Endo-sponge treatment of anastomotic leakage after colorectal surgery: A report of 29 cases compared to the main studies in the literature.
2 Department of Hepato-gastroenterology, Trousseau Hospital, Tours, France, Email: d.moussata@chu-tours.fr
3 Department of Surgical Lyon Sud Hospital, Pierre Benite, France, Email: d.moussata@chu-tours.fr
4 Department of Pharmacy, Lyon Sud Hospital, Pierre Benite, France, Email: d.moussata@chu-tours.fr
5 Department of Pharmacy, Trousseau Hospital, Tours, France, Email: d.moussata@chu-tours.fr
6 Department of Surgical Trousseau Hospital, Tours, France, Email: d.moussata@chu-tours.fr
Received: 10-May-2018 Accepted Date: Jun 20, 2018; Published: 25-Jun-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Anastomotic leakage is the main complication after colorectal surgery, inducing sepsis and often, further surgical intervention is needed. In the last few years, the vacuum-assisted closure therapy using the Endo-sponge system has been used in different types of complicated wounds and in anastomotic leakage after rectal resection. In this study, we reported our experience with Endo-sponge in leakage from colorectal anastomosis and compared it to other published series. PATIENTS AND METHODS: 29 patients with abscess or fistulas from anastomotic leakage after rectal resection or colectomy were retrospectively reviewed. The main clinical symptom was sepsis. Stoma was present at the beginning of Endo-sponge treatment in 21 patients (72% of cases). RESULTS: The mean size of the fistula was 7 ± 4.6 cm (2-20 cm). The mean time to closure of the cavity was 10 ± 6.5 weeks (2–28) and required on average 18.6 ± 13 sessions. We obtained a closure in 27 out 29 patients (93%), which were sustained in 24 out of 27 patients (89%) at 6 months. The treatment was well tolerated and performed on an outpatient basis without sedation. CONCLUSION: In our study, anastomotic leakage was treated efficiently with Endo-sponge without sedation or stoma in 31% of cases.
Keywords
Vacuum-Assisted Closure (VAC); Endo-sponge-anastomotic colorectal leakage; Colorectal surgery
Leakage is the major complication of colorectal anastomosis. Its reported incidence is between 1.5% and 17.5% [1-8]. Sometimes the fistula closes itself but at other times, it can be complicated by sepsis, thus increasing the morbidity and mortality with a risk of permanent stoma [9-11]. Treatment ranges from conservative management (antibiotics, transrectal rinsing and drainage) to surgical procedures (drainage, resection of the anastomosis with proximal colostomy or finally abdomino-perineal extirpation) [12]. To avoid a surgical resumption, transrectal drainage by vacuum-assisted closure (VAC) system placed endoscopically is a new approach to treat the anastomotic leakage. This technique is based on an open-pored polyurethane sponge (Endo-sponge, B. Braun Medical B.V., Melsungen, Germany) linked to a drainage tube connected to a vacuum system, which is introduced endoscopically into the wound. The negative pressure induces a removal of fluid with a reduction of the oedema. This, in turn, increases the local perfusion and helps the granulation tissue grow into the cavity and fill it up, eventually causing the fistula to close. We reported our experience with Endo-sponge in leakage from colorectal anastomosis and compared it to the main published series.
Patients and Methods
Patients
We retrospectively analyzed all patients with clinical symptomatic anastomotic leakage referred by different surgical teams and treated by Endo-sponge. In most cases, the leakage was diagnosed after a sepsis had been confirmed by a CT-scan.
