Electronic structure of carbon nanotubes interacting with Cu and Li atoms
2 South-West State University, 94, 50 let Oktyabrya st, 305040 Kursk, Russia, Email: Kuzrsquo@mail.ru
Received: 25-Jan-2018 Accepted Date: Jan 31, 2018; Published: 10-Feb-2018
Citation: Zavodinsky VG, Kuz’menko AP. Electronic structure of carbon nanotubes interacting with Cu and Li atoms. J Mater Eng Appl 2018;1(1): 14-18.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The density functional theory within the Kohn-Sham approach, the pseudopotential method, and sets of plane waves are using to compare the electronic structures of single-wall carbon nanotubes (SWCNT) interacting with Cu and Li atoms. It has been found that neutral Cu+SWCNT and Li +SWCNT systems (armchair or zigzag) possess the metallic electronic states near the Fermi level. The charged (either negative or positive) Cu +SWCNT and Li+SWCNT armchair systems also have the metallic-like electronic structure. However the charged Cu+SWCNT and Li+SWCNT zigzag systems have energy gaps at the Fermi region; and the negative charged systems are more favorable energetically than the positive charged systems. The Cu-zigzag system has the gap of 1.2 eV; and the gap of the Li-zigzag is 1.7 eV. As the energy gap at the level of Fermi prevents leaving of a charge from the charged Cu+SWCNT and Li+SWCNT systems, the systems can work as negative electrodes for the power accumulator. Thus, the electrical charge can be effectively accumulated in these systems. Electrical characteristics of the Cu+SWCNT system are close to those of the Li+SWCNT system, and at the same time the Cu +SWCNT systems are more stable and cheaper than Li+SWCNT ones, thus they are perspective for production of new power sources.
Keywords
Copper; Lithium; Semiconductor; Nanotubes
Introduction
Lithium can react reversibly with most carbonaceous materials to some extent, therefore they can be used as negative electrodes in Lithium-ion batteries [1]. However, current of Lithium-ion batteries is limited by the formation of an oxidation layer over the anode, increasing the internal resistance over time and the fact that Lithium must bond to at least six carbon atoms, a detriment to miniaturization. Besides, Lithium is a rather expensive material.
Copper is cheaper than Lithium and less sensitive to oxidation. In works [2,3] formation of charged Cu-carbon composites was described. It has been found that Cu atoms can diffuse from a copper electrode through the nanostructured graphite material and bring some negative charge. This accumulated charge can be used as a source of electric power. In the works [2,3] it was reported that in the system “Cu-carbon (dispersed graphite)” accumulation of charge has been registered enough for many-weeks shine of a diode lamp.
There are many examples of study of the Li interaction with carbon nanotubes [4-13]. Recently a very interesting papers devoted to the interaction of Cu with carbon were published [14-16]. In particular, there were considered [16] Cun nanoparticles (n=4, 5, 6, 7, 13) and concluded that Cu-carbon materials may be useful for the alternative energetic.
Our paper is devoted to comparable study the influence of Cu and Li atoms interacting with single-wall carbon nanotubes (SWCNTs). The main attention is paid to calculation of the electronic structure and to studying of the charge accumulation at adsorption of metal atoms on the surface of nanotubes due to formation of the energy gap at the Fermi level.
Material and Methods
All calculations which are carried out in this work are executed by means of the widely known FHI96md [17] package based on the density functional theory [18] within the Kohn-Sham [19] approach, the method of pseudopotentials [20] and sets of plane waves. Pseudopotentials were generated by means of the FHI98pp [21] package, the exchange and correlative interactions were considered in the approximation of the generalized gradients [22]; the cutting energy of the plane wave set was equal to 40 Rydberg (544 eV), the number of k-points was used up to 21. Densities of states (DOS) were calculated as sums of Gaussians centered on electronic energy levels.
It is well known [23-26] that SWCNTs may be formed with atomic structures of different chiralities. The main ones of them are the armchair and zigzag structures. Armchair SWCNTs have a metallic-like electronic structure, while zigzag SWCNTs posses a semiconductor-like gap between occupied and unoccupied states. Other chiralities lead to intermediate types of the electronic structure.
