Efficacy of administration of oral liposomal iron after a period of intravenous iron carboxymaltose in two groups of chronic kidney disease patients: home hemodialysis and conservative therapy
2 Department Pathology and Clinical Laboratories AUO Novara, Italy
3 Nephrology and Dialysis Unit Ospedale di Arezzo, Tuscany, Italy
Received: 16-May-2018 Accepted Date: Jun 11, 2018; Published: 18-Jun-2018
Citation: Duranti E, Duranti D, Panza F, Ralli C. Efficacy of administration of oral liposomal iron after a period of intravenous iron carboxymaltose in two groups of chronic kidney disease patients: home hemodialysis and conservative therapy. Clin Nephrol Res. 2018;2(1):33-35.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Anemia could be a frequent and early complication of Chronic nephropathy (CKD), and its prevalence will increase with the worsening of nephritic perform, involving over five hundredth of patients in predialysis (stage 5) and much nearly one hundred of patients in dialysis [1]. The administration of oral or blood vessel iron and glycoprotein (Epo) could be a key part for the correction of anemia each in patients with CKD and in patients on chronic dialysis. The aim of our work was to verify the effectiveness of oral liposomal iron in a very cluster of our home dialysis patients and in a very cluster of patients in conservative medical aid with CKD, examination it with a previous amount in blood vessel medical aid with carboxymaltose iron. Materials and methods: We screened 8 patients (5 F/3 M; age 62 ± 10 yrs) in chronic daily home hemodialysis for more than 12 months and 16 patients (9 F/7 M; age 75 ± 12 yrs) in conservative therapy, affected by CKD stage 3b-4, who were on treatment for a sideropenic anemia. All had been treated for at least 6 months, with carboxymaltose iron iv (500 mg/ month) and therefore the last hematological control was considered time 0. From that moment on, they were then passed to oral liposomal iron. The protocol lasted 6 months for home hemodialysis pts and 12 months for CKD pts. During these two periods, serum iron, serum Hb, Ferritin, Transferrin Saturation, CRP, Albumin, weekly consumption of erythropoietin were evaluated bimonthly. Statistical analysis was performed with Student's t test for paired data (Table 1). Results: Switching to the os iron periods, both in the group in dialysis that in conservative, compared to the end of the iv iron period, there was an improvement, even if not statistically significant, of the concentration of Hb and a reduction of CRP values. Ferritin, transferrin saturation, Albumin and weekly consumption of Erythropoietin did not change in the two pts groups.7 out of 16 CKD patients, throughout the period, did not use erythropoietin. No patient reported any side effects during intravenous or liposomal iron treatment and none has discontinued therapy.
Keywords
Anemia, chronic kidney disease, hemodialysis.
Introduction
Anemia is a frequent and early complication of Chronic Kidney Disease (CKD), and its prevalence increases with the worsening of renal function, involving over 50% of patients in predialysis (stage 5) and practically almost 100% of patients in hemodialysis [1]. The anemic state depends on an inadequate production of erythropoietin, however a fundamental importance is represented by the alterations of the martial state or due to an iron deficiency, as a consequence of inadequate intestinal absorption, or due to reduced bioavailability, linked to the systemic inflammatory state, characteristic of these patients or for uremic toxicity [2]. The administration of oral or intravenous iron and erythropoietin (Epo) is a key element for the correction of anemia both in patients with CKD and in patients on chronic hemodialysis [3,4]. The martial therapy, administered orally, whose intestinal absorption is for 15%-20% of the administered dose, is preferred in patients in the conservative phase, but presents frequent side effects especially of gastrointestinal type. In contrast, hemodialysis patients use almost exclusively the intravenous route that can promote even serious allergic phenomena, and can lead to an increase in the systemic inflammation with consequent functional anemia due to reduced use of the iron by the marrow [3,4]. The possibility of having a particular oral iron preparation, the liposomal iron, based on ferric pyrophosphate carried within a phospholipid membrane, appears to have a lower incidence of gastrointestinal side effects, without increasing the inflammation of the patient [5]. The aim of our work was to verify the efficacy of oral liposomal iron in a group of our home hemodialysis patients and in a group of patients in conservative therapy with CKD, comparing it with a previous period in intravenous therapy with carboxymaltose iron.