Methods
Without any sedation or under partially sedating inhaled gas Kalinox (Air Liquide Santé, Paris, France), an endoscope (gastroscope, 180H, Olympus, Japan) was introduced into the cavity to evaluate its length and the level of the anastomosis from the anal verge. At the same time, Endo-sponge treatment was initialized. During each procedure, the sponge was cut according to the size of the cavity, which was measured with the endoscope. It was inserted into the cavity through the over-tube, which was introduced with the endoscope. The drainage tube inserted into the sponge was connected to a low vacuum wound drainage system to which a sub-atmospheric pressure (125 mmHg) was applied. The system was changed every 3 to 5 days. Sometimes, the cavity was not roundish and presented some irregularities with small secondary tracks treated by pieces of sponge introduced with a biopsy forceps. The contact of the sponge with the mucosa induced the formation of a granulation tissue into the fistula. Before a new sponge was inserted, non-viable tissue was debrided with a biopsy forceps in order to eliminate fibrosa and obtain a wide contact of the sponge with the mucosa. No additional treatment such as fibrin glue injection or radiologic drainage was used. The treatment was stopped when the length of the cavity was close to 1 centimeter because, in that case, it was no longer possible to introduce the sponge and the fistula was considered healed.
Follow-up
After endo-sponge treatment, the patients were followed up endoscopically at 1, 3 and 6 months to assess the maintenance of the results. All participants have been treated according to clinical needs and gave informed consent to the protocol which was approved by our Institutional Review Board.
Statistical Analysis
Correlations were calculated using the Spearman test. Data from patients with or without stoma were compared using the Mann-Whitney test.
Results
Epidemiologic characteristics
Between January 2013 and December 2016, 29 patients (22 men, mean age ± SD, 68 ± 10 years) were treated in 2 endoscopic departments (Lyon Sud Hospital and Tours Hospital, France). The characteristics of the patients are summarized in Table 1. Twenty-three patients (23/29, 79.3%) were operated on because of rectal cancer with neo-adjuvant radio-chemotherapy in 19 cases (19/29, 65.5%). Three patients were operated on for a sigmoiditis, 1 for left colonic cancer and 2 for right colonic cancer with a peritoneal carcinosis treated by hyperthermic intraperitoneal chemotherapy and left colectomy with colorectal anastomosis. In 25/29 patients (86.2%), the fistula was detected after a sepsis, in 2 cases (6.9%) after rectal bleeding and in 1 case (3.4%) after diarrhea. In 12 cases (41.3%), the treatment by Endosponge was started in the month following surgery, while in the other cases the mean delay was 35 ± 56 weeks (8-260 weeks). In these cases, the patients were referred to our center because of failure of surgical or radiological treatments. At the inclusion stage, 21 patients (72.4%) were referred for Endo-sponge treatment with a stoma systematically performed by the surgical team at the time of anastomosis (12 cases) because of the high risk of fistula (neo-adjuvant radiotherapy, male, obesity, low anastomosis, [13]) or secondly (9 cases) to treat the sepsis. Twelve patients (41%) were under antibiotics when endo-sponge was performed. The drainage allows sepsis healing and after a few days (less than 10), the antibiotics can be stopped. A nutritional support has been used in 3 patients. In 1 case, the stoma was closed during the treatment after 11 weeks and the treatment continued for another 8 weeks. In another case, after 14 weeks of Endo-sponge treatment, the diameter of the fistula reduced more than its length and it became impossible to introduce the sponge. It was decided to enlarge the fistula but during the procedure, the colon was perforated and a stoma was performed (Table 1).