In present work only armchair and zigzag SWCNTs were investigated. Diameters of tubes were taken of 0.69 nm for armchair and of 0.80 nm for zigzag cases. Calculations were carried out in periodic representation therefore the lengths of studied tubes can be considered as infinite. The numbers of carbon atoms included in calculations was 60 for armchair and 40 for zigzag cases.
Results and Discussion
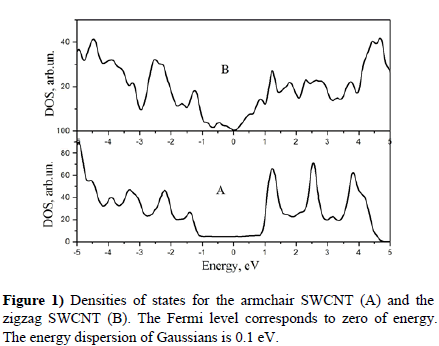
First of all we calculated the electronic structures of free armchair and zigzag SWCNTs. Results are presented in Figure 1.
Comparing of our plots with well-known published data [23-26], both theoretical and experimental, demonstrate that our methodic of calculation is rather correct and confidential. Namely, we obtained the constant density of states near the Fermi level for the armchair SWCNT in accordance with known published data [23-26]. In the zigzag case we have found the energy gap of 0.45 eV around the Fermi level; published data lie in the region of 0.47-0.52 eV [27].
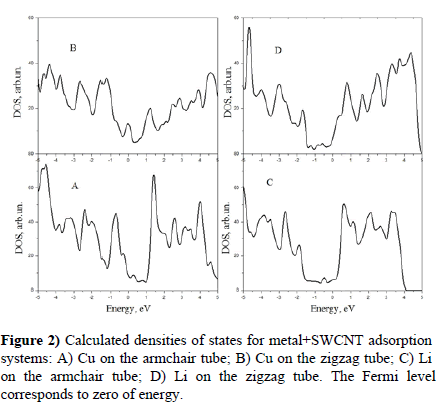
Then we studied adsorption of Cu and Li single neutral atoms on the walls of nanotubes. Atoms of copper were placed above surface C atoms and the equilibrium dCu-C distances of the systems were found of 0.178 nm for the armchair SWCNT and 0.177 nm for the zigzag SWCNT. For Li we have found the equilibrium positions above the hollow site with dLi-C=0.209 nm for the armchair SWCNT and dLi-C=0.225 nm for the zigzag SWCNT. Calculated densities of states are plotted in Figure 2.
Comparing Figures 1 and 2 we see that Lithium slightly changes metallic states of the armchair SWCNT forming a little peak under the Fermi level. However Li turns the semiconductor states of the zigzag SWCNT to the metallic ones. Copper disturbs the DOS of the zigzag SWCNT even more and also turning it to the metallic case. The copper adsorption on the armchair SWCNT also generates metallic states at the
Fermi level. Thus, any of cases, above studied, do not lead to emergence of energy gaps between occupied and unoccupied states.
The binding energies Eb of the neutral Cu and Li atoms absorbed on the surfaces of SWCNTs are collected in Table 1.
| Studied systems | Cu-armchair | Cu-zigzag | Li-armchair | Li-zigzag |
|---|---|---|---|---|
| Eb, eV | 0.4 | 0.76 | 1.21 | 1.33 |
Table 1: The binding energies Eb of the neutral Cu and Li atoms absorbed on the surfaces of SWCNTs.
Values of Eb were calculated as,
Eb=E(M+SWCNT)-E(M)-E(SWCNT)
Where, E(M+SWCNT) is the energy of a SWCNT with an adsorbed atom of metal (M=Cu or Li), E(M) is the energy of a single atom of metal, E(SWCNT) is the energy of a free SWCNT.
It is followed from Table 1 that neutral atoms of Cu and Li willingly join carbon nanotubes. However, the Li+SWCNT bonds are stronger than those of the Cu+SWCNT system.
Charged systems
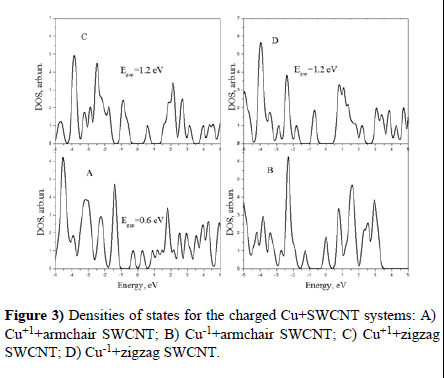
We have repeated the above described calculations, adding one electron to M+SWCNT systems (or taking it away from them). In the first case we received systems with a negative charge, in the second case with positive. Results of calculations are presented in Figures 3 and 4.