Materials and Method
We screened 8 patients (5 F/3 M; age 62 ± 10 yrs) in chronic daily home hemodialysis for more than 12 months and 16 patients (9 F/7 M; age 75 ± 12 yrs) in conservative therapy, affected by CKD stage 3b-4, who were on treatment for a sideropenic anemia: Hb<11 g/dl, Transferrin saturation <20% and ferritin <100 mcg/l. Patients with hereditary anemias, neoplasms, autoimmune diseases were excluded. All had been treated for variable periods, but not less than 6 months, with carboxymaltose iron iv (500 mg/month) and therefore the last haematological control was considered time 0. From that moment on, they were then passed to oral liposomal iron+vit C (30 mg/day, PL Pharma Italia). The protocol lasted 6 months for home hemodialysis pts and 12 months for CKD pts. During these two periods, serum iron, serum Hb, Ferritin , Transferrin Saturation, CRP, Albumin, weekly consumption of erythropoietin were evaluated bimonthly. Statistical analysis was performed with Student's t test for paired data.
Results
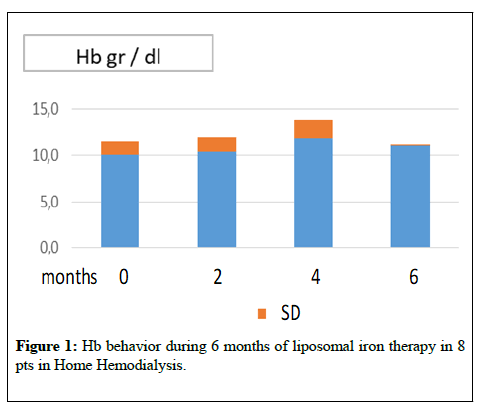
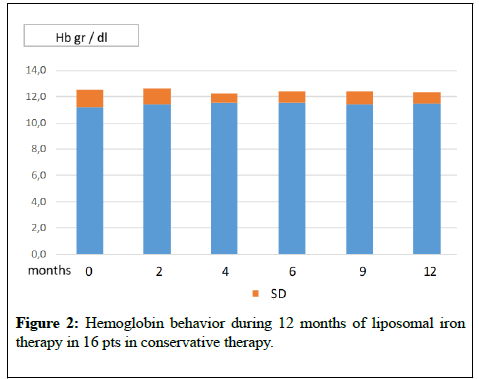
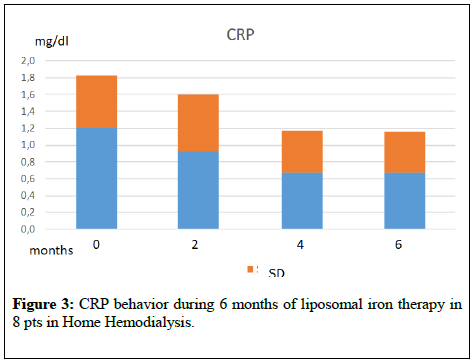
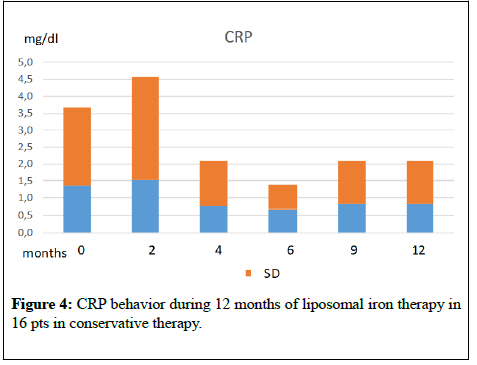
The graphs of (Figures 1-4) show the behaviour of Hb and CRP of the two patients groups during the two periods of treatment. From the mean values it was found that switching to the os iron periods, both in the group in dialysis that in conservative, compared to the end of the iv iron period, there was an improvement, even if not statistically significant, of the concentration of Hb and a reduction of CRP values. Ferritin, transferrin saturation and Albumin (Table 1,2) did not change in the two pts groups. Weekly consumption of Erythropoietin did not change in the two groups, however we emphasize that 7 out of 16 CKD patients, throughout the period, did not use erythropoietin. No patient reported any side effects during intravenous or liposomal iron treatment and none has discontinued therapy.