| Patients | Age (years) | Sex | Diagnosis | Neo-adjuvant RT-CT | Operation |
|---|---|---|---|---|---|
| 1 | 68 | M | RC | 1 | LAR |
| 2 | 68 | M | RC | 1 | LAR |
| 3 | 66 | M | RC | 1 | LAR |
| 4 | 52 | F | RC | 0 | LAR |
| 5 | 51 | F | RC | 0 | IAA |
| 6 | 74 | M | RC | 1 | LAR |
| 7 | 67 | M | RC | 1 | PCP |
| 8 | 71 | F | RC | 1 | LAR |
| 9 | 87 | F | RC | 0 | LAR |
| 10 | 61 | M | RC | 1 | LAR |
| 11 | 70 | M | RC | 1 | LAR |
| 12 | 68 | M | RC | 1 | LAR |
| 13 | 64 | M | RC | 1 | LAR |
| 14 | 59 | M | RC | 0 | LAR |
| 15 | 56 | M | RC | 1 | LAR |
| 16 | 75 | M | RC | 1 | LAR |
| 17 | 84 | M | RC | 1 | C |
| 18 | 62 | M | CC | 0 | C+HIC |
| 19 | 63 | M | CC | 0 | C |
| 20 | 60 | M | CC | 0 | C+HIC |
| 21 | 63 | F | Sig | 0 | Sigm |
| 22 | 82 | M | Sig | 0 | PCP |
| 23 | 88 | M | Sig | 0 | Sigm |
| 24 | 66 | M | RC | 1 | LAR |
| 25 | 56 | F | RC | 1 | LAR |
| 26 | 80 | F | RC | 1 | LAR |
| 27 | 83 | M | RC | 1 | LAR |
| 28 | 56 | M | RC | 1 | LAR |
| 29 | 81 | M | RC | 1 | LAR |
| Mean ±   SD | 68 ±   10 | 19 RT-CT |
RC: Rectal Cancer; CC: Colonic Cancer; Sig: Sigmoiditis; LAR: Low Anterior Resection Sigm: Sigmoidectomy; IAA: Ileo-Anal Anastomosis; C: Colectomy; PCP: Partial Colo-Proctectomy; HIC: Hyperthermic Intraperitoneal Chemotherapy.
Table 1: Characteristics of the patients
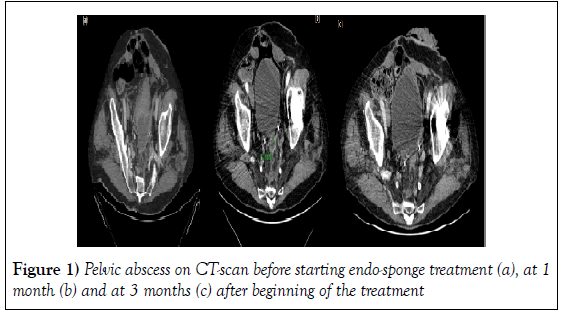
This patient was treated with antibiotics and the Endo-sponge treatment started 4 weeks after the surgery and lasted for an additional 3 weeks. Overall, 8/29 (27.6%) of the patients were treated without any stoma. One patient presented an abscess 2.5 years after the proctectomy for complicated diverticulitis, which was drained surgically. The length of the cavity was so long (20 cm) that we had to insert 3 Endo-sponges at the beginning, then 2 and finally 1 after 5 weeks. On the CT-scan the abscess disappeared totally after 3 months. The mean length of the fistula was 7 ± 4.6 cm (2-20 cm). The mean level from the anal verge was 6.2 ± 4.6 cm (2-20 cm). After a mean delay of 10 ± 6.5 weeks and a mean number of sessions of 18.6 ± 13 (range 4 to 57 sessions), the cavity was closed (less than 1 centimeter) in 27/29 (93%) patients (Figure 1 and Table 2).
