Enrichment of trace elements in urban soils and sediments in a typical sedimentary environment; Onitsha metropolis southeastern Nigeria
2 Department of Geology, University of Ibadan, Ibadan, Nigeria, Email: olatunji@hotmail.com
Received: 28-Aug-2018 Accepted Date: Sep 20, 2018; Published: 05-Oct-2018
Citation: Asowata TI, Olatunji AS. Enrichment of trace elements in urban soils and sediments in a typical sedimentary environment; Onitsha metropolis southeastern Nigeria. J Environ Geol 2018;2(2):47-60.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
This study was carried out to evaluate the concentration, spatial distribution, and fate of potentially harmful elements in soils and sediments in Onitsha metropolis [N=162]. The samples were air-dried, sieved and digested with Aqua regia prior to elemental analysis using ICP-MS. Five [5] potentially harmful elements [Pb, Zn, As, Cd, and Mn] were selected for geoenvironmental assessment. A five-step sequential extraction was carried out to ascertain the fate of these selected elements in the soils and sediments. Elemental concentrations revealed that Pb ranged from 7.2 ppm-2026.0 ppm; Zn, 3.0 ppm-8395.0 ppm; As, 0.5 ppm -27.3 ppm, Cd, Undetected-10.3 ppm and Mn, 10.0 ppm -2127.0 ppm. Elevated concentrations of these elements were found in soils when compared to the sediments. The result also revealed that there was a marked variation between the metal concentrations in the topsoil compared to the sub-soil. The result of the sequential extraction showed that Zn and Cd were more in the carbonate bound fraction, while As were higher in the Organic bound fraction with Mn and Pb more in the hydroxide and residual fraction respectively. It was observed that the concentration of elements that were leached at the demineralized fractions was generally low in all the elements. The relative bioavailability of the metals can be summarized as Cd>Zn>As>Mn>Pb. Owing to the extremely toxic nature of the selected metals and the ease with which they could be leached to the environments where they could become extremely bio-available, it is important that a monitoring programme is instituted in order to avoid catastrophic consequences.
Keywords
Bioavailability; De-mineralized fraction; Geo-environmental assessment; Potentially harmful elements
The increasing expansion of cities in Nigeria by the continuous migration of human beings from rural settlements to urban areas is of high rate. This becomes of great concern because of the possible influence on the environment, particularly with respect to the enrichment of trace elements on the geo-media such as soils, sediments, atmospheric particulate, surface and groundwater among others. The concentration and distribution of trace elements in soils and sediments of an environment can be traced to be from the underlying rock [geogenic] in such an area [1]. This is because rocks that make up the lithology of an area undergoes weathering get transported and deposited in favorable areas as sediments, and also forms soils. In a pristine environment, the trace elements concentration of the underlying rock will possibly be of close range in concentration, with that of the rock around. But researches have shown that the trace element concentrations of soils and sediments in an area can also be enriched as a result of human-related activities and waste [2]. Such activities include; industrialization, smelting, metal plating and fining, vehicles and transport activities, electronics, iron and steel work, general urban waste [3-6]. These human activities become a matter of concern especially in cities with very little physical planning and regulations. And the enrichment of trace elements in soils and sediment should be of concern to the people because of the relationship between these geo-media with the daily living of human beings in the area. Many works have been carried out that establishes the relationship between the quality of the environment and the biota, particularly human beings [7-10]. Soils and sediments are very important components of the earth’s ecosystem, relevant for many functions; serves as habitat for biota, act as filters, buffers, and transform of numerous organic and inorganic substances, control and shapes biogeochemical cycles for essential nutrients in the environment. The evaluation of the geochemical characteristics of potentially harmful elements in soils and sediments upon which large urban towns are developed are essential for the development and maintenance of healthy and sustainable habitat [1,11-13]. Large cities and towns affect greatly the quality of the environment, altering the fate and transport, increasing the concentration of trace elements, which can be potentially harmful, in soil and sediments that may increase the detrimental and toxicological effect of the biota, the environment and human health [1,7,9,12-19]. Soil and sediments in urban environment serve as both recipient and sink of the contaminant over a long period of time, through various sources of pollution and surface run-off resulting in both diffuse and point source pollution [20-27].
The effect of trace elements in soils and sediments in the environment and human health are a function of the mobility and bio-availability of these elements [12,28-30]. Certain environmental conditions such as redox, pH, salinity, organic matter content and temperature are determinant factors in the control of their deposition in soils and sediments. Hence, it is essential to identify the binding sites and phase association of these elements in assessing environmental quality and risk to human health in the urban environment.
Sequential extraction procedures has been accepted as a useful method for the prediction of long term adverse effects from soils and sediments that are contaminated [12,31,32] and can be used in assessing differences in mobility, bioavailability and ultimately the toxicity level in an environment situation.
The city that has witnessed such a tremendous increase in human population is Onitsha. It is the commercial hub of south-eastern Nigeria. And the city has continued to receive high human population. This has resulted in its expansion with most of the people living in relatively unplanned settlements. Investigation of the characteristics of trace elements in soils and sediments in this city is of great importance. Information about the geochemical characteristics of soils and sediments in the area appears rear, hence the relevance of this study. Also, the bioavailability of potentially harmful elements has not been carried out in this area of study. The objectives of this study are to determine the concentration of some potentially harmful elements in both soils and sediments, a variation of elements in different land use and ascertain the bioavailability of these elements.
Materials and Methods
Study area
Onitsha is located in south-eastern Nigeria. The city is about 45 km east to Awka. The city is characterized by an extensive flood plain that covers the entire Iyowa-Odokpa community in the southern part of the city and part of the Harbour industrial layout with an average elevation of 26 m while in the northeast around Trans-Nkisi, Ogigi and the GRA elevation ranges from 34 m to 159 m above sea level.
Onitsha is located within longitudes 6.5°E to 6.11°E and latitudes 6.45°N to 6.53°N (Figure 1). The total area coverage of Onitsha is about 72km2. The area as it is presently developed from merging of various indigenous communities. Onitsha is known for its attendant commercial activities, servicing most states and cities around it in Nigeria.
Figure 1: Study area with drainage [41]
Geology of the study area
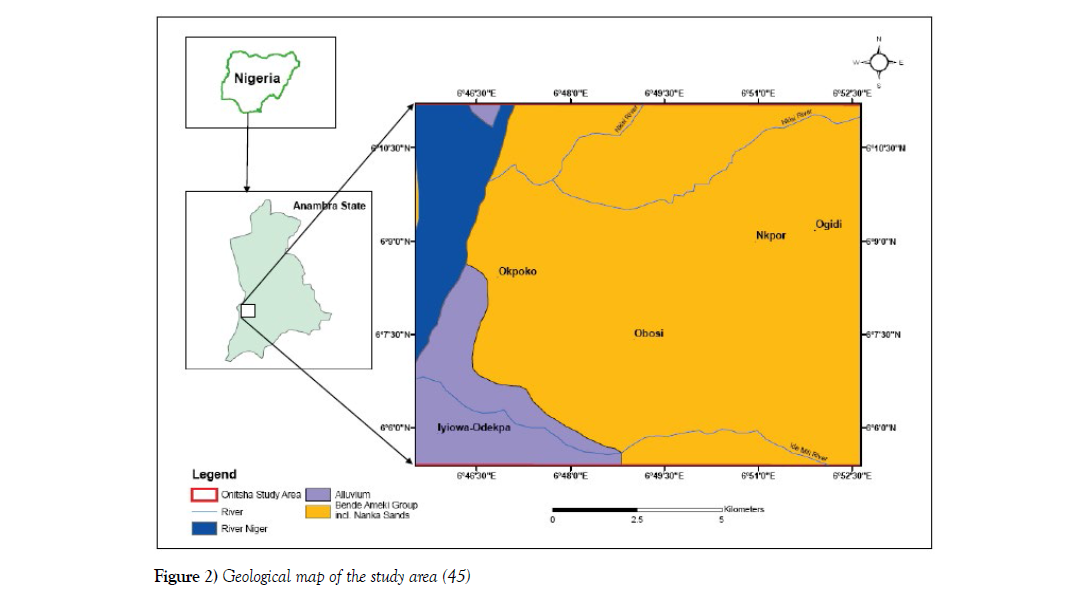
The Onitsha area is underlain by the Ameki Formation and at the western part of the area is bounded by the river Niger with the edge of the River housing the Alluvium deposits (Figure 2). The Ameki Formation is Eocene in age and its lateral facies equivalence is the Nanka Formation. The Ameki Formation consists of a series of highly fossiliferous grayish-green sandy clay with calcareous concretions and white clayey sandstone. It comprises two lithological groups. The lower groups are fine to coarse-grained sandstone with intercalation of calcareous shale and thin shaly limestone while the upper is coarse-grained cross-bedded sand-stone with bands of fine grey-green sands and sandy clay [33]. Nwajide CS [34] refers to the Ameki Formation to have between 1200 ft to 1500 ft with regressive facies, shallow marine environment as assigned. The formation overlies the Imo Formation. Its lateral equivalence is the Nanka sand, Figure 2.
Figure 2: Geological map of the study area [45]
Sampling
A total of 162 samples were collected for analyses. 83 topsoil [0-30 cm depth], 35 subsoil [30-100 cm depth], 20 stream sediment [11 from NdeMilli River and 9 from Nkisi River] at an interval of 250 m to 300 m, 21 Roadside drain sediment along high vehicle traffic roads at an interval of 250 m and 3 ferruginised sandstone samples as found from within the soil profile from the various quarry units in the study area. In other to achieve the said objectives, the sample locations were designed to follow land use pertain and appropriate code were given to these various land use. These include Residential Area [RA], Farmland and Garden [FLG], Active Waste Dump [AWD], Mechanic and Metal Workshops [MWM], Market and Abattoir [MAP], Schools and Office Complexes [SOC] and Control Samples [CT] from relatively pristine areas. While for the sediments samples, they include Roadside Drain Sediment [RSD], Ndemilli River [ID] and Nkisi River [NK]
The soil samples were collected with a stainless steel sampling auger of 1.5meter long this was to enable one get to the required depth of one [1] meter. While the sediment samples were collected with the aid of a clean plastic bowl. Three to four subsamples were collected and composited as one representative sample for every location for the soils and sediment samples. This was adopted in order to reduce point source contamination error. All the soil and Roadside drain sediment samples were stored into polythene bags and labeled at every location sampled.
