Engineering aspects of human blood
2 Assistant Professor, Mechanical Engineering, Okanagan Campus, Kelowna, Canada, Email: hadi.mohammadi@ubc.ca
Received: 05-Feb-2018 Accepted Date: Apr 10, 2018; Published: 18-Jun-2018
Citation: Earl E, Mohammadi H. Engineering aspects of human blood. Biomed Engg Cur Res 2018;1(1): 4-10.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Hematology is known as the study of blood regarding health and disease which consists of issues with the red blood cells (RBCs), white blood cells (WBCs), platelets, lymph nodes, blood vessels, bone marrow, and the proteins involved in bleeding and clotting. As biomedical engineers, it is especially vital to understand the mechanics of blood components to avoid unwanted results from implantations such as heart valves. A comprehensive review on the biomechanics of blood is discussed in this chapter. We will also discuss that even though the human blood is a non-Newtonian fluid, depending on the circumstances it can be assumed to be a Newtonian fluid.
Keywords
Blood stream; Viscosity; Hemodynamics; RBC; Multiphase Flow; Pulsatile Flow; Non-Newtonian Flow
Blood is known to be one of the connective tissues in the human body, connecting single cells, tissues, and organs in our body together (1). All necessary substances for human life are transported through the vascular system. The science of blood flow and its mechanics is known as hemodynamics. Hemodynamics is a significant element of cardiovascular mechanics and engineering as it simply clarifies the physical laws that direct the blood stream in the blood vessels (2). In a considerable number of cardiovascular diseases and disorders, dysfunctions such as hypertension and congestive heart failure are linked to systemic hemodynamic function. For example, clinical studies advocate that local wall shear stress rates and forms moderate the location and the advancement of atherosclerotic plaques.
Assessment of the values for shear stress and wall shear stress in the blood stream throughout the entire cardiovascular system has a significant impact on the physics and mechanics of blood. In particular, these values play an important role in the design and development of medical devices for cardiovascular applications. In the area of cardiovascular engineering and technology and medical devices, it is undisputedly vital to have a thorough understanding of hemodynamics and the mechanics of blood as to ensure effective application and development.
Composition of blood
The major components of blood are considered to be plasma, RBCs, WBCs, and platelets. The liquid component of blood equating to roughly 55% of total blood volume, known as plasma, is made up of water, salt, sugar, fat, and protein. Plasma is responsible for the transportation of blood cells throughout the body, allowing for connectivity between bodily systems, supporting the notion as to why blood is considered to be a connective tissue. Antibodies, oxygen, waste products, chemical messengers such as hormones and proteins, and clotting proteins are transported throughout the body within blood. The remaining majority of blood volume is taken by RBCs, composing roughly 40% - 45% of total blood volume. Comparatively, WBCs which are responsible for the protection of the body against infections, are much fewer in number than RBCs, yielding a ratio of approximately 1 WBC to 800 RBCs (3). The final major component of blood, platelets, are formed bodies, however are not considered to be cells. Platelets are cell fragments that mainly participate in the clotting process, also known as coagulation. Upon injury, platelets gather at the site of the injury and stick to the lining of the injured vessel, forming a frame-like structure known as a platelet plug, on which blood coagulation can take place. This process involves the formation of a fibrin clot, covering the wound and preventing and stopping the leakage of blood. In addition to the fibrin clot, fibrin also contributes to the healing process by forming the structure of a scaffold upon which the development and growth of new cells can take place.
Red blood cells
RBCs resemble biconcave disks with flattened centers and are composed of a compound known as hemoglobin, a protein that aids in the carrying of oxygen (4). Hemoglobin consists of two beta units and two alpha subunits where each subunit holds a heme group and each heme holds a Fe2+ ion, which can bind to an O2 molecule. When O2 molecules are bound to the heme components of hemoglobin, the hemoglobin becomes oxyhemoglobin. RBCs undergo two major states while circulating throughout the body: oxygenated and deoxygenated, however blood in its entirety is never actually deoxygenated as not all of the oxygen will ever be removed (5). Oxygenated cells are bright red in color and contain large quantities of oxyhemoglobin, whereas deoxygenated cells have less oxyhemoglobin existing in the hemoglobin compound. Oxygenated cells circulate through the body to deliver oxygen to bodily tissues. When a RBC reaches the intended tissue, oxygen molecules are removed from the hemoglobin where the first two O2 molecules are easier to remove than last two, causing a gradient of oxygen release.
Life cycle of RBCs
RBCs are produced in the bone marrow through a process known as erythropoiesis. The production of RBCs involves erythropoietin, monosaccharides, lipids, vitamin B12, amino acids, folic acid, and iron. RBCs are released into the blood stream once they are developed, after which they have a lifespan of approximately 120 days, expiring due to mechanical or structural damage. Dead RBCs are then removed from the blood stream through the spleen, liver and bone marrow. The dead cells are crushed into their main components known as heme, comprised of iron and bilirubin, and globin, comprised of amino acids. Amino acids and iron are reused by bone marrow for the erythropoiesis process, whereas the bilirubin is removed through feces and urine (6).
RBC configuration
RBCs have a discrete biconcave shape, similar to a disk (Figure 1). Normal RBCs are 7.5-8.0 μm in diameter and ~2.0 μm in height, however RBCs must adapt their shape in order to effectively travel through capillaries as capillaries have a diameter of only ~3 micrometers (6). This adaptation suggests that RBCs experience significant passive deformation through their 120-day lifespan and in order to accommodate for this, the properties of a RBC must be physically and mechanically stable as to resist disintegration. The mechanical properties that determine this stability are bending moduli, shear moduli, area expansion moduli and relaxation times.