| Patients | Stoma before Endo-sponge treatment | Time between surgery and Endo-sponge treatment (week) | Level of anastomosis (cm) | Initial length of the cavity (cm) | Nb of Endo-sponge sessions | Total time to closure (week) | Closure rate (size<1cm) | Sustained closure rate during the follow-up at 6 months |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 104 | 10 | 6 | 25 | 10 | S | F |
| 2 | 0 | 1 | 6 | 5 | 11 | 4 | S | S |
| 3 | 0 | 34 | 7 | 13 | 20 | 14 | S | S |
| 4 | 1 | 30 | 3 | 4 | 31 | 15 | S | S |
| 5 | 1 | 4 | 2 | 12 | 25 | F | F | F |
| 6 | 0 | 2 | 10 | 9 | 18 | 9 | S | S |
| 7 | 1 | 260 | 2 | 7 | 48 | 25 | S | S |
| 8 | 1 | 12 | 3 | 5 | 30 | 14 | S | S |
| 9 | 1 | 17 | 13 | 5 | 25 | 12 | S | S |
| 10 | 1 | 4 | 8 | 2 | 7 | 4 | S | S |
| 11 | 1 | 1 | 3 | 10 | 57 | 28 | S | S |
| 12 | 1 | 10 | 3 | 7 | 19 | 10 | S | F |
| 13 | 1 | 8 | 5 | 7 | 7 | 8 | S | S |
| 14 | 1 | 2 | 15 | 4 | 7 | 4 | S | S |
| 15 | 1 | 12 | 5 | 5 | 18 | 13 | S | S |
| 16 | 1 | 4 | 3 | 5 | 4 | 7 | S | S |
| 17 | 1 | 2 | 1 | 2 | 11 | 2 | S | S |
| 18 | 0 | 3 | 4 | 15 | 36 | 19 | S | S |
| 19 | 0 | 1.5 | 20 | 2 | 4 | 2 | S | S |
| 20 | 0 | 4 | 15 | 7 | 6 | 3 | S | S |
| 21 | 1 | 1 | 7 | 18 | 9 | 14 | S | S |
| 22 | 1 | 136 | 2 | 20 | 10 | 6 | S | S |
| 23 | 0 | 68 | 7 | 5 | 17 | 8 | S | S |
| 24 | 1 | 104 | 1 | 3 | 24 | 13 | S | F |
| 25 | 1 | 52 | 5 | 3 | 13 | 6 | S | S |
| 26 | 1 | 21 | 6 | 5 | 10 | 5 | S | S |
| 27 | 1 | 48 | 2 | 7 | 5 | 3 | F | F |
| 28 | 1 | 25 | 7 | 3 | 18 | 9 | S | S |
| 29 | 1 | 18 | 5 | 6 | 24 | 12 | S | S |
| Mean ± SD |
21 stoma | 35 ± 56 | 6.2 ± 4.6 | 7 ± 4.6 | 18.6 ± 13 | 10 ± 6.5* | 27S and 2F | 24S and 5F |
F: Failure; S: Success
Table 2: Endoscopic characteristicsd
The treatment failed in one patient with ulcerative colitis operated on for rectal cancer with an ileo-anal anastomosis. Within 4 weeks following the surgery, the patient presented a sepsis and the CT-scan showed an abscess in the pelvis. In endoscopy, we observed a large, nearly circumferential (270°) anastomotic leakage. We tried to treat the leakage with Endo-sponge during 14 weeks without success and we decided to perform a definitive ileo-stoma. There was a significant correlation between the delay of closure and the size of the fistula (Rho=0.45, p=0.03), but not with the delay of discovery and the age of the patients (Rho=0.31, p=0.12; Rho=0.14, p=0.63, respectively). There was no significant difference between the patients with and without stoma in the time to closure, the age of the patients, the size of fistula, neo-adjuvant radiotherapy, the level of anastomosis and the time between surgery and fistula treatment (Table 2).
Follow-up
During the follow-up, the fistula recurred at 1 month in 3 patients and endoscopic control was not performed in another patient who was lost for follow-up. These 4 cases were considered as secondary failures. At 6 months, 18 patients (85.7%) who presented a stoma experienced a closure of the protective stoma.