The stream sediments were first sampled into a plastic bucket with the aid of plastic bowl and wet sieved with < 75 μm mesh right inside or by the side of the stream with the aid of the stream water, and the clay fraction was stored inside a 75-liter plastic bottle. This sampling procedure was adopted to reduce the contamination effect.
All the field sampling was carried out between December and January 2012 and December and January 2013. The choice for this time of the year was for easy accessibility in the study area and to reduce dilution effect that may be caused by rainfall on metals and organic compounds in both the soils and sediments.
The soil and roadside drain sediment samples were subsequently air dried under room temperature for 72 hrs and stored. While the stream sediment collected in plastic bottles were allowed to settle down, the water in the plastic bottle was decanted and the sediment in clay fraction was allowed to dry under room temperature for 5 to 6 days, there-after stored for further preparation.
Sample preparation
The soil samples collected were air-dried, sieved with 200 μm to remove all large particles. This was then followed by grinding, with the aid of mortar and pestle and subsequently sieved with > 63 μm in other to get the clay fraction. The stream sediment samples were wet sieved with > 75 μm, stored in a plastic bottle, allowed to dry under room temperature, and later pulverized and sieved with >63 μm. Replicate samples [n=4] were successively added to the entire samples for quality check. All prepared samples were then analyzed for elemental compositions.
Determination of electrical conductivity, pH, and total dissolve solid in soil and sediment samples
Fifty [50] grams of air-dried soil and sediment samples were measured and transferred into 200 ml beaker, and 100 ml of distilled water was gradually added and left for 30 minutes to facilitate water movement through the samples. The samples were later stirred occasionally with a glass rod and allowed to stand for 24 hours. The Hach Eco 40 multi, Milwaukee hand held digital meter capable of measuring pH, Electrical conductivity [EC] and Total Dissolved Solid [TDS], was standardized with buffered appropriately after which the electrode was dipped into the samples for the measurements of the pH, EC, and TDS. These analyses were carried out at the department of geology, University of Ibadan, Ibadan.
Determination of metal content in soils and sediments
0.5 g of both soil and sediment samples were first digested with aqua-regia [HNO3 and HCl]. Thereafter, the digests were subjected to elemental [trace elements] analysis determination were carried out using Inductively Coupled Plasma-Emission Spectrometry [ICP- MS] at the ACME Laboratories, Vancouver Canada.
As part of quality control for precision and consistency, replicate samples and blank samples analyses of the samples were carried out in the course of the analyses of the samples.
Sequential extraction analysis
Sequential extraction is selective extraction, which is usually carried out to mimic the release of the selective metals into solution under various environmental conditions. The procedure was designed to quantify partitioned metals in different solid phase based on their chemical form [12,31,35-38].
At each of the step of the process, calculated concentrations of chemicals and buffers were added and the samples were shaken on an end-over-end shaker. The leachate from each step was then digested and analyzed with an inductively coupled plasma mass spectrometer [ICP/MS], This multi-step procedure assures that all the metals of concern are completely extracted from the sample. The results from all the different steps are calculated and used to determine the accurate concentrations under different conditions. Factors such as pH of the acid used for adjustment, temperature, and duration of extraction are the critical factors that control the concentration of metal extracted from the sample. Below are the five-step sequential extraction methods that were chosen for this research, ten [10] samples from locations with a relatively high concentration of potentially harmful elements.
(i) Demineralized H2O leach water soluble components [LH1], at pH 7 for 2h
(ii) Carbonate fraction [LH2] 1 M ammonium acetate leach cations adsorbed by clay and elements co-precipitated with carbonates; adjusted to pH 5.0
(iii) Organic and Sulfide fraction [LH3] 0.1 M sodium pyrophosphate leach for elements adsorbed by organic matter [humic and fulvic compounds]
(iv) Reducible fraction [LH4] 0.1 M hydroxylamine HCl leaches for elements adsorbed by amorphous Mn hydroxide, often the most reactive soil phase for scavenging mobile elements
(v) Residual fraction [LH5] 0.25M hydroxyamine HCl for elements adsorbed by amorphous Fe hydroxide and more crystalline Mn hydroxide
Data quality and data analysis
The various elemental results that were obtained from the analyses were subjected to statistical analyses. The tools used included Descriptive statistics [median, mean, range, percentiles, standard deviation, and coefficient of variance]. These statistical interpretations were done using Excel 2007, SPSS [Statistical Program for the Social Scientists] version 15.0, Grapher 8, Origin and Surfer 8 respectively.
Geochemical interpretative tools such as Geo-accumulation [I-geo], was used to assess the degree of contamination which is carried out by the concentration level at present with the preindustrial level of concentration in the area. 39. Muller G [39] designed the computation formula;
Igeo = Log2 Cn/1.5 Bn,
Where Cn is the measured concentrations of the element in the sample and Bn is the geochemical background value. While 1.5 is a constant which allows for natural fluctuations in the content of any environmental media and very little anthropogenic influences.
Normal elemental abundance in unmineralized geologic materials is known as background concentration. In geochemistry, there is no one natural background level that can be used on the earth in evaluating earth materials like soils and sediments as in this case. This is because the earth is geochemically non-homogeneous, uniform distribution of elements, soil type, and horizon, [40] so, in environmental geochemistry studies, natural background values must be evaluated on a local scale. It is based on this that samples were taken from relatively pristine locations away from the urban area of study and a below one [1] meter depth.
Results and Discussion
Physical-chemical analyses of soils and sediments
The summary of the pH, Electrical Conductivity [EC] and Total Dissolved Solids [TDS] is presented in Table 1. The pH values of the soils ranged from 4.8 To 7.7 while that of the sediments ranged from 6.1.to 7.3. The EC in [μS/ cm] for the soils ranges from 6.41 to 794 and sediments ranged from 31.6 to 1059. And the TDS for the soils ranges from 4.05 mg/l to 508 mg/l and the TDS value for the sediments ranges from 20.2 mg/l to 668 mg/l, (Table 1). The pH values for the soils as observed had relatively low pH in location CT 2 [4.8] which is one of the control samples. The maximum value of pH was found in location MWM/3B [7.7]. Other locations showed slightly acidic pH to slightly alkaline concentration. Examples of such locations include FLG 7B and FLG 8 with pH of [5] at both locations, while location MWM 2 [7.2] and MWM 7 [7.2] respectively. Generally, it was observed that the distribution of the pH was influenced to some extent by land use activities. It was found that while the soils collected from farmland and gardens recorded relatively slightly acidic pH, the soils collected from the mechanic and metal workshops exhibited slightly alkaline pH. The pH of the sediment samples also showed the same pattern of distribution, with no clear variation of pH base on sediment type except on location bases. The few locations with relatively lower pH may have been attributed to point source effect which may be as a result of high organic decay such as plants in the area, hence the possible reason while most soils from farmland recorded lower pH value. The pH of soil and sediment affect the possible mobility and bioavailability of trace element hence the relevance in understanding the acidity or alkalinity of soils and sediment. This is also a function of other physicochemical characteristics such as Total Dissolved Solids [TDS], Electrical Conductivity [EC] and Temperature [T]. Varying concentration of conductivity values was observed in the soils and sediments in Onitsha, where the conductivity value ranges from 7.67μS/cm as found in location CT/5 to 694 μS/cm in location AWD/7 for soil, Table 1. The highest EC value was recorded in location RSD/12 [1059.0 μS/cm] this may have been connected with the fact that roadside drain sediment is a major receptor to solute dissolving in the sediment more than the soil, Table 1. A similar trend is observed in most of the samples from roadside drain sediment. The result of the Total Dissolved Solid [TDS] shows that the highest value was found in location AWD/4 [508 .0 mg/L] and the lowest value was found in location CT/2 [4.05 mg/L] for soils while the highest value for TDS in sediment was found in location RSD/12 [668.0mg/L] and the lower value was found in location RSD/10 [20.2 mg/L]. Locations with relatively higher TDS showed the presence of higher dissolved solids, which can be influenced by activities being presently taking place in the area.
Table 1: Summary of the physical-chemical parameters of the soils and sediments of Onitsha area (N=162)
| Parameters | Sediments | Soils | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| pH | 6.1 | 7.3 | 4.8 | 7.7 |
| EC (µS/cm) | 31.6 | 1059 | 6.41 | 694 |
| TDS (mg/l)) | 20.2 | 668 | 4.05 | 508 |
Trace elements distribution for soil and sediments
The summary of the elemental concentration of the soils and sediments is presented in Table 2. The elemental distribution in the soils and sediments of Onitsha metropolis revealed varying concentration for observed elements. The soils showed significant heterogeneity in the concentration of Pb, Zn, Mn, As and Cd. The concentration [in ppm] of the elements revealed that Pb ranges from [6.2-2026.4], Zn [3.0-7563.1], Mn [7.0-1920.0], As [0.5-27.3] and Cd [Nil-10.3], Table 2. The result of elemental concentration [ppm] in the sediments revealed that Pb ranges from [19.7-540.1], Zn [95.0-555.9], Mn [185.0-1410.0], As [0.6-16.9], Cd [0.1-1.6], Table 2. The heterogeneity of the various trace elements in both soils and sediments were found to be influenced by land use activities.