Figure 1: The geometry of human RBC (A) The normal biconcave shaped RBC cut in half along the y-z plane (B) A RBC with the cytoplasm (7)
This deformability is studied by implementing microfluidic channels as a means of simulating human capillaries. The microfluidic channels are comprised of two wide channels on each edge and a cuboid channel in between the two. The wide channels are normally 20.0 μm wide and 3.0 μm high and the cuboid channel is 4.0 μm wide, 3.0 μm high, and 30.0 μm long (6). These microfluidic channels are filled with fluid particles containing RBCs, while the walls of the channel are formed by stationary DPD particles. Adaptive boundary conditions for fluid DPD particles are used to control density fluctuations.
RBC deformation
An individual RBC undergoes a continuous and severe transition from its normal biconcave shape to an ellipsoidal shape when travelling through the capillaries. This deformation includes elongation in the direction of flow (the longitudinal axis), and shortening in the cross-flow direction (the transverse axis) as the RBC enters into the narrow channel. Once the entire RBC is within the constricted vessel, it deforms further to efficiently pass through the capillary vessel. Three distinct cellular components contribute to the deformability of RBC’s: the cell geometry, the cytoplasmic viscosity and the membrane elasticity.
Cell geometry
The geometry of a cell determines its ratio of cell surface area to cell volume which is directly correlated to the deformability of that cell. This suggests that cells that possess a higher surface area to volume ratio can deform more easily. As RBC’s have a comparatively high surface area to volume ratio due to their biconcave shape and enucleated nature, this allows for heightened deformability and easier transfer through the capillary network.
Cytoplasmic viscosity
The cytoplasmic viscosity is the viscosity of the intracellular fluid of the RBC and represents how easily the fluid within the RBC can move. It is mainly regulated by the mean corpuscular hemoglobin concentration (MCHC). As the amount of hemoglobin within the cell increases, the viscosity of the intracellular fluid decreases due to the viscosity of hemoglobin being relatively low. This suggests that the viscosity of RBCs is directly related to changes in cell volume as cells with greater volumes can possess greater amounts of hemoglobin. Therefore, cells with greater volumes have higher cytoplasmic viscosities and deform with more difficultly compared to cells with lower volumes.
Membrane elasticity
The membrane of RBCs consists of a lipid bilayer supported by an attached spectrin-based cytoskeleton. The resistance of the lipid bilayer to elastic bending is controlled by the bending rigidity, (kc) and the resistance of the spectrin-based cytoskeletal network to shear strain is characterized by the inplane shear modulus, (μs). The deformability of the membrane, along with the mechanical stability of the cell, can be attributed to the elastic modulus, bending modulus, and yield stress.
Membrane Simulations
RBC membrane properties have the potential to reveal the complex behavior that takes place within the membrane as the cell deforms. A simulation known as Twisting Torque Cytometry (TTC) is used to simulate membrane rheology where desired properties of RBCs can be obtained, inclusive of the yield stress of the cell, shear thinning and viscoelasticity. To perform the test a microbead is bonded to the surface of a cell membrane and a magnetic twisting cytometry applies both static and oscillating magnetic fields, rotating the microbead. Wall adhesion is simulated by keeping 15% of vertices stationary on the bottom of the lipid bilayer component of the RBC membrane and microbead adhesion is simulated by including several RBC vertices in the lipid bilayer component near the bottom of the microbead in its rigid motion.
Elasticity of RBCs An example of the use of the TTC simulation is for the determination of the elastic moduli of a RBC. The complex elastic moduli of a RBC can be computed from the phase angle between the storage and loss moduli as such:
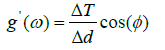 (1)
(1)
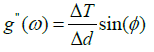 (2)
(2)
Where φ is the phase, g’ is the two-dimensional storage modulus, g” is the loss modulus, ΔT is the torque, and Δd is the microbead displacement amplitude.
Blood disorders
Blood disorders can affect any of the major components of blood: RBCs, WBCs, platelets and plasma. A few of the most common blood disorders occur due to abnormal hemoglobin function such as seen in thalassemia induced anemia, destruction of RBCs which occurs in individuals with malaria, and improper function of WBCs as present in blood cancers such as leukemia.
Anemia occurs when the number of red blood cells is lower than normal, which is commonly caused by an iron deficiency, a B12 deficiency, chronic diseases of the kidneys or bones, or the destruction of RBCs due to shearing forces. An example of a disorder that causes anemia is thalassemia. Thalassemia is an inherited blood disorder characterized by the abnormal formation of hemoglobin which leads to irregular oxygen transport and the destruction of RBCs (8). Hemoglobin is composed of 2 pairs of globin chains that vary from consisting of alpha, zeta, epsilon and gamma types in embryonic and fetal hemoglobin to consisting of only alpha, beta, and delta globin types in adult hemoglobin. Thalassemia occurs due to the defective synthesis of at least one of the globin chains because of genetic mutations of one or more of the globin genes (9).
The two main forms of thalassemia are known as α-thalassemia and β-thalassemia (10). α-thalassemia occurs due to mutations in one or more of the alpha globin genes, whereas β-thalassemia occurs due to mutations in one or more of the beta globin genes. Both main types of thalassemia have two subtypes pertaining to the number of missing globin genes or present mutations within the globin genes. The more severe form of α-thalassemia is known as Hydrops Fetalis and occurs when all four alpha globin genes are altered or missing, often resulting in stillbirth of the fetus or death shortly after birth due to severe anemia, hypoxia, and heart failure (11). The less severe form of α-thalassemia is known as Hemoglobin H which occurs when 3 of the alpha hemoglobin genes become inactive or are missing and often yields moderate anemia, microcytosis, and hypochromia (12). β-thalassemia major, the more severe subtype, occurs when there is a complete absence of beta globin genes, leading to symptoms including anemia, paleness, poor appetite and jaundice. In comparison, β-thalassemia intermedia occurs when the beta globin genes are present, however are altered or mutated, leading to present but reduced symptoms.