Discussion
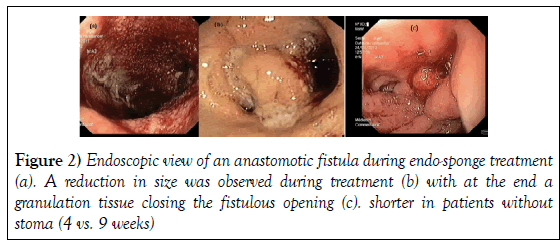
Anastomotic leakage inducing a sepsis is the main complication of rectal surgery. Sometimes, a percutaneous or surgical drainage is performed but in the majority of cases, a diverting stoma or Hartmann’s procedure is required because it is difficult to come back surgically especially as many patients have been treated by radiotherapy. In this context, Endo-sponge appears as a new therapeutic option. In the literature, there are some case reports and few studies including a low number of patients treated by Endosponge [14-32]. In the 4 previously published series, patient characteristics, level of anastomosis and cavity length were comparable to those in the present study (Table 3) [15,17-19]. In our series, the delay between fistula discovery and Endo-sponge treatment was longer, the number of Endosponge sessions was higher and the number of patients with stoma was smaller. We hypothesize that it could be due to a larger size than in other studies or a long delay between the surgery and the start of the Endo-sponge treatment even if we did not find a significant correlation probably due to the small number of patients. We did not use sedation which allowed us to perform Endo-sponge treatment in outpatients and we verified the healing rate 6 months after closure. The percentage of protective ileo-stoma closure was similar to that reported in other studies (Table 3). In previous series, the healing rate ranged between 56 and 96%. In our series, one failure appeared in one patient with ulcerative colitis who presented a very large anastomotic leakage from ileo-anal anastomosis and in another one, the fistula relapsed. Van Koperen et al. have reported that the healing rate depended on the delay between surgery and fistula discovery. They reported more failures in patients in whom the delay was longer than 6 weeks. In our study, the failures occurred in one patient in whom the time between surgery and fistula diagnosis was 4 weeks and in the other one, the delay was longer (10 weeks). Glitsch et al. have reported that the duration of this treatment was correlated with the size of the cavity (>6 cm) and with the age of the patient (more than 62 years old). In our series, we found a significant correlation between the delay of closure and the size of the fistula but not with the delay of therapy after the surgery or the age of the patients. Compared with In others series, we have a higher number of patients who were successfully treated without stoma. In our study, 21 patients presented a stoma at the inclusion. During the treatment, the stoma was closed in 1 case after 11 weeks of treatment because of the wish of the patient. In the 8 patients without stoma, we did not encounter any difficulties in Endosponge exchange, which was performed after 2 rectal enemas such as before a rectoscopy. Interestingly, Glitsch et al. have reported that the duration of endo-sponge treatment shorter in patients without stoma (4 vs. 9 weeks) (Figure 2).
| Studies | Year | Nb patients | Mean age (range), Sex | Neo-adjuvant Radiotherapy | Patients with stoma | Mean delay of fistula discovery (week) |
Level of anastomosis (cm) | Cavity length (cm) | Closure (%) | Nb sessions | Exchange frequency (days) | Mean delay of closure (week) | Sedation | Hospitalisation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weidenhagen et al. (15) | 2008 | 29 | 66 (42-79), 24 M | 9/29 (31%) | 25/29 (86%) | 1.1 ± 0.5 (0.4-2.4) | 5.3 (1-12) | 7.4 ± 5.1 (2-20) | 28/29 (96%) | 11.4 ± 6.3 | 2-4 | 24 ± 11.5 (1-45) | 100% | 100% |
| Glitsch et al. (18) | 2008 | 17 | 61 (42-84), 14 M | 9/17 (53%) | 13/17 (76%) | 2 (0.4-5.5) | 5.3 (0-11) | 7 | 16/17 (94%) | 5.4 (1-24) | 2 | 7.5 (1.4-31.5) | - | 47% |
| Mees et al. (28) | 2008 | 5 | 46 (33-65), 4 M | 0/5 (0%) | 5/5 (100%) | 2.2 (1.8-2.7) | 4-7 | 5.4 (4.3-7.2) | 5/5 (100%) | 7 (6-13) | 3-5 | 3.8 (2.5-5.2) | 20% | 100% |
| Van Koperen et al. (17) | 2009 | 16 | 64 (19-78), 9 M | 11/16 (68%) | 16/16 (100%) | 5.8 (2-228) | 5 (2-8) | - | 9/16 (56%) | 13 (8-17) | 3-4 | 5.7 (4-12.8) | 56% | - |