Table 2: Summary of trace element composition for soils and sediments in the study area (N=162)
| Land Use | Pb | Zn | Mn | As | Cd |
|---|---|---|---|---|---|
| Residential Area (RA) | |||||
| Min | 12.5 | 15 | 7 | 0.5 | 0.1 |
| Max | 257.3 | 2557 | 2127 | 9.1 | 2.2 |
| Mean | 68.7 | 422.6 | 596.5 | 5.2 | 0.5 |
| Std.Dev | 66.2 | 591.9 | 431.4 | 1.8 | 0.6 |
| School and Office complex (SOC) | |||||
| Min | 6.4 | 11 | 98 | 0.5 | 0.08 |
| Max | 253.1 | 452.3 | 779 | 13.2 | 0.9 |
| Mean | 61.01 | 173.11 | 402.65 | 5.34 | 0.35 |
| Std.Dev | 69.08 | 166.42 | 193.27 | 3.48 | 0.24 |
| Control (CT) | |||||
| Min | 7.2 | 3 | 10 | 0.5 | Nil |
| Max | 28.1 | 35.5 | 241 | 27.3 | Nil |
| Mean | 18.2 | 14.9 | 81.5 | 8.2 | Nil |
| Std.Dev | 6.2 | 10.2 | 72.2 | 8.4 | Nil |
| Farmland and gardens (FLG) | |||||
| Min | 10.9 | 17 | 109 | 0.8 | 0.09 |
| Max | 366.2 | 8395 | 1320 | 10.3 | 1 |
| Mean | 51.44 | 654.95 | 423.18 | 4.9 | 0.35 |
| Std.Dev | 84.71 | 2008.57 | 332.77 | 3.11 | 0.33 |
| Market and Abattoir (MAP) | |||||
| Min | 16.3 | 249 | 470 | 0.8 | 0.5 |
| Max | 270.2 | 5169 | 947 | 7.2 | 2 |
| Mean | 111.9 | 2161.1 | 679.2 | 4.6 | 1.1 |
| Std.Dev | 97.1 | 1932.1 | 211.6 | 3.2 | 0.6 |
| Active waste dump (AWD) | |||||
| Min | 20.4 | 52 | 181 | 1.1 | 0.2 |
| Max | 448.4 | 4298 | 1128 | 9.6 | 5.6 |
| Mean | 149.1 | 956.2 | 564.7 | 5.1 | 2.1 |
| Std.Dev | 136.8 | 1211.3 | 327.8 | 2.3 | 2.1 |
| Mechanic and metal works (MWM) | |||||
| Min | 22.4 | 24 | 117 | 2 | 0.1 |
| Max | 2026.4 | 7568.1 | 1920 | 19.8 | 10.3 |
| Mean | 379.1 | 1081.6 | 565.6 | 5.8 | 2 |
| Std.Dev | 545.9 | 1706 | 417.7 | 3.7 | 2.9 |
| Ndemili river (ND) | |||||
| Min | 19.7 | 101 | 204 | 0.6 | 0.1 |
| Max | 180.3 | 776 | 395 | 7.9 | 0.9 |
| Mean | 106.95 | 482.15 | 282.2 | 5.02 | 0.57 |
| Std.Dev | 53.66 | 227.93 | 60.28 | 1.97 | 0.25 |
| Nkisi river | |||||
| Min | 24.1 | 116 | 185 | 8.4 | 0.1 |
| Max | 83.7 | 555.9 | 1178 | 16.9 | 0.75 |
| Mean | 50 | 263.5 | 443.6 | 11.4 | 0.3 |
| Std.Dev | 21.7 | 149 | 337.4 | 2.6 | 0.2 |
| Roadside drain sediment (RSD) | |||||
| Min | 45.7 | 95 | 250 | 2.6 | 0.1 |
| Max | 540.1 | 1173 | 1410 | 8.6 | 1.6 |
| Mean | 151.37 | 502.49 | 451.38 | 5.29 | 0.65 |
| Std.Dev | 134.07 | 304.48 | 248.52 | 1.73 | 0.44 |
Zn exhibits the highest concentration in the soil sample collected around the vicinity of farmlands and gardens, location [FLG 3A] 8395.0 ppm. It was also observed that other locations with relatively higher concentration of Zn were found in Mechanic and metal works [MW] land use, these locations include MWM [17A, 4A, 5, 9, 10, 11, 16B, 16A and 6A] with Zn concentration in ppm of 7568.1, 3477.4, 2079.2, 5218.0, 1228.0, 2208.1, 2026.4, 1730.5 and 1444.3 respectively. Other areas with relatively high concentration were found in locations RA [4, 14, 18] with concentrations in [ppm] of 1221.1, 2557.0 and 1259.5 respectively, MAP [1A, 3A and 3B], 1274.3, 5169.0 and 2899.0 respectively as well as AWD [5A and 8 ] 1506.0 and 1337.0 respectively. Comparing the results of these locations with the control samples [CT] [2, 3, 4 and 6] with Zn concentration in ppm of 35.5, 10.0, 9.0 and 3.0 respectively, it shows that these locations are in 10 to 20 fold higher than the control samples which were sampled from the outskirt of the urban area with relatively no influence of urban activities [pristine locations]. Other locations show relatively low to the moderately high concentration of Zn in the study area, but all locations are found to have higher Zn concentration compare to the Zn concentration of the control samples. This shows significant enrichment of Zn which may have been affected by anthropogenic sources because of the presence of some out-lier values and relatively wide range in values of the Zn content in soils of the study areas relative to the results of samples collected from the outskirt of the study area.
The concentration of Pb in the soils in the study area was also relatively high is some locations. The highest concentration of Pb in [ppm] was found in location MWM 16b [2026.4 ppm] Table 2. Other locations with relatively high concentration of Pb [ppm] include locations MWM [6A, 9, 14, 16A, 5] with Pb concentration of 1444.3, 1067.5, 1048.1, 1730.5 and 580.5 respectively. Other areas include AWD 8 [448.4ppm], AWD 6A [311.9 ppm] and FLG 6A [366.2ppm]. While other locations showed relatively moderate to the low concentration of below 250 ppm. But comparing most of these results with results of control samples, CT [2-6] with Pb content in ppm of 15.7, 7.2, 22.1, 18.6, and 16.1 respectively, it is observed that most of the locations are 12 to 20 fold higher than most samples collected as control samples. This suggests that Pb content may have been enriched by anthropogenic activities as shown by the presence of wide spatial values in the Pb content of the soils in the study area.
High concentration of Mn was also observed in some locations of the study area. Very high concentration of Mn above 1000ppm were found in locations RA 5A [1069.0 ppm], RA 14 [2127.0 ppm], FLG 11 [1320.0 ppm], AWD 1 [1128.0 ppm], AWD 5A [1073.0 ppm], MWM 9 [1698.0 ppm] and MWM 11 [1920.0 ppm] which was the location with the highest concentration of Mn. many other location had relatively high concentration below 1000 ppm, such locations include RA 13B [969.0 ppm], RA 4 [876.0 ppm], RA 17 [875.0 ppm], FLG 9B [879.0 ppm], MAP 1B [860.0 ppm], MAP1B [947.0 ppm], AWD 6A [974.0 ppm], MWM 6A [992.0 ppm], MWM 16B [998 0ppm] and MWM 17B [900.0 ppm] among others that exhibited relatively moderate to low Mn content in the soil. Comparing the results of Mn content in most of the locations with control samples CT [2-4, 6] with Mn content in ppm of 241, 104, 66, and 10 respectively, it shows that most of the locations are significantly enriched by Mn content.
The content of As in the soils of Onitsha were observed to be of varying spatial distribution, with relatively high concentration found in some locations as against some other locations with low concentration. Locations RK 2 and RK 1 had As content of 27.3 ppm and 12.0 ppm respectively while other locations with relatively high concentration of As include MWM 11 [19,8 ppm], MWM 15A [9.3 ppm], MWM 2A [10.8 ppm], MWM 15A [9.3 ppm], AWD 8 [9.6 ppm], FLG 5B [10.3 ppm], FLG 3A [9.8 ppm], SOC 12B [13.2 ppm], SOC 12A [11.0 ppm], SOC 6 [12.8 ppm] and RA 14 [9.1 ppm], Table 2. Other locations were found to have relatively moderate to low concentrations, while some other areas recorded below detection limit [BDL]. One striking observation in the samples analyzed were the relatively high concentration of RK 2 and RK 1. These locations are the ferrogenised sandstone within the sedimentary profile in the study area, the samples recorded high concentration of As content, suggesting secondary geogenic enrichment of As within the ferruginous sandstone. But other locations with relatively high concentrations of As followed the influence of land use activities which is anthropogenic effect.
Cd content in the soils was also found to be of varying concentration from one location to the other. The highest concentration of Cd was found in location MWM 9 [10.30 ppm] closely followed by location MWM 17 [10.19ppm]. Other locations with relatively high concentration include location MWM 14 [6.0 ppm], MWM 11 [5.30 ppm], MWM 6 [4.3 ppm], MWM 4A [3.39 ppm] and AWD 8 [5.6 ppm], Table 2. Relatively moderate to low concentration of Cd were found in other location, while in some locations below detection limit were observed. Comparing the result of most of the locations with the control samples CT 2 to CT 6 all BDL. One main observation on the enrichment of Cd content in the soils was the influence of anthropogenic effect relating to land use.
Similarly the concentration of the metals investigated for sediments also exhibited spatial distribution with some locations showing more enrichment of metal content relative to the other. The sediments as earlier explained include the Nde-Milli River [ID], Nkisi River [NK] and Roadside drain sediments [RSD]. Lead [Pb] exhibits high concentration in location RSD/7 [540.1 ppm], RSD 8 [331.4 ppm]. Other locations include RSD/ 12, RSD /14, ID/ 5, ID /4 and 6 with Pb concentrations of 320.5, 180.3, 150.0, 147.8 and 140.9 ppm respectively. Comparing the results with the control samples CT 2 [9.83 ppm], CT3 [5.3 ppm] and samples collected far away from built up areas RSD [54.4 ppm] and NK 19 [19.9], it showed that most locations for the sediment samples have been enriched by Pb to between 5 to 10 folds.
Zinc content in the sediment samples also exhibited spatial distribution of metal enrichment, with location RSD 8 [1045.0 ppm], (Table 2) as such with the highest concentration of Zn. Other locations with relatively high concentration of Zn include location ID 1 [518.0 ppm], ID 2 [599.0 ppm], ID 3 [596.6 ppm], ID 4 [686.9 ppm], NK 14 [555.9 ppm], NK 20 [357.0 ppm], RSD 7 [439.0 ppm] RSD 24 [410.1 ppm], RSD6 [306.0 ppm], RSD 4 [365.0 ppm] and RSD 12 [320.5 ppm]. Comparing the results with the control samples CT/2, 3 and 4 with Zn content of 35.5, 10.0 and 9.0 ppm respectively it is observed that most of the locations for the sediments have been enriched greatly to between 8 to 15 folds.
The concentration of Pb in the sediments also showed varying concentration from one location to the other. The highest concentration of Pb was found in location RSD/7 [540.1 ppm], with other locations having lesser enrichment of Pb content but relatively high; RSD24 [410.1 ppm], RSD 8 [331.4 ppm] and RSD 12 [320.5 ppm] as against the the results of the Pb content in the control samples location CT 3 [7.2 ppm] and CT 2 [15.7 ppm] and other locations with relatively low concentration of Pb such as location ID 7 [19.7 ppm] and ID 8 [25.2 ppm], Table 2. This suggests that most of the result of Pb content in the sediment may have been enriched by activities relating to urbanization.