Thalassemia is treated by regular blood transfusions once every 2 to 4 weeks, however due to the blood transfusions the patient develops elevated iron levels which must be mitigated with iron-chelating agents that bind to the excess iron and aid in the removal of it from the body (13,14). If ironchelation therapy is not used this can lead to iron overload, damaging the liver and heart.
Malaria is a mosquito-borne infectious disease where parasite-encoded proteins are exported into the cytoplasm of RBCs (15). In an attempt to invade more cells, the parasites reproduce in the RBC which causes swelling and degradation of the RBC cytoplasm, eventually leading to explosive lysis, scattering components of the malaria parasite capable of further reproduction and host RBC control (16). This causes a chain reaction as more and more parasitic components are available to attack host cells. The decrease in healthy red blood cells can cause anemia to occur, however in addition to anemia other minor symptoms can include fever, fatigue, vomiting, and headaches and severe symptoms consist of seizures, coma and possibly death. Though the parasite attacks the body at a fairly fast pace, dependent on the strain of malaria in addition to the time taken to realize the parasite is present within the host, it can be treated with drugs, causing the parasites to disappear from the blood of the host within 2 or 3 days.
The main cancer associated with blood is leukemia which develops in bone marrow and results in high numbers of abnormal non-fully developed WBCs, referred to as blasts. As the blood is overcrowded with these abnormal WBCs, there is a lack of blood platelets and regular WBCs which produces an inhibited ability for the blood to clot and the body to fight infection. Patients suffering from leukemia generally suffer from poor blood clotting, anemia, and a weak immune system. Other symptoms that can be experienced are nausea, fever, chills, night sweats, flu-like symptoms, weight loss, bone pain, and tiredness (14).
Leukemia is defined by 4 subgroups: acute lymphocytic leukemia, acute myelogenous leukemia, chronic myelogenous leukemia and chronic lymphocytic leukemia (17). The variation between acute versus chronic leukemia subtypes relates to the speed of the development of the cancer; chronic leukemia develops slowly whereas acute leukemia develops quickly. In addition, the leukemia type is different dependent on which type of WBC it affects. Lymphocytic leukemias show abnormailities in lymphocytes opposed to myelogenous leukemias which show abnormalities in granulocytes or monocytes (18). Treatments for leukemia vary depending on the classification of the cancer as well as the age and general health of the patient. Treatments for acute leukemia should begin as soon as possible due to the aggressive nature of the cancer, inclusive of chemotherapy and in some cases bone marrow transplants. Chronic leukemia treatment varies dependent on the cancer stage; however types of treatment include targeted therapy, interferons, chemotherapy, radiation therapy and stem cell transplants (14).
Hemodynamics And Flow Types
Blood circulation
Blood flow in the circulatory system is determined by the pulsing drive that is developed from the heart, the individual mechanical and flow properties of the fluid, and the structure and mechanical properties of blood vessels. These factors combined at appropriate levels ensure that the cells of the body receive adequate amounts of oxygen as well as maintain waste management.
Flow Pulse Development
The heart’s main function is to circulate blood throughout the human body. It is composed of 4 chambers: 2 chambers known as ventricles on the lower half of the heart and 2 chambers known as atria composing the upper section. Upon the propagation of bioelectricity through these different components of the heart, contraction of each chamber occurs, moving blood throughout the body in a system known as the cardiac cycle (19). The cardiac cycle can be easily separated into two main time events: systole and diastole. These two events refer to the action of either the heart pumping blood into the circulatory system or receiving blood from the venous system. In addition to these events, other factors of blood such as velocity are initialized from the cardiac output of the heart.
Systole and Diastole
Systole is when the pressure in the circulatory system is the highest due to the force of the heart pumping the blood into the aorta and pulmonary artery, whereas during diastole the pressure in the circulatory system is lowest as the heart is considered to be in a “resting” state where blood is moved into the heart due to a pressure difference between the vena cava and the right atrium (20).These periodic variations in pressure is what causes blood flow to be considered “pulsatile” (21). This pulsatile action is the cause of the inability to effectively model blood flow by standard flow models unless specific assumptions are applied.
Cardiac Output
The amount of blood that flows out of the heart in one minute is know as the cardiac output which varies based on the weight of an individual. Standard values of cardiac output are within the range of 4.0 to 8.0 L/ min (21). Cardiac output is dependent on 4 main components: heart rate, contractility, preload and afterload.
The heart rate is directly proportional to the velocity of the blood moving throughout the body because under normal circumstances blood maintains a constant volume. As heart rate increases the velocity increases, respectively affecting the viscosity and turbulent effects of the flow. A similar relationship is seen with contractility; the more force the heart initially enacts while emptying the left ventricle the larger the pressure will be that is pushing the blood from the heart, respectively increasing the initial blood velocity. Preload is the degree of myocardial extension prior to shortening which maintains a direct relationship with cardiac output as the heart is stretched to its greatest dimensions (3). Afterload is the force that the ventricle must overcome in order to push the blood into the system of blood vessels which maintains an indirect relationship to cardiac output as greater forces to eject the blood will limit the amount of blood the heart can expel. These components combined affect the initial velocity, pressure, and forces applied on the blood flow.
Blood flow
Blood circulation begins by the heart pumping deoxygenated RBCs to the lungs through the pulmonary circuit which are then oxygenated and released back to the heart. These oxygenated RBCs are then pushed through the systemic circuit by the heart to deliver oxygen to the body’s tissues. After releasing and depositing oxygen within the tissues the RBCs become deoxygenated and travel back to the heart through the systemic circuit to repeat the cycle.
The flow of fluid within the circulatory system is dependent on a variety of factors, however can be characterized by considering the laminar and turbulent properties of the flow. In addition to the laminar and turbulent properties of the flow, it is also important to consider the motion of the suspended particles within the heterogeneous fluid allowing it to cohere to adequate blood flow needs.