| Riss et al. (16) | 2009 | 9 | 63 (50-71), 5 M | 6/9 (67%) | 7/9 (78%) | 11 (4.3-102) | - | - | 6/9 (66%) | - | 2-4 | - | 100% | - |
| von Bernstorff et al. (26) | 2009 | 26 | 62(42-84), 21 M | 14/26 (54%) | 18/26 (69%) | 1.5 (0.1-4.8) | - | 39.5 (4-120) | 23/26 (88.5%) | 10 (3-41) | 2-3 | 7.14 (1.4-31) | - | 100% |
| Nerup et al. (29) | 2013 | 13 | 64(36-71), 11 M | 6/13 (46%) | 13/13 (100%) | - | 9 (6-12) | - | 13/13 (100%) | 8(1-18) | - | 2.5 (0.3-5.7) | - | 100% |
| Srinivasamurth et al. (25) | 2013 | 8 | 66.5 (45-79), 7M | 6/8 (75%) | 8/8 (100%) | 4.1 (1.4-19.4) | - | - | 6/8 (75%) | 4 (1-7) | - | 3 (1-7) | 100% | 100% |
|  Strangio et al.(30) | 2015 | 25 | 67 (37-89), 18 M | 18/25 (72%) | 13/25 (52%) | 172.4 (0-14.5) | - | 5.6 (1.5-10) | 22/25 (88%) | 9 (1-39) | 2-4 | 4 (1-18) | - | - |
| Milito et al. (31) | 2015 | 14 | - | - | 14/14 (100%) | 2 | 3-7 | 8.1 | - | 3-14 | - | 5.2 | - | 0% |
| Borstlap et al.(32) | 2017 | 30 | 66 (40-79), 19 M | 19/30 (63%) | 20/30 (67%) | 2 (0.4-10) | - | - | 28/30 (93%) | 3.5 (2-15) | 3-4 | 18.1 (2-103) | 0% | - |
| Present study | 2016 | 29 | 68 (51-88), 22 M | 19/29 (66%) | 21/29 (72%) | 35 ± 56 (1-260) | 6.3 ± 4.6 (2-20) | 7 ± 4.6 (2-20) | 24/29 (82.7%) | 18.6 ± 13 (4-57) | 3-5 | 10 ± 6.5 (2-28) | 0% | 0% |
Table 3: Comparison between the present series and previously published series
However, in Glitsch’s study [19], the size of the cavity was larger in patients with a diverting stoma and most of them had been treated by neo-adjuvant radiotherapy, a treatment that may slow down self-healing [16] and lengthen the Endo-sponge treatment. In our study, there was no significant difference in the duration of treatment, size of the cavity and percentage of patients treated by neo-adjuvant radiotherapy between patients with and without stoma. Endoscopically, the system is easy to introduce into the wound through the anus. In our study, the examination is performed in ambulatory conditions without any sedation. Some authors [17,23] have evaluated the patient’s comfort using visual analogic scale including the patient’s satisfaction, the alteration in daily life activity and pain during Endo-sponge treatment. They have concluded that Endo-sponge is well tolerated. In our study, we performed in 6 patients the examination under Kalinox, an equimolar mixture of oxygen (O2) and nitrous oxide (N2O) in ambulatory conditions and even if the duration of treatment was longer, we did not report any loss of compliance. In 2 patients, the exchange was painful and required antalgics as morphinic at the beginning of the treatment. However, when the procedure was finished, the patients did not present any pain at home. In our practice, we encountered some difficulties when there were several tracks along the fistula as previously reported by Weidenhagen et al. or when there was stenosis at the origin of the fistula requiring its enlargement (in one patient). In one case, we noticed a new fistulous track created by the suction of the distal extremity of the drainage tube, which was in direct contact with the mucosa. That is why we recommend that the central drainage tube is not applied in direct contact with the mucosa and we advise to cut it shorter than the sponge. We acknowledge that our study has some limitations. Firstly, it was uncontrolled and we cannot rule out that some fistula would have closed without Endosponge treatment. However, about half of patients were referred to our center late after failure of common management of anastomotic leakage. Secondly, it is a retrospective study in which only patients who were treated by Endosponge have been included. The number of patients was small and the group was somewhat heterogeneous. Lastly, criteria for faecal diversion are not universally admitted and largely dependent on subjective appreciation of the risk of fistula and on different surgical teams’ processes. However, when comparing patients treated by Endo-sponge with or without stoma, their characteristics and those of the fistula group do not appear to be significantly different (Table 4).