Similar spatial variations where found in Mn, As and Cd, with most of the locations within the metropolis recording relatively higher concentration of trace metals compare the the results of the control samples, Table 2.
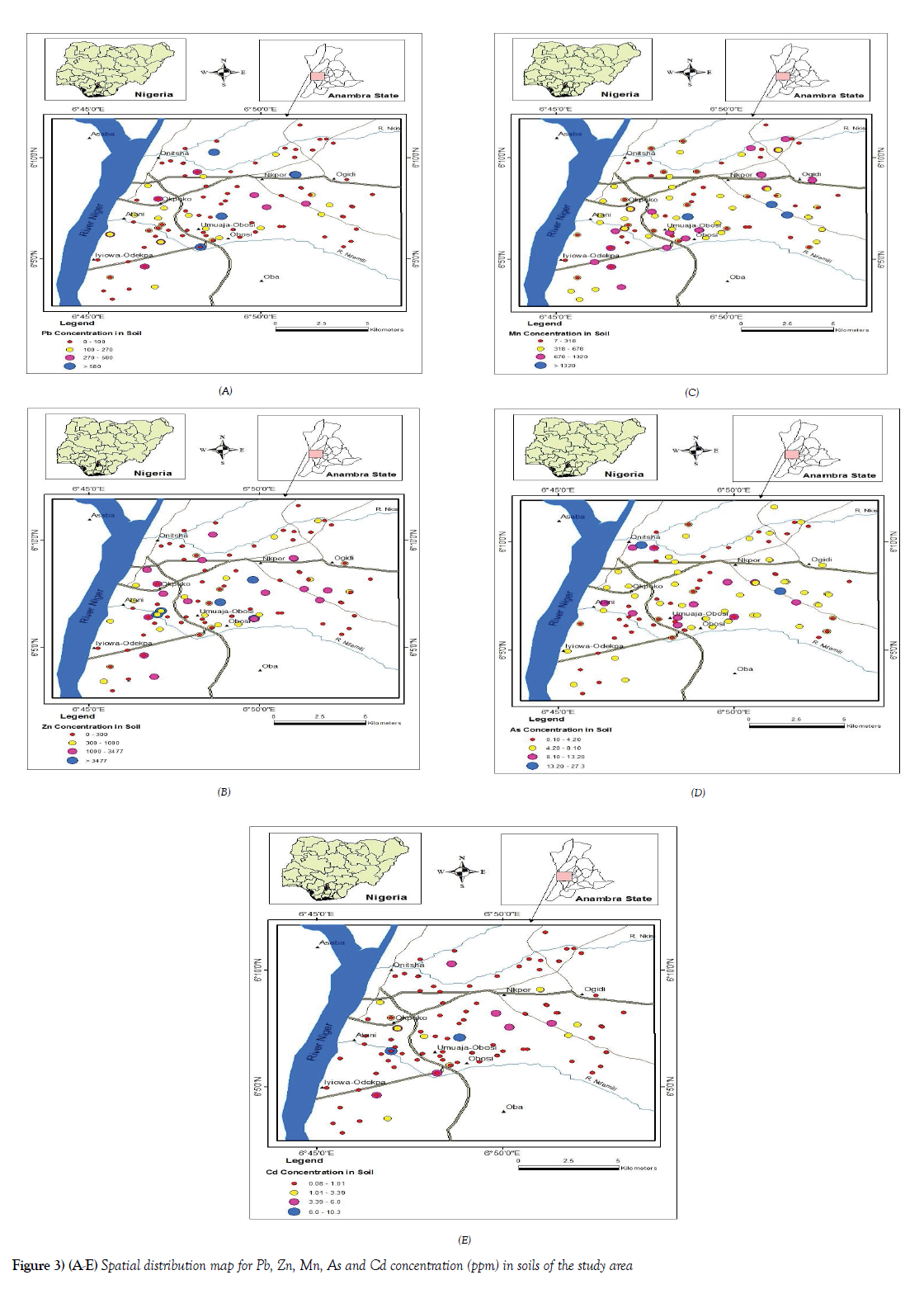
Spatial distribution of elements in soils
Geochemical maps using ArcView GIS software were created to visualize the spatial distribution of trace elements studied in soils in the study area (Figures 3A-3E). The concentrations were plotted as graduated points to reduce overgeneralization errors.
The spatial distribution of Cd concentration in Onitsha soils showed some locations with a relatively high concentration of Cd (Figure 3E). These areas are such with relatively high human activities, Okpoko, Umuaja-Obosi, Atani road area among the areas with an elevated concentration of Cd. Relatively moderate to low concentration were found around the residential areas as well as outskirt of the town with relatively low human influence. Similarly, geochemical maps of the spatial distribution of Pb, Zn, As, and Mn, show that the areas with relatively high concentration of these elements are within the core of the town, Upper Iweka, Nkpor, New Tazan, old Port High court, Okpoko, Umuaja- Obosi areas of the metropolis, relative to the soils collected at the outskirt of the town as seen in the control samples. Virtually the whole element exhibited the same pattern of enrichment distribution, showing the influence of urbanization and industrialization on the enrichment of the metals in the study areas. Following the land use pattern, elements such as Pb, Zn, and Mn, were found to be elevated in mechanic and metal workshop locations [MWM], active waste dump [AWD] site as well as farmland and garden [FLG] areas respectively. Since this type of land use is common within the city center and in some location a little at the outskirt of the town, such activities are never the less major contributors to the enrichment of these metals to soils in an urban environment. As concentration distribution though had some locations with higher elevation at the core of higher human activities (Figure 3D), some of the locations with relatively high elevation were the ferrogenised sandstone within the sedimentary lithology at Trans Nkisi road, at the back of the Nigeria prison and near formal toll gate at Ogidi area, which were used as part of the control samples. Hence the observations of such in the distribution map.
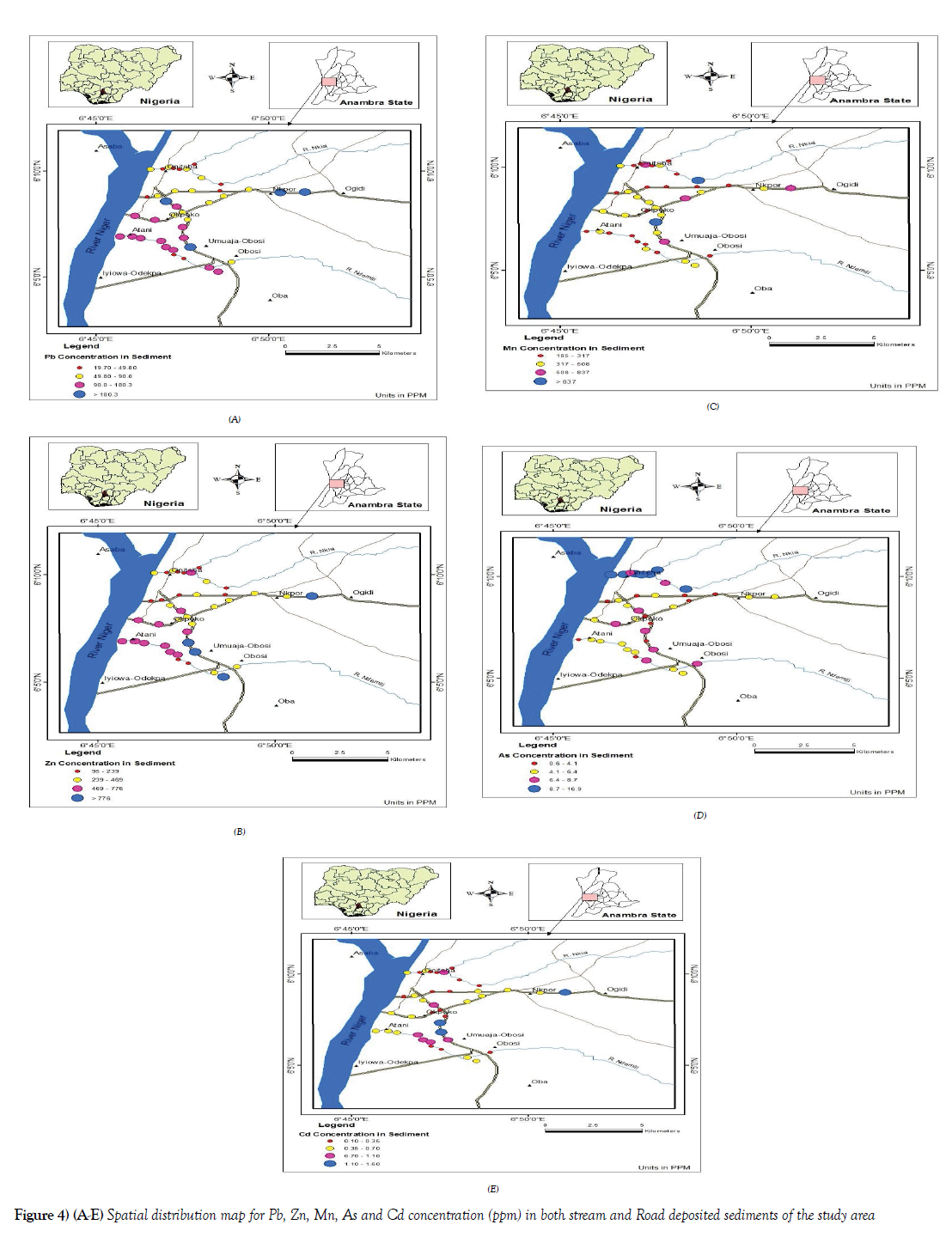
Spatial distribution map of sediments in Onitsha metropolis
Geochemical maps were also created for the trace element investigated for sediments which were also aimed at visualizing the spatial distribution of trace elements in Roadside drain sediments [RSD], NdeMilli river [ID] and Nkisi river [NK] sediments in the study area (Figures 4A-4E]. A point source type was also used for the same reason stated above in the case of soils geospatial map. Base on the spatial distribution of all the elements. it is observed that most of the elements with elevated concentrations found on the roadside drain sediment [RSD], are on the highway road drains, the drains on the Onitsha – Enugu expressway, the Onitsha- Owerri expressway and the old PH road relative to the concentration of those elements assessed on less vehicular traffic way like the old Onisha- Enugu road and Nnamdi
Azikiwe road. Again, it was observed that the Ndemilli River recorded relatively high elevation.