Laminar and Turbulent Blood Flow
In homogenous fluids, flows are laminar up to a Reynolds number of roughly 2300 and become turbulent at a Reynolds number of 4000. This logic cannot be applied to the flow of blood as blood is not a homogenous fluid and blood vessels are not perfectly cylindrical and possess viscoelastic properties. Though directly corresponding to Reynolds Numbers will not accurately represent the type of flow in blood, it is generally considered that the possibility for turbulence will increase as the Reynold’s number increases, regardless of the precise critical Reynolds number values for transition. Adhering to this logic, as seen in Eq 3, the possibility for turbulent flow will increase as the velocity increases, the diameter increases, the density increases and as the viscosity decreases.
As a flow develops into turbulence, unsteady vortices appear and interact with each other leading to the development of eddy currents, small currents where the flow differs from that of the general flow. Due to their chaotic nature, turbulent flows require more energy in order to move throughout the system as much of the energy is lost due to misaligned flows and eddy currents. Turbulence occurs naturally in locations of the circulatory system where the Reynolds numbers are comparatively elevated such as the ventricles and ascending aorta. In addition to this, turbulence can also be initiated due to branches or curves in the flow, irregularities due to surgical implants, and improper function of circulatory valves (22). Under diseased or abnormal conditions other segments in the circulatory system can experience turbulent flow which can have negative effects on epithelial function (22).
In individuals with conditions affecting the viscosity of their blood, such as anemia, the opportunity for one’s blood to enter turbulence is increased due to the increased Reynolds number (23). Other individuals that experience an increased opportunity for unwanted turbulence are those with foreign objects present in their circulatory system, such as those with replaced heart valves. This turbulence is caused by an increased contact area between the blood flow and the valve which can sometimes lead to the development of heart murmurs.
Heart Murmurs
A heart murmur is a sound that is developed in the heart that occurs due to the presence of turbulent flow near the heart valves (24). Heart murmurs can be classified into two main types: innocent and abnormal. These murmurs can then be broken down based on if they occur during systole or diastole and by what type of flow characteristic they are caused by, namely regurgitation or ejection (24). Due to the fact that heart murmurs occur because of turbulent flow they have the tendency to be increased in those who are diagnosed with anemia and those with heart valve defects. In addition to these two cases, anything that causes irregular or disturbed flow has the potential to increase turbulence in the flow and therefore increase the possibility of causing heart murmurs. Examples of such include replaced heart valves which introduce new stresses and area contact points in the flow and infections in heart valves causing inflammation (24).
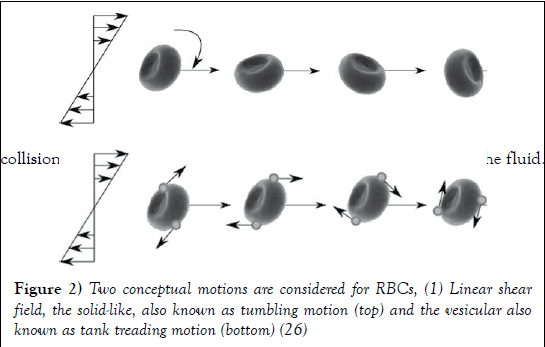
Movement of RBCs
RBCs go through a series of motions and deformations which helps sustain efficient blood flow. Dependent on shear rate, RBCs can move throughout the circulatory system in one of three manners known as tumbling, swinging and tank-treading (25). At the lowest shear rates RBCs have the tendency to move in a pattern known as tumbling (Figure 2). This is where the RBCs spin completely around their axis and maintain little to no deformation. As shear stress is increased RBCs experience a transition motion known as swinging where they undergo quasi-deformation and their rotation abilities alter from 180 degrees at tumbling to a range of 5-26 degrees while swinging (23). Following this, RBC motion develops completely into tank-treading motion which is defined by large amounts of RBC deformation and quasi-steady motion. During tank treading, RBCs explore a small volume of the flow, leading to less collisions and disturbances, respectively decreasing the viscosity of the fluid.
Figure 2: Two conceptual motions are considered for RBCs, (1) Linear shear field, the solid-like, also known as tumbling motion (top) and the vesicular also known as tank treading motion (bottom) (26)
Viscosity
The viscosity of blood is due to the internal friction between the flow, incorporating the effects of the suspended particles present in blood; inclusive of RBCs, WBCs, and platelets. As this internal friction increases, more force is required from the heart in order for it to maintain the desired cardiac output of blood in the circulatory system. This requires a heightened contractility from the muscles of the heart which can result in the fatigue of the heart and in major cases, heart collapse (27). The opposite case where there is a lack of proper internal friction in blood flow will cause a decrease in the ability of one’s blood to clot, which imposes risk when blood vessels are damaged and blood continues to flow out of the site of damage for a prolonged period of time.
The viscosity of blood is dependent on many factors such as the properties of blood plasma, the hematocrit levels, and the individual mechanical properties and influence of the suspended particles in the flow, however this is inherently dependent on whether blood is considered as a Newtonian or Non-Newtonian fluid. The true nature of blood is that it exhibits Non- Newtonian properties under specific conditions, however these conditions arise at very few locations throughout the circulatory system.
Newtonian and Non-Newtonian Conditions
Blood possesses Non-Newtonian properties when the shear rate is above 100 s-1 (28) and follows shear thinning effects. High shear rates occur in the capillaries and the larger arteries coming directly from the heart because the shear rate of a fluid through a vessel increases with increasing velocity and decreasing diameter as shown in Eq. 4. However, due to the fact that the main Non-Newtonian properties of blood occur in small diameter vessels, it is argued that the Non-Newtonian effects that occur within the largest arteries can be ignored (29,30). In blood vessels that possess a diameter in the more medial range or where there is decreased velocity, such as veins and arterioles, these Non-Newtonian factors have minute effects on the properties of the flow, causing them to be neglected for these areas as well.