One can see that in all cases (except the negative armchair Cu-1+SWCNTsystem there is the energy gap in the Fermi level region.
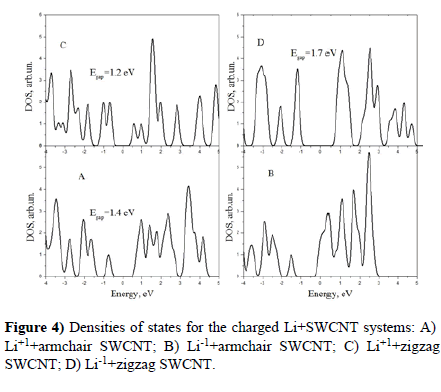
Densities of states for the charged Li+SWCNT systems are presented in Figure 4.
We see that influence of charged Lithium on the electronic structure of SWCNTs looks very like that of copper. It induces the energy gap in the Fermi level region in all cases except the negative armchair system.
We calculated binding energy for all charged systems and collected results in Table 2.
| Studied systems | Cu-armchair | Cu-zigzag | Li-armchair | Li-zigzag | |
|---|---|---|---|---|---|
| Eb, eV | Positive charged | 2.3 | 6.6 | 0.6 | 0.8 |
| Negative charged | 1 | 5.4 | 1.7 | 1.9 | |
| Egap, eV | Positive charged | 0.6 | 1.2 | 1.4 | 1.2 |
| Negative charged | No | 1.2 | No | 1.7 | |
Table 2: The binding energies Eb and the energy gaps Egap of the charged Cu and Li atoms absorbed on the surfaces of SWCNTs.
For the Li systems the negative zigzag and armchair cases are favorable; however the only zigzag system has the energy gap. For the Cu systems the zigzag system are very favorable; however the energy gap has only the negative one.
Thus, transition of negative charge to SWCNT together with Cu or Li atoms is energetically favorable. This transition is followed by formation of the energy gap in the Fermi-level region that prevents leaving of the charge from the metal-SWCNT composite and promotes its accumulation. The values of the energy gaps are collected in Table 2. The 1.2 eV gap of the Cu-zigzag system is a little smaller than 1.7 eV of the Li-zigzag gap, however it is enough to keep electrical charge.
Conclusion
Using density functional pseudopotential calculations we compared the electronic structures of Cu+SWCNT and Li+SWCNT systems. It has been found that neutral Cu+SWCNT and Li+SWCNT systems (both armchair and zigzag) possess the metallic electronic states near the Fermi level. The charged (either negative or positive) Cu+SWCNT and Li+SWCNT armchair systems also have the metallic-like electronic structure. However the charged Cu+SWCNT and Li+SWCNT zigzag systems have energy gaps in the Fermi region; and the negative charged systems are more favorable energetically than the positive charged and neutral systems. The Cu-zigzag system has the gap of 1.2 eV; and the gap of the Li-zigzag is 1.7 eV. As the energy gap at the level of Fermi prevents leaving of a charge from the charged systems, they can work as electrodes for the power accumulator. Thus, the electrical charge can be effectively accumulated in those systems. Electrical characteristics of the charged Cu +SWCNT system are close to those of the Li+SWCNT system, and at the same time the Cu+SWCNT systems are more stable and cheaper than Li +SWCNT ones, thus they are perspective for production of new power sources.
REFERENCES
- Dahn JR, Zheng T, Liu Y, et al. Mechanisms for Lithium insertion in carbonaceous materials. Science. 1995; 270:590-3.
- Kuz’menko AP, Silyutin IA, Kovalenko SV, et al. Original carbon-copper compound as a new source of electric power. In Proceedings of the International VIII Russia-China Symposium “Modern materials and technologies 2007”. Khabarovsk, Russia. 2007;1:153-6.
- Kuz’menko AP, Zavodinsky VG, Kuz’menko MA, et al. Formation of electric potential at solid-phase dissolution of copper in the nanostructured graphite. Izvestiya YuZGU. 2012;2:38-46.
