conclusion
Rousvastatin treatment rather than lifestyle modification reduce serum visfatin and increase serum vaspin levels in CAD patients. Those pleiotropicassociated effects of Rousvastatin are of clinical importance. Moreover, low vaspin and high visfatin levels were significantly correlated with CAD severity suggesting a relation between atherosclerosis and adiposity.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics Approval
The study was approved by the ethical committee of Tanta University. All patients were informed about the study, and their written consent forms were obtained.
Acknowledgements
We thank Dr Naglaa Khedr, Assistant Professor of Biochemistry, Faculty of pharmacy, Tanta University, for her collaboration in experimental work.
REFERENCES
- Kones R. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des Devel Ther 2011;5:325-80.
- Kotlega D, Ciecwiez S, Turowska-Kowalska J, et al. Pathogenetic justification of statin use in ischaemic stroke prevention according to inflammatory theory in development of atherosclerosis. Neurol Neurochir Pol 2012;46(2):176-83.
- Wright RS, Murphy JG, Bybee KA, et al. Statin lipid-lowering therapy for acute myocardial infarction and unstable angina: efficacy and mechanism of benefit. Mayo Clin Proc 2002;77(10):1085-92.
- Kostapanos MS, Liberopoulos EN, Goudevenos JA, et al. Do statins have an antiarrhythmic activity? Cardiovasc Res 2007;75:10-20.
- Sahebkar A. Head-to-head comparison of fibrates versus statins for elevation of circulating adiponectin concentrations: A systematic review and meta-analysis, Metabolism 2013;62(12):1876-85.
- Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005;307(5708):426-30.
- Romacho T, Azcutia V, Vazquez-Bella M, et al. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia 2009;52(11):2455-63.
- Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 2007;115:972-80.
- Hida K, Wada J, Eguchi J, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci 2005;102(30):10610-5.
- Jung CH, Lee WJ, Hwang JY, et al. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem Biophys Res Commun 2011;413(2):264-9.
- Phalitakul S, Okada M, Hara Y, et al. Vaspin prevents TNF-alpha-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-kappaB and PKCtheta activation in cultured rat vascular smooth muscle cells. Pharmacol Res 2011;64(5):493-500.
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51(3):606.
- Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome. Diseases 2018;16;6(1):9.
- Sahebkar A, Giorgini P, Ludovici V, et al. Impact of statin therapy on plasma resistin and visfatin concentrations: A systematic review and meta-analysis of controlled clinical trials. Pharmacol Res 2016;111:827-37.
- Kadoglou NP, Gkontopoulos A, Kapelouzou A, et al. Serum levels of vaspin and visfatin in patients with coronary artery disease-Kozani study. Clin Chim Acta 2011;412(1-2):48-52.
- Kobat MA, Celik A, Balin M, et al. The investigation of serum vaspin level in atherosclerotic coronary artery disease. J Clin Med Res 2012;4(2):110-3.
- Li HL, Peng WH, Cui ST, et al. Vaspin plasma concentrations and mRNA expressions in patients with stable and unstable angina pectoris. Clin Chem Lab Med 2011;49(9):1547-54.
- Aust G, Richter O, Rohm S, et al. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis 2009;204(1):262-266.
- Fu H, Zhu Y, You GY, et al. Detection of visfatin level of plasma in patients with coronary artery diseases. Sichuan Da Xue Xue Bao Yi Xue Ban 2009;40(2):322-4.
- Liu SW, Qiao SB, Yuan JS, et al. Association of plasma visfatin levels with inflammation, atherosclerosis and acute coronary syndromes (ACS) in humans. Clin Endocrinol (Oxf) 2009;71(2):202-7.
- Cortese F, Gesualdo M, Cortese A, et al. Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol Res 2016;107:1-18.
- Castro PF, Miranda R, Verdejo HE, et al. Pleiotropic effects of atorvastatin in heart failure: role in oxidative stress, inflammation, endothelial function, and exercise capacity. J Heart Lung Transplant 2008;27(4):435-41.
- Lei Z, Shuning Z, Hong J, et al. Effects of statin therapy on inflammatory markers in chronic heart failure: A Metaanalysis of randomized controlled trials. Archives of Medical Research 2010;41:464-71.
- Seeger J, Ziegelmeier M, Bachmann A, et al. Serum levels of the adipokine vaspin in relation to metabolic and renal parameters. J Clin Endocrinol Metab 2008;93(1):247-51.
- Wang X, Zhao X, Li L, et al. Effects of combination of ezetimibe and rosuvastatin on coronary artery plaque in patients with coronary heart disease. Heart Lung Circ 2016;25(5):459-65.
- Everett BM, Zeller T, Glynn RJ, et al. High-sensitivity cardiac troponin I and B-type natriuretic Peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation 2015;131(21):1851-60.
- Zhou R, Ma P, Xiong A, et al. Protective effects of low-dose rosuvastatin on isoproterenol-induced chronic heart failure in rats by regulation of DDAH-ADMA-NO pathway. Cardiovasc Ther 2017;35(2).