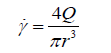 (4)
(4)
Eq. 4 is not an accurate representation of proper blood flow as blood flow is subject to fluctuations based on the viscoelastic properties of the vessel walls, the alternating pressures from systole and diastole, and when present the Non-Newtonian properties of the blood itself, however is used here to outline the relationship between the shear rate and vessel size and diameter.
Considering the above, the Non-Newtonian effects of blood are only active within a small portion of overall blood flow and acquire more importance when blood flow at those selected areas are specifically studied. When being considered as a Newtonian fluid, the viscosity of blood, in addition to possessing variations dependent on the vessel size and blood velocity, is also varied based on the blood plasma itself and the concentration of suspended particles in the flow.
Factors of Blood as a Newtonian Fluid
The blood plasma is mainly composed of water (roughly 91% by volume), proteins, hormones, and glucose and acts as a Newtonian fluid with standard values between 1.1 and 1.3 mPas at the human body temperature of 37 degrees Celsius (31). Due to the incredibly high water content in the substance, the viscosity of plasma is highly affected by the hydration levels of an individual. As a human becomes dehydrated this percentage decreases and blood becomes more viscous (32). In addition to hydration levels, blood plasma viscosity is also directly affected by the amount of proteins and lipids in the blood post consumption. The higher the concentration of these elements, the more viscous the plasma will become (33).
The shear in the flow is also determinant on the amount of suspended particles in the flow. In heterogeneous fluids where particulates are present, these particulates alter the velocity profile of the fluid due to the increased shear at the fluid particle interface. Due to the fact that aside from plasma, a majority of the remaining volume of blood is composed of RB Cs, RBCs are the particles that impose this effect to the flow with the greatest magnitude. Aside from the direct viscosity of the plasma itself, plasma also affects the viscosity of blood by the housing of certain proteins such as fibrinogen that cause aggregation in the suspended particles (34).
Factors of Blood as a Non-Newtonian Fluid
The percentage comparison of the volume of RBCs to the total volume of blood is known as hematocrit and is the main factor contributing to the viscosity of blood as the blood’s ability to flow is highly dominated from the ease of movement of RBCs. At high shear rates the deformability of RBCs is what effectively determines the viscosity of the fluid, however at low shear rates the viscosity is controlled by the unique property of RBCs to aggregate (35).
Physical Capabilities and Tendencies of RBCs
As previously mentioned, the deformability of RBCs is controlled by 3 main factors: the relatively high surface area to volume ratio due to RBCs enucleated nature, the viscosity of the cytoplasm, and the viscoelastic properties of the cell membrane (36). The viscoelastic properties of the cell membrane are dominated by 3 moduli known as the shear elastic modulus, the area compressibility modulus (K ) and the bending modulus. The definitions of the previous as well as determined experimental values for healthy RBCs are denoted in Table 1. The bending modulus (EK ) is calculated as such:
| Modulus Type | Definition (38) | Tested Value (39) |
|---|---|---|
| Shear Elastic Modulus | The ratio of shear stress to shear strain. | 5.5 +/- 3.3 (μN/m) |
| Area Compressibility Modulus | The energy per unit area required to uniformly stretch an interface to produce an area change according to Hooke’s law. | 399 +/- 110 (mN/m) |
| Bending Modulus | The energy per unit area required to produce a mean curvature (H) according to Equations 5 and 6. | 1.15 +/- 0.9 (x10-19Nm) |
Table 1: Viscoelastic factors for RBCs
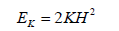 (5)
(5)
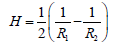 (6)
(6)
During the same experiment as the calculation for the elastic moduli the cytoplasmic viscosity was tested as well, producing a value with an average that is approximately 6 times greater than the viscosity of plasma.
This viscosity is important because it outlines how quickly the cell can reshape itself. Similar to the viscosity of plasma, RBC viscosity is dependent on the hydration levels of the individual in which the blood is present (39). Deformability of RBCs is relevant in locations of high shear rates such as the capillaries because in certain locations the cells are larger than the vessels themselves and in order to maintain proper blood flow they must adhere to the vessel to sustain motion. To do this effectively, the RBCs fully elongate into ellipsoids and align with the flow, reducing the possibility of collisions and decreasing the overall viscosity.
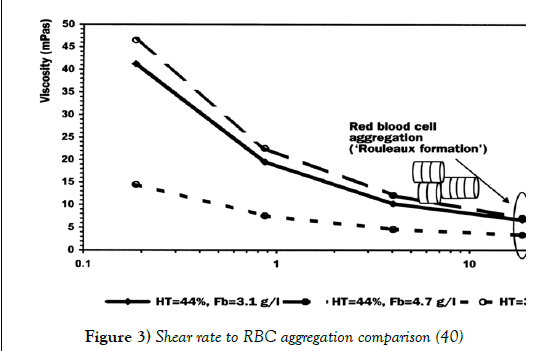
At low shear rates RBCs have the tendency to aggregate together, most commonly into stacks called rouleaux. It is suggested that this specific formation occurs due to the incredibly high surface area RBCs possess. This combining of RBCs severely increases the frictional resistance between flow streamlines, increasing the viscosity of the fluid. However, as seen in Figure 3 at high shear forces this tendency is overruled and the blood cells separate and re-align in the direction of the flow (40).
Figure 3: Shear rate to RBC aggregation comparison (40)
Aside from RBCs other suspended particles such as platelets and WBCs are present in the blood which also maintain aggregative properties, however due to the fact that they compose roughly 1/800th and 1/600th of the volume of the blood respectively, they are often not considered a vital part of the viscosity of the blood (40-43).