The relatively higher concentration of these elements on most of the samples collected from the Ndemilli river may have been influenced by the fact that the Ndemilli river drains over 60 % off of the total developed area of Onitsha metropolis relative to the Nkisi river which drains smaller portion of the metropolis and the areas that the Ndemilli drains are more of industrial layout, commercial areas as well as places with huge human and its related activities thereby generating different kinds of liquid and solid waste that eventually find its way into the river, on like the Nkisi river with relatively lesser human activities.
Effect of land use on the distribution of trace elements
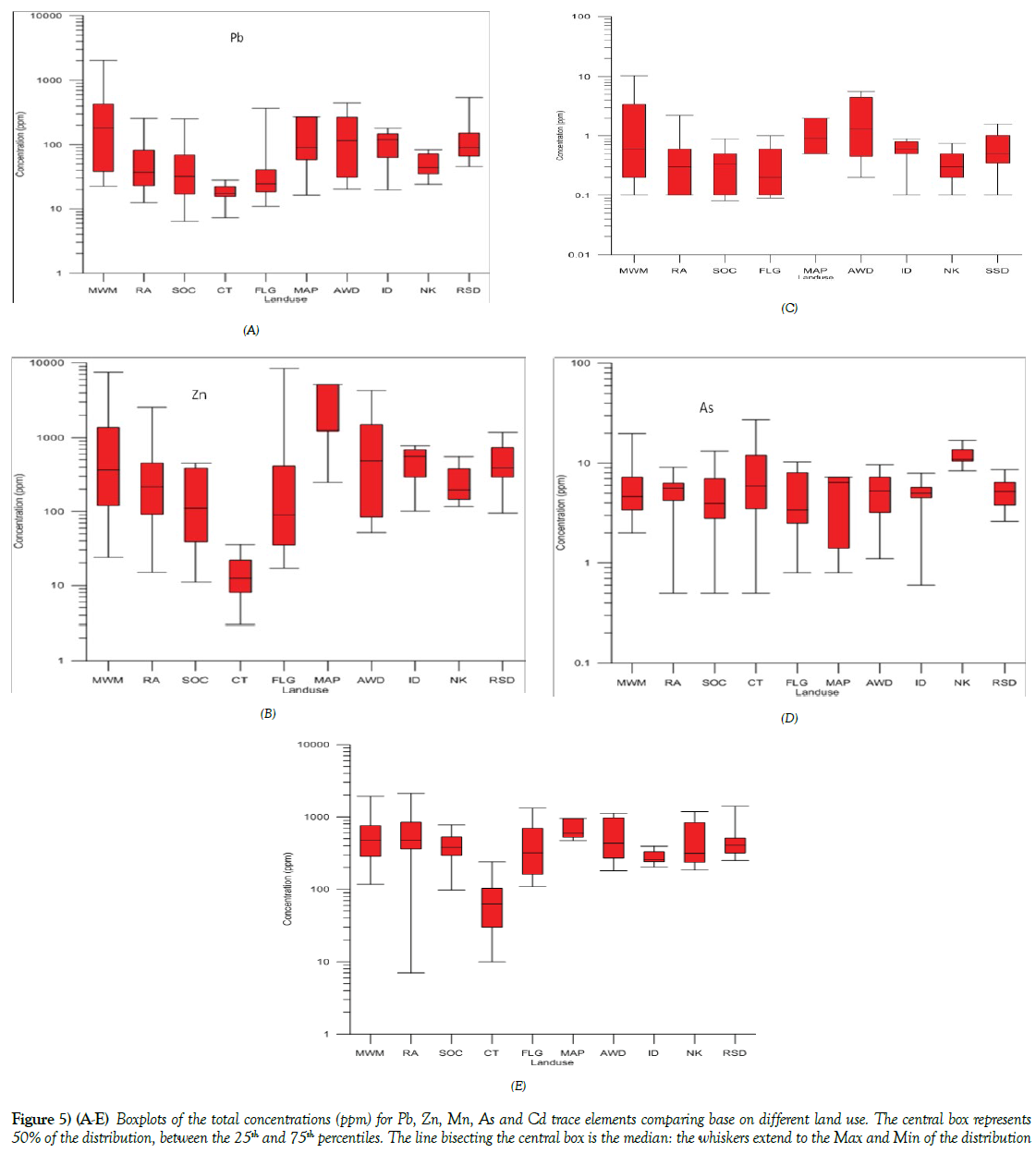
The highest concentration of Zn in the study area was found to be from the soils collected within the vicinity of FLG 3A with [8395.0 ppm], with a mean concentration of 654.95 ppm standard deviation of ± 2008.57 ppm, (Tables 3 and 4) This result showed that activities that enriched Zn content may have been used in the farmland and garden [FLG] land use in Onitsha; this includes the use of fertilizer to enhance the garden, a practice that is common because the garden land area always been used continuously on annual bases. Again the land may have been enriched with Zn because of waste iron and steel materials that are been emptied in the farm area which contain corrosive protection materials. The relatively higher concentration of Zn [24.1-7568.1 ppm], mean [1081.6 ppm] was also found in soils collected from Mechanic and Metalworks [MWM] land use. This area is such that contain used gasoline, diesel, engine oil, iron, and steel materials, paint as well as other vehicular activities taking place in the area. This kind of anthropogenic activities greatly enriches Zn in the soil of the study area. comparing the mean concentration of the various land use (Figures 5A-5E) which include; SOC [173.11 ppm], RA [422.6 ppm], MAP [2161.1 ppm], AWD [956.2 ppm], ID [482.15 ppm], NK [263.5 ppm] and RSD [502.49 ppm] and the CT, [14.9 ppm], it is found that most of the land use soils and sediments have been enriched with Zn concentration that calls for concern. Figure 5B is a boxplot showing the various statistical distribution of Zn in the various land use.
Figure 5 (A-E): Boxplots of the total concentrations (ppm) for Pb, Zn, Mn, As and Cd trace elements comparing base on different land use. The central box represents 50% of the distribution, between the 25th and 75th percentiles. The line bisecting the central box is the median: the whiskers extend to the Max and Min of the distribution
The same pattern of metal enrichment was observed in Pb content in the soils of various land use. From the results, Pb was found to be highest in the soils from MWM [2026.4 ppm] the mean and standard deviation in ppm of the various land use; for residential area [RA] [68.7 ± 66.2 ppm], school and office complexes [SOC] [61.01 ± 69.08], control samples [CT] [18.2 ± 6.2], farm land and gardens [FLG] [51.4 ± 84.7], market and abattoirs [MAP] [111.9 ± 97.1], active waste dump [AWD] [149.1 ± 136.8], mechanic and metal workshops [MWM] [379.1 ± 545.9], Ndemili river [ID] [106.9 ± 53.44], Nkisi river [NK] [50 ± 21.7] and Roadside drain sediments [RSD] [151.37 ± 134.07], Table 2, it is observed that while the soils from the control [CT] has the lowest concentration, all other lands uses have higher Pb concentration, indicative of differential influence of land use to the enrichment of Pb content in the soils and sediment. The enrichment of Pb in the environment may have been influenced by anthropogenic like petroleum/gasoline usage, paints, storage batteries, antiknock agent etc. hence the relatively high concentration of Pb content in the soils collected within the vicinity of MWM compare to other land use (Table 2 and Figure 5A).
The concentration of Cd in the different land use shows the influence of land use activities on the presence of Cd in the soils and sediment. It was observed that the samples collected within the vicinity of mechanic and metal works [MWM] exhibited the highest concentration of Cd [10.3 ppm] followed by active waste dumps land use [5.6 ppm], compared to the results of the soils collected from the control areas with no detection of cd [Nil], Table 2 and Figure 5E. Other land areas like the market and abattoirs areas [MAP] [2.0 ppm] showed relatively moderate to the low concentration of Cd. The areas with the enriched content of Cd may have been as a result of urban waste like electrical components, domestic burning of coal-rich materials, storage batteries and allows of metals.
The mean concentration of As for the control samples were found to be relatively high in control samples [CT], [8.2 ppm] compare to the other land use, (Table 2 and Figure 5D). The ferrogenised sandstone sample in the sedimentary profile was found to have high concentration of As, RK 1 [12.0 ppm] and RK 2 [27.0 ppm] relative to the concentration of other control samples CT 2 [3.8 ppm], CT 5 [3.5 ppm] and CT 6 [0.5 ppm]. The enrichment of As in the ferrogenised sandstone may have been as a result of secondary geogenic enrichment of the As in the ferogenised rock samples. The other land use also showed differential mean concentration, with MWM [5.8 ppm] showing relatively higher concentration of As compare to other land use.