| Variables | Patients with stoma (n=21) | Patients without stoma (n=8) | p-value |
|---|---|---|---|
| Age (years) | 68 ± 11 | 68 ± 9 |  0.25 |
| Neo-adjuvant CT-RT | n=14 | n=5 | 0.67 |
| Time between surgery and fistula diagnosis (week) |  36.7 ± 62 | 30.8 ± 41 | 0.47 |
| Level of anastomosis (cm) | 4.8 ± 3.7 | 9.8 ± 5.2 | 0.32 |
| Initial length of the cavity (cm) | 6.7 ± 4.7 | 7.7 ± 4.3 | 0.64 |
| Nb of VAC sessions | 19 ± 14 | 17 ± 10 | 0.39 |
Table 4: Comparison between patients treated by Endo-sponge with or without stoma (mean ± SD)
Core Tip
In the literature, there are few series reporting a good efficiency of endosponge in anastomotic colorectal leakage that’s we confirmed. The main difference with other published studies is the presence of a diverting stoma. Our series is the first one in which half of the patients do not have a diverting stoma and we have shown that endo-sponge treatment is feasible, efficient without any sepsis and that the duration of treatment tends to be shorter in these patients.
Conclusion
Our study confirms that Endo-sponge is an interesting option in the treatment of anastomotic leakage. This method is well tolerated and is an easy-to-handle therapy in ambulatory conditions without any sedation.
Author’s Contribution
Dr. Boschetti, MD, has collected, analyzed the data and written the manuscript. Dr. Chauvenet has performed the statistics. Dr. Belkhodia, MD and Dr. Nancey, MD PhD, have performed the endoscopies. Dr. Passot, MD, Dr. Vaudoyer, MD, Dr. Cotte, MD PhD, Dr. François, MD, Dr. Bourlier MD, and Dr. Glehen, MD PhD, have taken care of the patients. Dr. Cabelgenne, MD PhD and Dr. Stephanie Benaim MD have collected the data. Dr. Valette, MD PhD, has interpreted the CT-scan. Dr. Moussata MD PhD and Dr. B Flourié, MD PhD have critically reviewed the manuscript and managed the study.
Conflicts of Interest
All the authors declared no conflict of interest.
REFERENCES
- Heald RJ, Karanjia ND. Results of radical surgery for rectal cancer. World J Surg. 1992;16:848-857.
- Hallbook O, Sjodahl R. Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg. 1996;83: 60-62.
- Fielding LP, Stewart-Brown S, Blesovsky L, et al. Anastomotic integrity after operations for large-bowel cancer: A multicentre study. Br Med J. 1980; 81:411-414.
- Gastinger I, Marusch F, Steinert R, et al. Working Group "Colon/Rectum Carcinoa" Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg. 2005;92:1137-1142.
- Graf W, Glimelius B, Bergstrom R, et al. Complications after double and single stapling in rectal surgery. Eur J Surg. 1991;157:543-547.
- Tuson JR, Everett WG. A retrospective study of colostomies, leaks and strictures after colorectal anastomosis. Int J Colorectal Dis. 1990;5:44-48.
- Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355-358.
- Averbach AM, Chang D, Koslowe P, et al. Anastomotic leak after double-stapled low colorectal resection. Dis Colon Rectum. 1996;39:780-787.
- Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48:1021-1206.
- Pakkastie TE, Luukkonen PE, Jarvinen HJ. Anastomotic leakage after anterior resection of the rectum. Eur J Surg. 1994;160:293-300.
- Lee WS, Yun SH, Roh YN, et al. Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg. 2008;32:1124-1129.
- Keighley MRB, Williams NS. Surgery of the anus, rectum and colon. P. Saunders, Editor. 1993;1024-1026.
- Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis and treatment. J Am Coll Surg. 2009;208:269-278.
- Bemelman WA. Vacuum assisted closure in coloproctology. Tech Coloproctol. 2009;13:261-263.
- Weidenhagen R, Gruetzner KU, Wiecken T, et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22: 1818-1825.
- Nagell CF, Holte K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int J Colorectal Dis. 2006;21:657-660.
- Riss S, Stift A, Meier M, et al. Endo-sponge assisted treatment of anastomotic leakage following colorectal surgery. Colorectal Dis. 2009;12:104-108.
- Van Koperen PJ, Van Berge Henegouwen MI, Rosman C, et al. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc. 2009; 23:1379-1383.
- Glitsch A, Von Bernstorff W, Seltrecht U, et al. Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): An optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy. 2008;40:192-199.
- D'Hondt M, De Hondt G, Malisse P, et al. Chronic pelvic abscedation after completion proctectomy in an irradiated pelvis: Another indication for Endo-sponge treatment? Tech Coloproctol. 2009;13:311-314.
- Richterich JP, Heigl A, Muff B, et al. Endo-sponge--A new endoscopic treatment option in colonoscopy. Gastrointest Endosc. 2008;68:1019-1022.
- Durai R, Ng PC. Perirectal abscess following procedure for prolapsed haemorrhoids successfully managed with a combination of VAC sponge and Redivac systems. Tech Coloproctol. 2009;13:307-309.
- Mees ST, Palmes D, Mennigen R, et al. Endo-vacuum assisted closure treatment for rectal anastomotic insufficiency. Dis Colon Rectum. 2008;51:404-410.
- Einenkel J, Holler B, Hoffmeister A. Sonographic diagnosis and Endo-sponge assisted vacuum therapy of anastomotic leakage following posterior pelvic exenteration for ovarian cancer without using a protective stoma. J Gynecol Oncol. 2011;22:131-134.
- Srinivasamurthy D, Wood C, Slater R, et al. An initial experience using transanal vacuum therapy in pelvic anastomotic leakage Tech Coloproctol 2013;17:275-281.
- Bemelman WA. Vacuum assisted closure in coloproctology Techniques in Coloproctology. 2009;261-263.
- Manta R, Caruso A, Cellini C. Endoscopic management of patients withpost-surgical leaks involving the gastrointestinal tract: A large case series United European Gastroenterology Journal. 2016;4:770-77.
- Mees ST, Palmes D, Menninger R, et al. Endo-vacuum assisted closure treatment for rectal anastomotic insufficiency Diseases of the Colon and Rectum. 2008;404-410.
- Nerup N, Johansen JL, Alkhefagie GA, et al. Promising results after endoscopic vacuum treatment of anastomotic leakage following resection of rectal cancer with ileostomy Danish Medical Journal. 2013;60:A4604.
- Strangio G, Zullo A, Ferrara EC. Endo-sponge therapy for management of anastomotic leakages after colorectal surgery: A case series and review of literature. Dig Liver Dis. 2015;47:465-4699.
- Milito G, Lisi G, Venditti D, et al. Endoluminal vacuum therapy as treatment for anastomotic colorectal leakage. Surg Technol Int. 2017;30:125-130.
- Borstlap WAA, Musters GD, Stassen LPS. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc. 2017.