Flow Effects on RBCs
As blood is moving from a large vessel to a vessel less than 0.3mm (44) the RBCs re-align to the centre of the vessel. Due to this, the velocity of the centric RBCs is increased relative to the layer of plasma present at the wall of the vessel and the RBCs leave the vessel at a faster rate at which they enter them; this causes the hematocrit level to decrease through the vessel, also known as the Fahraeus Effect. This causes another effect known as the Fahraeus-Lindkvist Effect which states that the viscosity of blood decreases as the vessel size decreases. Though as viscosity in extremely small vessels is affected by the deformability of RBCs as discussed earlier, the increase in velocity of the RBCs increases the velocity of the entire flow, respectively causing the viscosity to decrease.
Viscoelasticity of Blood
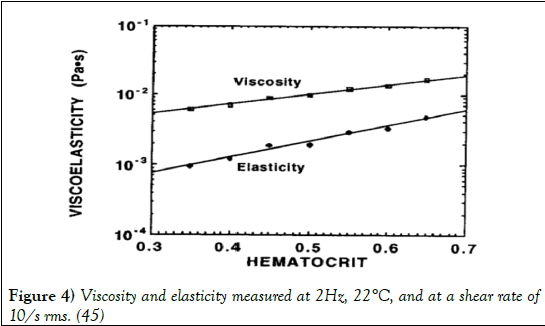
Viscoelasticity is a property that defines materials that exhibit both viscous and elastic characteristics when undergoing deformation (45-50). Viscous materials resist shear flow and strain linearly with time when a stress is applied, whereas elastic materials strain as a stress is applied, however quickly return to their original shape after the stress is removed. Blood is viewed most commonly as a viscous fluid, however does possess viscoelastic properties due to the ability of RBCs to store elastic energy (50). Due to this relationship, the elastic behaviour of blood increases as the hematocrit levels increase as shown in Figure 4 (51).
Figure 4: Viscosity and elasticity measured at 2Hz, 22°C, and at a shear rate of 10/s rms. (45)
Additionally, blood plasma has also been shown to exhibit certain viscoelastic properties which may contribute slightly to the overall viscoelasticity of the blood, however the viscoelasticity of blood is highly controlled by the amount of RBCs present in the flow (52).
Hemostasis
Hemostasis is the process by which the bleeding of an injury is stopped while still attempting to maintain normal blood flow in other areas of the body. Hemostasis occurs in three main steps: vasoconstriction, platelet activation, and blood clot formation (53). Upon the injury of a vessel the smooth muscle cells in the vessel wall contract to restrict blood flow through the site of injury. The injury of the vessel causes exposure of the sub endothelial matrix and tissue factor, initiating the adhesion and aggregation of platelets (54). As platelets aggregate at the site of the wound they become activated, synthesizing factors such as thromboxane A2 which acts as a stimulus for further platelet aggregation and helps reduce the flow of blood through the rupture by the development of a platelet plug (55). After the platelet plug is formed, a chain reaction of secreted factors activating one another work together to strengthen the site of injury by forming a fibrin clot in a process known as the coagulation cascade (56).
Blood Diluters
If the clotting ability of blood is heightened, this can cause blood to aggregate and clot without an initial injury, restricting the normal movement of blood throughout the circulatory system. This prevents or decreases the rate of the exchange of nutrients and wastes between the blood and the tissues at the location that the clot affects. Dependent on the location and size of the clot, this has the potential to cause traumatic tissue damage and possibly death. The clotting ability of blood can be reduced and mitigated by the use of blood thinners such as anticoagulant drugs and antiplatelet drugs which are commonly used for patients suffering from heart disease, poor blood circulation, abnormal heartbeat, and congenital heart defect (10,13,14,57,58). Anticoagulant drugs inhibit the coagulation cascade, preventing further clot propagation, whereas antiplatelet drugs prevent the aggregation of platelets, inhibiting the formation of clots. Antiplatelet drugs are commonly issued in patients with heart disease or who have had prior heart attacks and anticoagulant drugs are used prior to surgery on heart valves and congenital heart defects (58). Though blood thinners can be used to alter the clotting ability of blood, various side effects such as increased bruising, red or pink colored urine, bloody stools, increased bleeding during menstrual periods, purple toes, and blackish areas in fingers, toes or feet can occur (6). In addition to these side effects, the use of blood thinners also inhibits the body from clotting naturally which can pose issues if an individual who is being treated with blood thinners experiences an injury in which bleeding cannot be stopped.
Blood Clot Factors
In stagnant flow regions or where the blood flow moves very slowly the risk for blood to clot increases (59). This occurs due to a heightened exposure time of RBCs to large variations in shear stresses, regardless of if the stress values are small themselves. It has been shown that the pulsatile flow of blood is significant in the regulation of stagnation areas and blood clot formation (59,60). Additionally, blood clotting is known to be caused from both the jet velocity and turbulent shear stress where the Reynolds number is high in addition to stagnation regions (61). Factors that are said to be dominant in the triggering of blood clot formation are listed in Table 2.
| Factor | Triggering criteria for blood clots |
|---|---|
| Cavitation | Water hammer and squeeze flow |
| Reynolds shear stress | >>200 dynes/cm22 (67) |
| Cardiac output | Slow movement of leaflets |
| Stagnant flow | If occurring adjacent to prosthetic valves, can promote the deposition of damaged blood elements, leading to thrombus formation on the prosthesis (68) |
| Vortex shedding | Yields repeated vortex pairing within the wake, which is responsible for the formation of larger platelet aggregates (69) |
| Recirculation | Allows many platelets to be trapped (70) |
| Pressure drop | A larger pressure drop means that the heart with the MHV prosthesis has to work harder (71), thereby reducing cardiac output |
Table 2 : Blood clot factors (66)
Numerical models for blood clot formation
The process of blood clotting begins by activated platelets aggregating to a damaged blood element. It is well known that the level of platelet activation and blood cell damage are significantly related to the magnitude and duration of the applied shear stress which is known as the residual time (68).