Comparison between the top soils and sub soils metal content of Onitsha Area
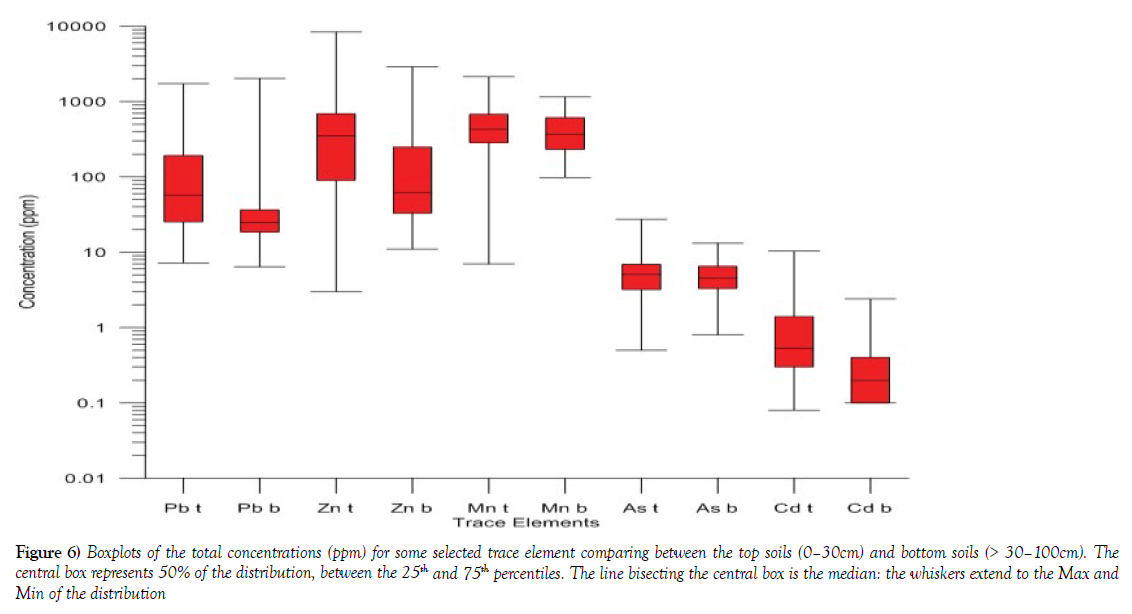
Comparative analyses of Pb, Zn, Mn, As and Cd trace elements were carried out using the boxplot to evaluate the variation that exists between the soils collected at a depth of 0-30 cm and > 30-100 cm depth. The trace element composition in both the top soils and subsoils shows the varying concentration of these elements (Table 5 and Figure 6). The mean concentration of Zn showed that topsoil had [847.12 ppm mean] compare to the subsoil [259.00 ppm mean]. This suggests that the topsoil is much more enriched with Zn content at the topsoil relative to the subsoil. Pb means content in the topsoil [167.99 ppm] showed higher mean concentration that in the subsoils [98.66 ppm]. Similar metal content characteristics were observed in almost all the element investigated, (Table 5 and Figure 6), which implies that the relatively high concentration of most of this element may have been enriched by urban and industry related activities hence the wide variation between the topsoil and subsoils to in some instance two to threefold higher in the topsoil than the subsoil.
Figure 6: Boxplots of the total concentrations (ppm) for some selected trace element comparing between the top soils (0–30cm) and bottom soils (> 30–100cm). The central box represents 50% of the distribution, between the 25th and 75th percentiles. The line bisecting the central box is the median: the whiskers extend to the Max and Min of the distribution
Comparison of the mean concentration of soils of selected cities in the world with this study
The mean concentrations of some potentially harmful elements in soils of some urban cities in the world with this study are presented in Table 3. From the observation, it was observed that in most of the elements being discussed, there is a considerably higher concentration of some of the elements in the present study area [Onitsha] with most of these referenced cities to an alarming rate that calls for concern. For example, the mean concentration of Zn in Onitsha [present area of study] is found to be higher than all the referenced cities. A similar phenomenon is being observed in Pb where except for Benin City, the mean Pb concentration in this study is found to be higher than all other cities (Table 3). The mean concentration of As in this study is higher than other cities referenced except that of Idrija [Slovenia] were that of Idrija is more than 4 fold higher than that this study. A similar observation is made for Cd and Mn, Table 3.
Table 3: Comparison of mean concentration (ppm) of selected potentially harmful elements in soils of some urban areas of the world with this study area
| City | As | Cd | Mn | Pb | Zn | Reference |
|---|---|---|---|---|---|---|
| Idrijaa | 20.2 | 0.6 | 49.4 | 120 | Bavec, et al [46] | |
| Berlinb | 3.9 | 0.35 | 76.6 | 129 | Bavec, et al [46] | |
| Ibadanc | 3.9 | 8.4 | 1097 | 95.1 | 228.6 | Odewande, et al [42] |
| Norwayd | 3 | 0.24 | 52 | 151 | Andersson, et al [47] | |
| Murciae | 0.13 | 135 | 21.9 | 16.6 | Acosta, et al [48] | |
| Benin Cityf | 1.6 | 3.01 | 425.29 | 232.31 | 533.1 | Olatunji et al [43] |
| Kowloog | 0.62 | 94.6 | 125 | Lee, et al [23] | ||
| This study | 5.61 | 1.4 | 514.46 | 167.99 | 847.12 |
Comparison of the mean concentration of sediments of selected cities in the world with this study
Similarly, Table 4 shows the mean concentration of some potentially harmful elements in urban cities of the world with this study. From the results, As mean concentration in the two rivers and the roadside drain sediment studied is higher than the mean concentration in the As reported in the Lagos lagoon. The mean concentration of Cd in the study area is lower than most of the cities referenced except for the Lagos lagoon but with close level of concentration, Table 4. the mean lead [Pb] concentration in the study area is similar to most of the cities referenced for comparison except cities like Seoul and Uijeongbu City sediments were their Pb mean concentration is significantly higher than the study area sediments to between 2 to 3 folds. However, the results of Pb in the area are of concern.
Table 4: Comparison of mean concentration of selected potentially harmful elements in sediments of some urban areas of the world with this study area
| City | As | Cd | Mn | Pb | Zn | Reference |
|---|---|---|---|---|---|---|
| Seoul Citya | - | 4.3 | - | 214.3 | 2665 | Lee et al [12] |
| Uijeongbu Cityb | - | 1.2 | - | 534 | 334 | Chon et al [20] |
| Birminghamc | - | 1.62 | - | 48 | 534 | Charlesworth et al [49] |
| Lagos Lagoond | 2.99 | 0.27 | 494.98 | 20.27 | 72.33 | Olatunji et al 2010 [26] |
| Kottuli [Indian]e | - | - | 127.8 | 43.46 | 2848 | Harikumar et al [50] |
| Aqaba Cityf | - | 2.5 | 96.5 | 206 | 153 | Al-Khashman [29] |
| Jordang | - | 1.87 | - | 270.5 | 350 | Al-Mumani [51] |
| Calcuttah | - | 3.12 | - | 536 | 159 | Chatterjee and Banerjee [52] |
| This Study* | 5.02 | 0.57 | 282.2 | 106.95 | 482.5 | NdeMilli Rv |
| This Study** | 11.4 | 0.3 | 443.6 | 50 | 263.5 | Nkisi Rv |
| This Study*** | 5.29 | 0.65 | 451.38 | 151.37 | 502.5 | Roadside drain Sediment |
Table 5: Comparative statistical result of trace elements (ppm) for Top and Sub soils in Onitsha area
| Heavy Metal | Top Soils (N=83) (ppm) 0 -30cm | > 30 - (N=35) Sub Soils 100cm (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Std. Dev | Min | Max | Mean | Std. Dev | |
| Pb | 7.2 | 1730.5 | 167.99 | 292.8 | 6.4 | 2026.4 | 98.66 | 335.72 |
| Zn | 3 | 8395 | 847.12 | 1542 | 11 | 2899 | 259 | 543.78 |
| Mn | 7 | 2127 | 514.46 | 392 | 98 | 1148 | 451.78 | 295.15 |
| As | 0.5 | 27.3 | 5.61 | 4.01 | 0.8 | 13.2 | 5.24 | 2.55 |
| Cd | 0.08 | 10.3 | 1.4 | 2.17 | 0.1 | 2.4 | 0.42 | 0.61 |
Zn means concentration in this study is found to be lower than cities like Seoul and Kottuli [Indian] where the mean Zn concentration is 4 to 5 fold higher than the results in the three areas of this study. But significantly higher than most other cities sediments as referenced Table 4. Similarly, variation in mean concentration is found in Mn, Table 4, where the influence of human population, urbanization, and industrialization play a significant role in the relative variation of the enrichment of these elements.
Environmental assessment of the study area
Geo-accumulation index assessment
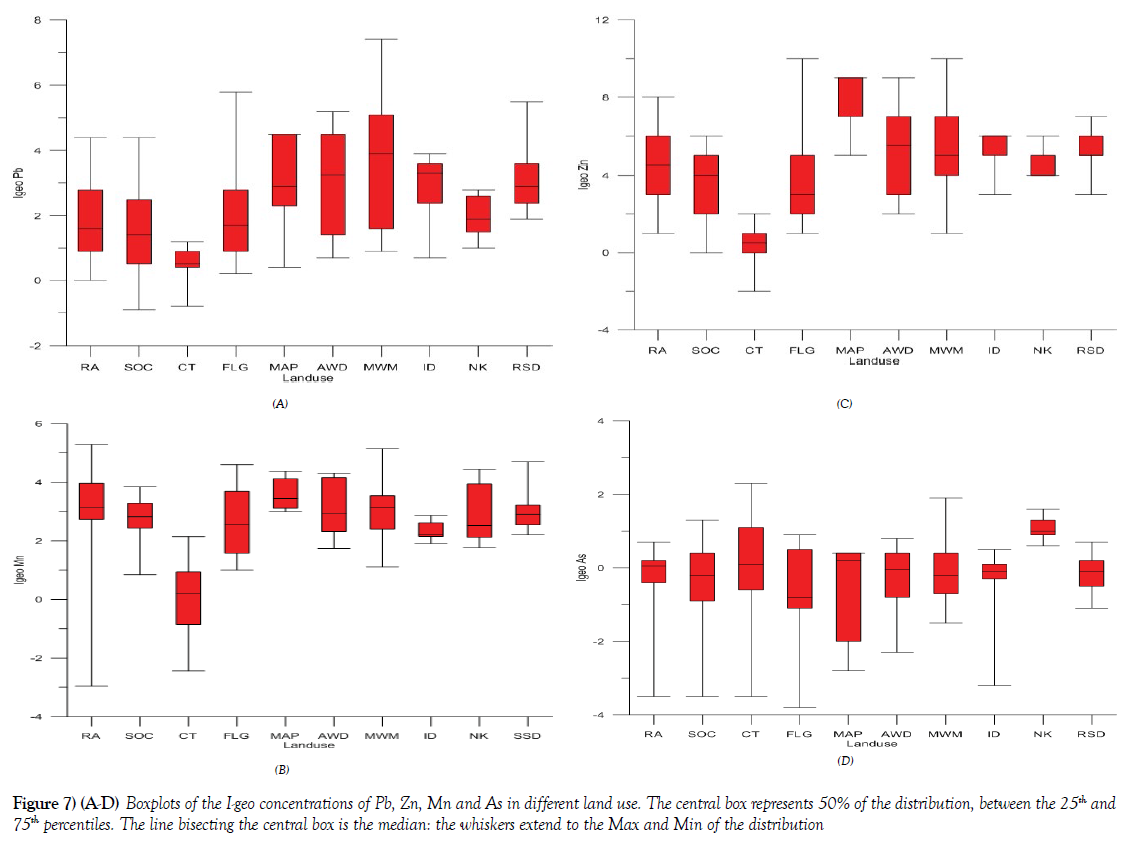
The summary and detailed results of the calculated Geo-accumulation Index Assessment [I-geo] values from selected metals in the Onitsha metropolis according to different land use are presented in Figures 7A-7D. The selected metals presented in box plot include Pb, Zn, Mn, and As. The results of the I-geo of these summarized elements revealed that the soils and sediments from different land use vary from practically unpolluted, as exhibited by most of the samples collected from control area [CT] to highly polluted land use, as exhibited by soils and sediments from MWM, AWD, ID, and RSD. Pb I-geo in the various land use showed that MWM exhibited the highest pollution status in most of the samples analyzed, relative to other land use with characteristics of moderate to heavily contaminate as against the I-geo characteristics of practically uncontaminated to moderately contaminated for samples of the control samples. The summary result for Mn, Zn and As of the I-geo characteristics are presented in Figures 7A-7D respectively. These results are in agreement with other published work with similar studies [41-43], were enrichment and pollution status of most of these potentially harmful elements are influenced by a factor like urbanization, land use type among others.