- Wu GT, Wang CS, Zhang XB, et al. Structure and Lithium insertion properties of carbon nanotubes. J Electrochem Soc. 1999;146(5):1696-1701.
- Sozykin SA, Beskachko VP. Interaction of carbon nanotubes (7,7) and (8,8) with embedded atoms. Vestnik Yu-UGU 2010;9(2):87-91.
- Koh W, Ji Choi I, Donaher K, et al. Mechanism of Li adsorption on carbon nanotube-fullerene hybrid system: A first-principles study. ACS Appl. Mater. Interfaces 2011;3(4):1186-94.
- Xiong Z, Yun YS, Jin HJ. Applications of carbon nanotubes for Lithium ion battery anodes. Materials 2013;6:1138-58.
- Zhou Z, Gao X, Yan Y, et al. Enhanced Lithium absorption in single-walled carbon nanotubes by boron doping. J Phys Chem B. 2004;108(26):9023-6.
- de las Casas C, Li W. A review of application of carbon nanotubes for Lithium ion battery anode material. J Power Sources. 2012;208:74-85.
- Sehrawat P, Julien C, Islam SS. Carbon nanotubes in Li-ion batteries: A review. Mater Sci Engineer B. 2016;213:12-40.
- Zhang WJ. A review of the electrochemical performance of alloy anodes for Lithium-ion batteries. J Power Sources. 2011;196:13-24.
- Nalimova VA, Sklovsky DE, Bondarenko GN, et al. Lithium interaction with carbon nanotubes. Synthetic Metals. 1997;88:89-93.
- Landi BJ, Ganter MJ, Cress CD, et al. Carbon nanotubes for Lithium ion batteries. Energy & Environ Sci 2009;6:549-712.
- Sepahvanda S, Safaeia P, Sanaee Z. Growth of carbon nano tubes on copper substrate suitable for Lithium ion battery anode. Proced Mater Sci. 2015;11:634-8.
- Hannula PM, Peltonen A, Aromaa Ja, et al. Carbon nanotube-copper composites by electrodeposition on carbon nanotube fibers. Carbon. 2016;107:281-7.
- García-Rodríguez DE, Mendoza-Huizar LH, Díaz C. A DFT study of Cu nanoparticles adsorbed on defective graphene. Appl Surf Sci 2017;412:146.
- Beckstedte M, Kley A, Neugebauer J, et al. Density functional theory calculations for poly-atomic systems: electronic structure, static and elastic properties and ab initio molecular dynamics. Comp Phys Commun. 1997;107:187-205.
- Hohenbeg H, Kohn W. Inhomogeneous electron gas. Phys Rev. 1964;136:864-71.
- Kohn W, Sham JL. Self-consistent equations including exchange and correlation effects. Phys Rev. 1965;140:1133-8.
- Cohen ML, Heine V. Pseudopotential theory of cohesion and structure. In: Solid State Physics. New-York: Academic Press. 1970;24:250.
- Fuchs M, Scheffler M. Ab initio pseudopotentials for electronic structure calculations of poly-atomic systems using density-functional theory. Comput Phys Commun. 1999;119:67-98.
- Perdew JP, Wang Y. Accurate and simple density functional for the electronic exchange energy. Phys Rev B. 1986;33:8800-2.
- Eletskiy AV. Carbon nanotubes. Uspekhi Fizicheskikh Nauk. 1997;167(9):945-72.
- Terrones M. Science and technology of the twenty-first century: Synthesis, properties, and applications of carbon nanotubes. Annu Rev Mater Res. 2003;33:419-501.
- Reich S, Thornsen C, Maultzsch J. Carbon Nanotubes: Basic Concepts and Physical Properties. 2004 WILEY-VCH Verlag GmbH & Co. Weinheim, Germany.
- Salehi H, Gharbavi K. Ab initio study of electronic properties of an armchair (7,7) carbon nanotube. Advanc Mater Phys Chem. 2012;2:159-162.
- Kim P, Odom TW, Jin-Lin Huang, et al. Electronic density of states of atomically resolved single-walled carbon nanotubes: Van Hove singularities and end states. Phys Rev Lett. 1999;82:1225-8.