There have been a few models developed based on the measured residual time and the amount of shear stresses as outlined in Table 3.
| Model | Expression | |
|---|---|---|
| Linear damage accumulation/BDI | 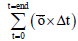 dynes/cm2 dynes/cm2 |
(73) |
| Platelet activation state (PAS) | Non-dimensional level of platelet activation within the interval of (0, 1), in which 0 and 1 correspond to non-activated and fully activated platelets, respectively | (74) |
| Power-law model | 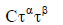 |
(75) |
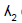 criterion criterion |
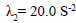 is responsible for blood clot formation is responsible for blood clot formation |
(76,77) |
| Adhesion model | 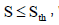 , where , where  is shear rate threshold, taken as 100 is shear rate threshold, taken as 100 |
(78) |
Table 3: Available models for the estimation of blood clot formation and threshold (10)
Conclusion
Biological processes are amazing in their complexity and optimization. Blood, being no exception, is extremely evolved and adapted to the various scenarios necessary to maintain bodily function. Consisting of plasma, WBCs, platelets and RBCs, it is able to transport vital molecules around the body including oxygen, and clot in the case of injury by means of platelet aggregation and coagulation. Since RBCs make up approximately half of the volume of blood, blood flow mechanics are largely related to the properties of RBCs defined under a soft solid. Experiencing large deformability is essential in the life cycle and function of red blood cells as capillaries are extremely small and a common way of studying this deformability is through simulations.
REFERENCES
- Mohammadi H, Mequanint K. Prosthetic aortic heart valves: Modeling and design. Med Eng Phys.2011;33(2):131-147.
- Mohammadi H, Fradet G. Prosthetic aortic heart valves, Cardiovascular Sys. 2017;5(2):1-31.
- Vincent JL. Understanding cardiac output. Crit Care. 2008;12(4):174.
- Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327-87.
- Dupire J, Socol M, Viallat A. Full dynamics of a RBC in shear flow. Proc Natl Acad Sci U S A. 2012;109(51):20808-13.
- Kesmarky G, Kenyeres P, Rabai M, et al. Plasma viscosity: A forgotten variable clinical hemorheology and microcirculation. Clin Hemorheol Microcirc. 2008;39(1-4):243.
- Chee CY, Lee Hp, Lu C. Fluid–structure interaction model to analyse the biomechanical properties of erythrocyte, Phys lett A. 2008;372(9):1357-62.
- Cole L, Kramer PR. Genetic Defects. In: Sam Young, Stacy Masucci, editors. Human Physiology, Biochemistry and Basic Medicine. Boston Academic Press: Mica Haley; 2015;p.187-91.
- In S. Brenner, J. H. Miller editors. Encyclopedia of Genetics. New York: Academic Press; 2001;p.1958–61.
- Holm G, Cherney K. Thalassemia. Healthline Media. USA. 2017.
- Chui DHK, Waye JS. Hydrops Fetalis Caused by α-Thalassemia: An Emerging Health Care Problem. Blood. 1998;91(7):2213-22.
- Chui DHK, Fucharoen S, Chan V. Hemoglobin H disease: not necessarily a benign disorder. Blood. 2003:101(3):791-800.
- Mir MA. Transfusion-induced iron overload medication. Medscape. 2016.
- Nordqvist C. Leukemia: causes, symptoms, and treatment. Medical News. 2017.
- Mohandas N, An X. Malaria and human red blood cells. Med Microbiol Immunol. 2012;201(4):593-8.
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124(4)755-66.
- Ludwig CD. Leukemia. MedSurg Nursing. 2009.
- What are the differences between lymphocytic and myelogenous leukemia. Dana–Farber Cancer Institute. Boston. 2016.
- Cherry EM, Fenton FH. Heart structure, function and arrhythmias. Cornell University, Ithaca, NY. 2017.
- How the heart works: get facts about the human heart. MedicineNet. 2017.
- Sabbah HN, Stein PD. Turbulent blood flow in humans its primary role in the production of ejection murmurs. Circ Res. 1976:38(6):513-25.
- Kruger T, Gross M, Raabe D, et al. Crossover from tumbling to tank-treading-like motion in dense simulated suspensions of RBCs. Soft Mater. 2013;9:9008-15.
- Dupire J, Socol M, Viallat A. Full dynamics of a RBC in shear flow. Proc Natl Acad Sci USA. 2012;109(51):20808-13.
- Heart murmurs: Abnormal heart sounds are often harmless. Mayo Clinic. US. 2018.
- Kumar D, Vinoth R, Raviraj A. Non-Newtonian and newtonian blood flow in human aorta: A transient analysis. Biomed Res. 2017;28(7):3194-203.
- Melchionna S. A Model for Red Blood Cells in Simulations of Large-scale Blood Flows, Macromol Theor Simul. 2011;20(7):54861.
- Janoschek F, Toschi F, Harting J. Simplified particulate model for coarse-grained hemodynamics simulations. Phys Rev E. 2010;82:056710.
- Bernaschi M, Melchionna S, Succi S, et al. A parallel multi physics/scale code for high performance bio-fluidic simulations. Comput Phys Commun. 180(9):1495-1502.
- Perktold K, Peter R, Resch M. Pulsatile non-newtonian blood flow simulation through a bifurcation with an aneurism. Biorheology. 1989; 26(6):1011-30.
- Ballyk PD, Steinman DA, Ethier CR. Simulation of non- Newtonian blood flow in an end-to-side anastomosis. Biorheology. 1994;31(5):565-86.
- Kesmarky G, Kenyeres P, Rabai M, et al. Plasma viscosity: A forgotten variable. Clin Hemorheol Microcirc. 2008;39(1-4):243-6.
- Jeukendrup A, Gleeson M. Dehydration and its effects on performance. Human-kinetics. 2017.