Geochemical partitioning of trace elements: Result of sequential extraction
Chemical fractionation is a natural phenomenon in trace elements within the geologic environment, which are coursed by dissolution, precipitation, sorption and complexation [43-45]. The use of sequential extraction method to evaluate phase characteristics within the geologic media is essential, which help to determine the bioavailability of the determined total elemental composition in the environment.
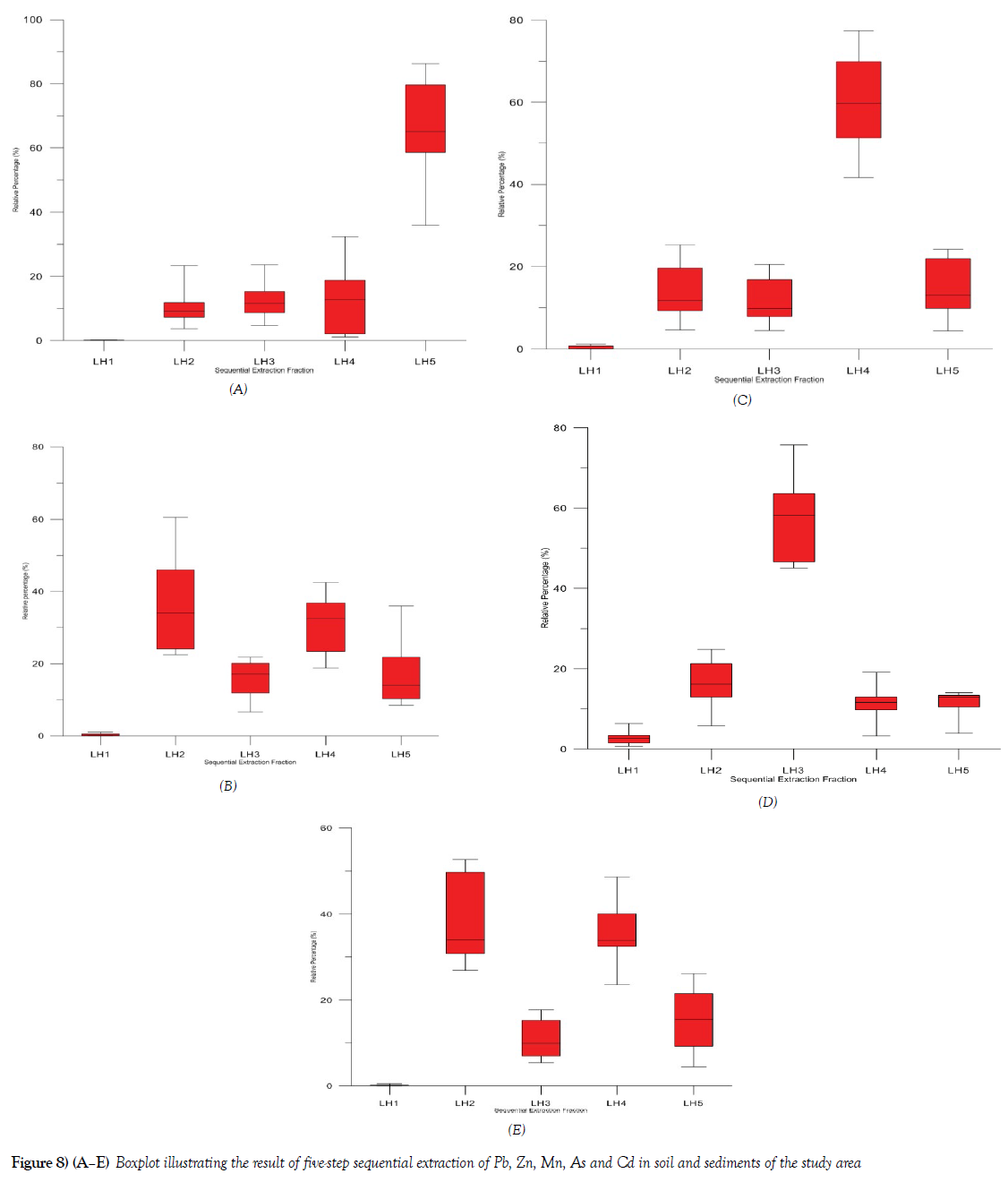
A five-step sequential extraction methods fraction was used for both the soils and sediment in the study area. Ten [10] samples that had relatively very high concentration of most of the potentially harmful elements were selected for the sequential extraction using LH1 [Demineralised H2O], LH 2 [Carbonate fraction], LH 3 [Organic and Sulfide fraction], LH 4 [Reducible fraction], LH 5 [Residual fraction]. The geochemical fractionation pattern of Pb, Zn, Mg, As and Cd in the soils and sediments are shown in Figures 8A-8E. The results are expressed in graphical form as leaching percentage, which shows the individual fraction that is removed relative to the sum of all fractions. The selected trace elements showed different chemical fractional availability, which suggests that at the different chemical form, element varies relative to the total fractional composition. The chemical fraction of As (Figure 8D) is dominated by the organic and Sulfide fraction [LH 3], with average fractional composition of about 58% of the total As that were analyzed and the Ammonium acetate fraction LH 2 average 18%, exhibiting that these two fractions are the most important fractions of consideration in terms of its ability to leach such quantity in both soils and sediments. It also becomes important to note that within the environment, any slit decrease in the pH of the water, it can react with the organic material and As can easily be available to any possible biologic [plants and animals] materials within the environment. The remaining chemical fraction of As are relatively negligible < 22% though of concern, especially that of the demineralize fraction, with < 7% can be said to be negligible to the biota.
Cd was identified to be bound to the LH2 fraction, Figure 8E, with an average concentration of about 34% of the total mean Cd in the media. Others with relative fractional affinity are LH 4, 33%, LH 5, 15% and LH 3, 11% of the total Cd respectively. Cd did not show many leaches with the demineralized fraction with <1% of total Cd in the media. Mn exhibits a fractional pattern that shows that substantial fractions of the total Mn are bound to the LH 4 phase, with an average of 61% of the total Cu, while the remaining fraction has 39% of the total Mn in the samples. LH5, 13%, LH 2 13%, LH3 11% and LH1, < 2% respectively. The relatively high proportion of Mn in the LH 4, fraction suggest that Mn is bounded more to the silicate lattice of the media analyzed in the study area (Figure 8C).
The geochemical phases exhibited by Pb shows that Pb is bounded more to the LH 5 fraction, accounting to an average of 66% of the total Pb in the study media. LH 4 accounted for 14%, LH 3, 13%, LH 2, 11% while LH 1 fraction is significantly less than 1% respective on the average of total Pb concentration in the study media. The relatively high proportion of Pb bound to LH 5 fraction shows that Ph in the study media is bound to the silicate lattice, which suggest that it present does not show much bioavailability under normal environmental condition (Figure 8A).
The geochemical association of Zn in the study media is dominated by LH 2 [average 33%], and LH 4 [average 32%], Figure 8B, of the total Zn concentration respectively. LH 3 fraction accounted for [average 19%], LH 5 fraction [average 14% and LH 1 [average 1%]. Zn concentration in the soils and sediment in many of the sample location had relatively high concentration, with the relatively high proportion of Zn in LH 2 accounting for [average 33%] of the total Zn, this calls for concern since any slight decrease in the pH in the environment of over 30% of total Zn will be released to the environment
Geochemical and Environmental importance of solid phase partitioning
The results of the solid phase partitioning of the soil and sediment analyzed suggest that comparative mobility and bioavailability of the metals in Onitsha metropolis tend to decrease in the following order Zn > Cd > AS > Mn > Pb. From the results of metal partitioning in the soils and sediment from Onitsha indicates that any change in the pH in the environment changing toward weak acidic conditions may possibly remobilize substantial percentage of Zn [21%–62%] for both LH1+LH11, for Cd, [> 56%]. Hence acid rain [pH < 5.8] may appreciably dissolve the substantial quantity of total Zn and Cd in the soil and sediment. This same chemical characteristic is been exhibited for As were as LH1+LH2 apparently releases up to 30% of total As content in the soil and sediment. This cannot be said of Mn and Pb were much of the total concentration is leached at LH4 and LH5 respectively, which are more of higher strength of extraction reagent [cold and hot hydroxyamine HCl] respectively, of which such conditions though possible, are always very rear.
In terms of eco-toxicity, Zn, Cd, and As are much of great concern, hence need frequent environmental monitoring for possible potential hazard. This is because of the quantum of the fractions of these elements release from weaker reagent which can possibly be exhibited by the soils and sediment as against the other elements analyzed that are mainly leached in the reducible and residual fractions. Lead [Pb] was found to be mainly contained in the residual fraction, suggesting that the bioavailability health risk of Pb leaching is comparatively little, which suggests that Pb is of minor concern as it relates to eco-toxicity. Trace elements that are leached from soils and sediment eventually are drained through both the artificial and natural drains to the River Niger. Again, other pathways of metals such as the continuous cultivation of non- seasonal crops along the banks of the rivers, a wetland in the area, rearing of animals that frequently feed on surface water, grasses in the streams and respiratory pathway from dust particles among other possible means are what should be considered.