| Months | 0 | 2 | 4 | 6 |
| Serum Iron | 72.5 ± 20.9 | 65.5 ± 31.5 | 60.3 ± 25.7 | 63.9 ± 29.7 |
| Trsf. Sat % | 22.3 ± 10.8 | 20.3 ± 11.6 | 18.3 ± 13.3 | 19.3 ± 11.6 |
| Ferritin ng/ml | 159 ± 183 | 88.3 ± 90.8 | 62.3 ± 52.7 | 63.3 ± 53.8 |
| Albumin gr/dl | 4.2 ± 0.3 | 4.13 ± 0.3 | 4.30 ± 0.3 | 4.13 ± 0.2 |
| Epo/week IU | 7500 ± 3000 | 7500 ± 3000 | 7500 ± 3000 | 7500 ± 3000 |
Table 1: Iron, Transferrin saturation, Ferritin, Albumin and Epo/ week consumption during 6 months on oral liposomal iron, regarding 8 home hemodialysis pts.
| Months | 0 | 2 | 4 | 6 | 9 | 12 |
| Serum Iron mcg/dl | 52.9 ± 18.8 | 52.7 ± 24.1 | 63.9 ± 19.1 | 56.4 ± 19.1 | 55.3 ± 16.8 | 55.3 ± 17.6 |
| Trsf. sat % | 16.2 ± 7.3 | 16.6 ± 8.6 | 22.0 ± 9.2 | 18.5 ± 8.2 | 18.7 ± 6.3 | 18.7 ± 6.5 |
| Ferritin ng/ml | 130 ± 115 | 181 ± 190 | 170 ± 127 | 153 ± 103 | 147 ± 97 | 153 ± 95 |
| Albumin gr/dl | 3.5 ± 0.6 | 3.5 ± 0.6 | 3.7 ± 0.5 | 3.7 ± 0.4 | 3.6 ± 0.5 | 3.7 ± 0.4 |
| Epo/week IU | 3067 ± 3693 | 3333 ± 3903 | 3333 ± 3903 | 3333 ± 3903 | 3333 ± 3903 | 3333 ± 3903 |
| number of no Epo pts | 7 | 7 | 7 | 7 | 7 | 7 |
Table 2: Iron, Transferrin saturation, Ferritin, Albumin and Epo /week consumption during 12 months on oral liposomal iron, regarding 16 CKD pts, stage 3b–4. 7 pts did not receive Epo during the observation period.
Figure 1: Hb behavior during 6 months of liposomal iron therapy in 8 pts in Home Hemodialysis.
Figure 3: CRP behavior during 6 months of liposomal iron therapy in 8 pts in Home Hemodialysis.
Figure 4: CRP behavior during 12 months of liposomal iron therapy in 16 pts in conservative therapy.
Discussion
In patients with conservative CKD oral iron administration is preferred, but sometimes for intestinal malabsorption or the appearance of side effects, such as abdominal pain, gastralgia, nausea, vomiting, diarrhea, we are forced to pass to the administration via intravenous [6]. In patients on chronic hemodialysis, intravenous iron is preferred for practical reasons. However, allergic phenomena may occur up to severe anaphylactic reactions, potential cytotoxicity, hepatic disease with iron accumulation in various forms of hereditary hemochromatosis, an increased risk of developing cirrhosis with levels of ferritin >1000 ng/ml and an increase in the systemic inflammation with a decrease in antioxidant defenses [7]. The increased production of inflammatory markers, such as CRP, IL-6, TNF-alpha, promotes the release of hepcidin, a protein produced by the liver that acts by binding to another protein, called ferroportin, which regulates the escape of iron from cells, blocking the passage of iron from cells to blood resulting in functional iron deficiency, so-called inflammatory anemia [8,9]. Furthermore, recently they have been emphasized the medico-legal issues related to intravenous iron administration: a recent note dated 25 October 2013 from the Italian Medicines Agency (AIFA) underlined the risk of intravenous administration with potentially fatal reactions, especially in patients with known allergies and in patients with inflammatory diseases, including the immune system, as well as in patients with asthma, eczema, atopic allergies [10]. Therefore, according to these notes, hemodialysis patients undergoing intravenous therapy should be monitored closely during and at least 30 min after administration in the presence of a doctor, in addition to the nursing staff, and all this could create organizational problems of considerable complexity especially if such indications should be extended to the Dialysis Centers for limited and / or decentralized assistance, where the presence of the doctor is notoriously circumscribed at times that do not cover all the dialysis sessions of the day. Although on one hand, among the various preparations, iron gluconate and carboxymaltose seem to be the one with fewer side effects, the Work Group KDIGO 2012 did not show a definite benefit of the intravenous route compared to the oral [1]. On the other hand, as already mentioned, the use of oral iron has not had much development until now due to its low efficacy and to the gastro enteric side effects linked to the compound which usually contains iron or iron sulphate, which has made it can be used only for a limited