- Dintenfass L, Blood Viscosity. Netherlands: Springer Science & Business Media; 1985;p.482.
- Michaelides, Stathis EE. Nanofluidics - Thermodynamic and transport properties. Springer; 2014.
- Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. J Geriatr Cardiol . 2013;10(3):291–301.
- Mouritsen OG. Life - As a matter of fat: the emerging science of lipidomics. Springer Science & Business Media, 2006;P.276.
- Tomaiuolo G. Biomechanical properties of RBCs in health and disease towards microfluidics. Biomicrofluidics. 2014;8(5):051501.
- Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327-87.
- Effros RM, Chang RS, Silverman P. Effect of osmolality on RBC viscosity and transit through the lung, J Appl Physiol. 1977;42(6):941-45.
- Pop GAM, Duncker DJ, Gardien M, et al. The clinical significance of whole blood viscosity in (cardio) vascular medicine. Neth Heart J. 2002;10(12):512-6.
- Bessonov N, Sequiera A, Simakov S, et al. Methods of blood flow modelling. Math. Model Nat Phenom. 2016;11(1):1-25.
- De Roeck RM, Mackley MR. Dynamics of complex fluids. In: Adams MJ, Mashelkhar RA, The rheology and microstructure of equine blood. London: Imperial College Press; 1998;P.339-345.
- Popel AS, Johnson PC. Microcirculation and hemorheology. Annu Rev Fluid Mech. 2005;37:43-69.
- https://en.wikipedia.org/wiki/F%C3%A5hr%C3%A6us_effect
- How TV Advances in hemodynamics and hemorheology. Greenwich: JAI Press; 1996.
- Fung YC. Mechanical properties of blood vessels. Biomech. 1981;261–301.
- Canfield TR, Dobrin PB. Mechanics of blood vessels. Electronics World.
- Classification & structure of blood vessels. SEER Training Modules.
- Capillaries: Function & Definition. Study.com.
- https://en.wikipedia.org/wiki/Blood_vessel
- Thurston GB. Viscoelasticity of Human Blood. Biophys J . 1972;12(9):1205–17.
- Brust M, Schaefer C, Doerr R, et al. Rheology of human blood plasma: viscoelastic versus newtonian behavior. PhysRev Lett. 2013;110(7):78305.
- Crampton, Linda. How blood clots: platelets and coagulation cascade. Owlcation-Education. 2017.
- Gale AJ. Current understanding of hemostasis. Toxicol Pathol. 2011;39(1):273-80.
- Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515-23.
- The clotting process. World Federation of Hemophilia. 2014.
- Disease & Treatment. Thalassemia Foundation. Canada.
- Sugerman DT. Blood Thinners. JAMA.2013;310(23):2579-80.
- Corbett SC, Ajdari A, Coskun AU. Effect of pulsatile blood flow on thrombosis potential with a step wall transition. ASAIO J. 2010;56(4):290-5
- Hashimoto S, Manabe S, Matsumoto Y, et al. The effect of pulsatile shear flow on thrombus formation and hemolysis. In: 22nd annual EMBS international conference, Chicago. 2000;2461-2.
- Fallon AM, Dasi LP, Marzec UM, et al. Procoagulant properties of flow fields in stenotic and expansive orifices. Ann Biomed Eng. 2008;36(1):1-13.
- Zakaria MS, Ismail F, Tamagawa M, et al. Review of numerical methods for simulation of mechanical heart valves and the potential for blood clotting. Med Biol Eng Comput. 2017;55(9):1519-48.
- Yoganathan AP. Cardiac valve prostheses. In: Bronzino JD editors. The biomedical engineering handbook. Chapter 127, 2nd ed. Boca Raton: CRC Press; 2000.
- Krishnan S, Udaykumar HS, Marshall JS, et al. Two-dimensional dynamic simulation of platelet activation during mechanical heart valve closure. Ann Biomed Eng. 2006; 34(10):1519-34.
- Bluestein D, Rambod E, Gharib M. Vortex shedding as a mechanism for free emboli formation in mechanical heart valves. J Biomech Eng. 2000;122(2):125-34.
- Yun BM, Aidun CK, Yoganathan AP. Blood damage through a bileaflet mechanical heart valve: A quantitative computational study using a multiscale suspension flow solver. J Biomech Eng. 2014;136(10):101009(1-17).
- Hong T, Kim CN. A numerical analysis of the blood flow around the bileaflet mechanical heart valves with different rotational implantation angles. J Hydrodyn Ser B. 2011;23(5):607-14.
- Grigioni M, Morbiducci U, Avenio GD, et al. A novel formulation for blood trauma prediction by a modified power-law mathematical model. Biomech Model Mechanobiol 2005;4(4):249-60.
- Xenos M, Girdhar G, Alemu Y, et al. Device thrombogenicity emulator (DTE)—design optimization methodology for cardiovascular devices: a study in two bileaflet MHV designs. J Biomech. 2010;43(12):2400-9.
- Sheriff J, Soared JOS, Xenos M, et al. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41(6):1279-96.
- Simon HA, Ge L, Sotiropoulos F, et al. Numerical investigation of the performance of three hinge designs of bileaflet mechanical heart valves. Anna Biomed Eng. 2010;38(11):3295-3310.
- Biasetti J, Hussain F, Gasser TC. Blood flow and coherent vortices in the normal and aneurysmatic aortas: a fluid dynamical approach to intra- luminal thrombus formation. J R Soc Interface. 2011;8(63):1449-61.
- Naimah W, Ab W, Balan P, et al. Prediction of thrombus formation using vortical structures presentation in Stanford type B aortic dissection: a preliminary study using CFD approach. Appl Math Model. 2016;40(4):3115-27.
- Tamagawa M, Kaneda H, Hiramoto M, et al. Simulation of thrombus formation in shear flows using lattice Boltzmann method. Artif Organs. 2009;33(8):604-10.