range of patients, certainly not affected by Chronic Renal Insufficiency, in which the incidence of gastritis and gastralgia is constant [6]. Recently the possibility of having a particular oral iron preparation, the liposomal iron, based on ferric pyrophosphate carried within a phospholipid membrane, showed a lower incidence of gastrointestinal side effects, thanks to the liposomal microencapsulation, for which iron does not come into contact with mucous membranes with better intestinal absorption bypassing the block induced by hepcidin, moreover the absorbed pyrophosphate iron has a greater affinity for the transferrin and is directly transferred to the bone marrow [5]. Our work, even if performed on a small number of hemodialysis patients and in conservative therapy, has shown an equivalent efficacy, compared to intravenous iron, with the maintenance and in many cases the increment of hemoglobin values and Erythropoietin dosages. These results confirm previously performed nephrological work on patients on conservative treatment [10-12]. A particularly interesting aspect is the reduction of PCR, although not statistically significant, during treatment with liposomal iron, which is explained by a lower activation / production of inflammatory markers that increase with the use of intravenous iron through the production of the species reactive oxygen that exacerbate systemic inflammation, with decreased antioxidant defenses and increased TNF and IL-6 release [13]. The absence of these effects in the case of the use of liposomal iron also explains the maintenance of weekly doses of erythropoietin, recalling that 7 patients out of 16 in conservative therapy, throughout the period, did not use erythropoietin and that this has certainly contributed to a reduction in costs for the use of liposomal iron. Another argument in favor of liposomal iron is the reliability of oral iron also on the appearance of its intestinal absorption in an almost constant percentage (20%) compared to the intravenously administered iron of which the percentage of use is not certain, with an amount that it certainly precipitates at the tissue level, once the transferrin is saturated.
Conclusion
In conclusion, our experience demonstrates the possibility of replacing intravenous iron administration with liposomal oral iron, well tolerated, effective, and with significant economic savings. Furthermore, we would like to underline the important relapse also on the medico-legal level, due to the lower clinical risk in the use of liposomal oral iron compared to intravenous iron.
REFERENCES
- Locatelli F, Nissenson AR, Barrett BJ, et al. KDIGO clinical practice guidelines for anemia in chronic kidney disease. Kidney Int. 2008;74:1237-40.
- Bregman DB, Morris D, Koch TA, et al. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88:97-101.
- Taylor JE, Peat N, Porter C, et al. Regular low-dose intravenous iron therapy improves response to erythropoietin in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1079-83.
- Del Vecchio L, Cavalli A, Tucci B, et al. Chronic kidney diseaseassociated anemia: new remedies. Curr Opin Investig Drugs. 2010 Sep;11:1030-38.
- Visciano B, Nazzaro P, Tarantino G, et al. Il ferro liposomiale: una nuova proposta per il trattamento dell’anemia nell’insufficienza renale cronica. G Ital Nefrol. 2013;30.
- Auerbach M, Goodnough LT, Picard D, et al. The role of intravenous iron in anemia management and transfusion avoidance. Transfusion. 2008;48:988-1000.
- Zager RA, Johnson AC, Hanson SY. Parenteral iron nephrotoxicity: potential mechanisms and consequences. Kidney Int. 2004;66:144-56.
- Zager RA, Johnson AC, Hanson SY. Parenteral iron therapy exacerbates experimental sepsis. Kidney Int. 2004;65:2108-12.
- Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378-82
- Van Wyck DB, Roppolo M, Martinez CO, et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68:2846-56.
- Van Wyck DB, Roppolo M, Martinez CO, et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68:2846-56.
- Charytan C, Qunibi W, Bailie GR, et al. Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin Pract. 2005;100:55-62.
- Agarwal R, Rizkala AR, Bastani B, et al. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26:445-54.