Conclusion
A total of 162 soil and sediment samples were analyzed for Zn, Pb, Mn, As and Cd. Generally, it was found that most of the elements have relatively high concentrations of these metals that indicate a potential environmental hazard. The result exhibits much more high concentration of these harmful elements being influenced by human, industrial and commercial activities as against samples used as a control which was collected at the outskirt of the city which were used as control samples. The results of Pb and Zn in soils collected from mechanic and metal workshops, Active waste dump, farmland, and garden, market and Abattoir up to between 10 to 8 fold higher than the control area. Mn, and Cd, metal content is found to be high in the soils and sediment in MWM, AWD, FLG among other land use. Compare to the control samples. Similarly, the metal content of the element analyzed was found to be of 2 to 4 fold higher in the top soils compare to the subsoil samples. The implication of these results suggests that the soils and sediment have received considerable inputs of anthropogenic enrichment of these metals, primary from vehicular activities, industrial waste, and commercial activities. This poses great threat health wise to the inhabitant and pedestrians in the metropolis.
Results for the sequential extraction shows that most of the metals selected for this research have varying potential mobility and bioavailability characteristics. From the result, significant quantities of most of the metals did not occur in the demineralized fraction. But significant quantity was found in the organic fraction [LH2] making a combination of LH1 and LH2 to be significant as exhibited by Zn and Cd [21% - 62%] and [>56%] respectively of their total metal concentration. As [>58%] is possibly leached at the organic and sulfide fraction [LH3], which suggest that whenever there is a relative decrease of the pH to the acidic value, will bring about metal remobilization of such percentage stated above.
Pb and Mn exhibited limited metal mobility characteristics except under very low pH conditions because a larger percentage of the total metal content could only be reached by the reducible [LH4] and residual [LH2] fractions respectively which suggest that they are mostly bound to the silicate lattice. Base on environmental significance, Zn, Cd, and As can possibly be mobile and bioavailable with slight acidic environmental conditions because they can be removed by a combination of [LH1, LH2, and LH3] with more than 50% of the total metals. While Pb and Mn, have relatively low potential to remobilize except with strong [acidic], very low pH conditions.
Acknowledgements
The authors of this paper are very grateful to the anonymous reviewers for their work in reading the manuscript very carefully as well as their valuable comments made.
REFERENCES
- Nriagu JO, Pacyna JM. Qualitative assessment of world-wide contamination of air, water and soils by trace metals. Nature. 1988;333:134-39.
- Nriagu JO. Human influence on the global cycling of trace metals. Palaeogeogr Palaeocol. 1990;82:113-20.
- Alloway BJ. Heavy metals in soils. Blackie, John Wiley and Sons Inc. 1990:339.
- Alloway BJ. The origin of heavy metals in soils. In B.J. Alloway [Ed] Heavy metals in soils. London, UK: Blackie Academic and Professional. 1995:38-57.
- Poggio LVB, Rainer S, Erwin HAF. Metals pollution andhuman bioaccessibility of topsoils in Grugliasco [Italy]. Environ pollut. 2009;157:680-89.
- Okorie IA. Determination of potentially toxic elements [PTEs] and an assessment of environmental health risk from environmental matrices. Doctoral thesis, Northumbria University. 2010:201.
- Harrison RM, Laxen DPH, Wilson SJ. Chemistry Association of Lead, Cadmium, Copper, and Zinc in street dust and roadside soil. Environ Sci Technol. 1981;15: 1378-83.
- Thornton I. Metal contamination of soils in urban areas: in: Bullock P, Gregory PJ [Eds]. Soils in the urban environment. Blackwell. 1991:47- 75.
- Schumacher M, Meneses M, Granero S, et al. Trace element pollution of soil collected near a municipal solid waste incinerator: human risk. Environ Contam Toxicol. 1997;59: 861- 67.
- Okafor EC, Opuene K. Preliminary assessment of trace metals and polycyclic aromatic hydrocarbons in the sediments. Intl J Environ Sci Technol. 2000;4(2):233-40.
- Peckey H. Heavy metals pollution assessment in sediments of the Izmir bay, Turkey. Environ Monit Assess. 2006;123: 219-31.
- Lee PK, Yu YH, Yun ST, et al. Metal concentration and solid phase partitioning of metals in Urban roadside sediments. Chemosphere. 2005;60:673-89.
- Lu Y, Yin W, Huang L, et al. Assessment of bioaccessibility and exposure risk of arsenic and lead in urban soils of Guangzhou City, China. Environ Geochem Health. 2011;33:93-102.
- Zheng W, Lichwa J, Yan T. Impact of different land uses on polycyclic aromatic hydrocarbon contamination in coastal stream sediments. Chemosphere. 2011;84:376-84.
- Thornton, I. 1991. Metal contamination of soils in urban areas: in: Bullock, P., Gregory ,P J. [Eds]. Soils in the urban environment Blackwell. PP 47- 75
- Tiller KG. Urbansoils contamination in Australia. Aust J soil Res. 1992;30:937- 57.
- Southerland R A. Bedsediment associated trace metal in an urban stream, Ohau, Hawaii. Enveron Geol. 2000;39: 611- 27.
- Laluraj CM, Nair SM. Geochemical index of trace metals in the surficial sediments from the western continental shelf of India, Arabian Sea. Environ Geochem Hlth. 2006;28:509-18.
- Lyons WB, Harmon RS. Why urban geochemistry? Elements. 2012;8:417 -22.
- Chon HT, Ahn JS, Jung MC. Seasonal variations and chemical forms of heavy metals soils and dusts from the satellite cities of Seoul, korea. Environ Geochem Health. 1998;20:77-86.
- Banat KM, Howari FM, Al-Hamad AA. Heavy metals in urban soils of central Jordan: should we worry about their environmental risks? Environ Res. 2005;97:258-73.
- Akujieze CN. Effects of Anthropogenic activities on urban groundwater system and aquifer vulnerability assessment in Benin City, Edo state, Nigeria. Ph.D. Thesis. University of Benin. 2004.
- Lee CS, Li X, Shi W, et al. Metal contamination in urban, suburban, and country park soils of Hong Kong: a study based on GIS andmultivariate statistics. Sci Total Environ. 2006;356:45-61.
- Johnson CC, Demetriades A, Locutura J, et al. Mapping the chemical environment of urban areas. John Wileya and Sons, West Sussex, UK. 2011:616.
- Jones JM. Sequential extraction method: a review and evaluation. Environ Geochem Hlth. 1993;5:2-3.
- Olatunji AS, Abimbola AF. Geochemical evaluation of the lagos lagoon sediments and water. World Appl Sci J. 2010;9 [2]:178-93.
- Demetriades A. Medical geology in Hellas: the Lavrion urban environmental pollution study. In: Selinus O, Finkelman RB, Centeno JA [Eds.], Medical Geology A Regional Synthesis. Springer, Dordrecht. 2010:355-90.
- Glennon M, Scanlon RP, O'Connor P, et al. Dublin SURGE Project: geochemical baseline for heavy metalsand organic pollutants in top soils in the greater Dublin area. Technical Report. Geological Survey of Ireland. 2012:198.
- Al-Khashman OA, Shawabkeh RA. Metals distribution in soils around the cement factory in southern Jordan. Environ Pollut. 2006;140:387-94.
- Charlesworth S. De Miguel E, Ordonez A. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ Geochem Health. 2011;33:103- 23.
- Andersson M, Ottesen RT. Levels of dioxins and furans in urban surface soil in Trondheim, Norway. Environ Pollut. 2008;152:553-8.
- Ahnstrom Z S, Parker DR. Development and assessment of a sequential extraction procedure for the fractionation of Soil Cadmium. Soil Sci Soc Am J 1999;63:1650-8.
- Abimbola AF, Laniyan TA, Okunola OW, et al. Water quality test of areas surrounding selected refuse dumpsites in Ibadan, southwestern Nigeria. Water resources journal of Nigeria Association of Hydrogiologist. 2005;16:39-48.
- Reyment RA. Aspects of the Geology of Nigeria Ibadan University Press. 1965:145.
- Nwajide CS. Geology of Nigeria’s Sedimentary Basins, A CSS Bookshops Limited Lagos, Nigeria. 2013:565.
- Jones JM. Sequential extraction method: a review and evaluation. Environ Geochem Health. 1993;15:2-3.
- Thums CR, Farago ME, Thornton L. Bioavailability of trace metals in brownfield soils in an urban area in the UK. Environ Geochem Health. 2008;30:549 -63.
- http/www.caslab.com/news/sequentialextractionhtml6/25/2001.
- Zimmerman AJ, Weindorf DC. Heavy metal and trace metal Analysis in soil by sequential extaction: A review of procedures. Int J Anal Chem. 2010;2010:1-7.
- Muller G. Schwermetalle in den sedimenten des Rheins - Veranderungen Seit 1971.Umschau 1979;79[24]:778-83.
- Eby GN. Principles of Environmental geochemistry, New York: Thomson, Ellis, S. and Mellor, A Federal Survey Nigeria, Sheet 263, map publication 1964. 2004.
- Odewande AO, Abimbola AF. Concentration indices and heavy metal concentrations in Urban soil of Ibadan metropolis, Southwestern Nigeria. Environ Geochem Health. 2008;30:243-54.
- Olatunji AS, Abimbola AF, Asowata IT. Geochemical evaluation of soils and road deposited sediments of benin city using gis and multi-variance approaches. Br J Appl Sci Technol. 2014;4(18):2590-606.
- Abdel-Ghani NT, Elchaghaby GA. Influence of operating conditions on theremoval of Cu, Zn, Cd and Pb ions from wastewater by adsorption. Int J Environ Sci Tech. 2007;4(4):451-56.
- Nigeria Geological Survey Agency. The geological map of Onitsha area. Published by the Director of Geological survey, sheet 71.1957.
- Bavec S, Gosar M, Biester H, et al. Geochemical investigation of mercury and other elements in urban soil of Idrija (Slovenia). J Geochem Explor 2015;154:213-23
- Andersson M, Ottesen RT, Langedal M. Geochemistry of urban surface soils -Monitoring in Trondheim, Norway Geoderma. 2010;156:112-8.
- Acosta JA, Faz A, Martinez S, et al. Enrichment of metals in soils subjected to different land uses in a typical Mediterranean environment (Murcia City, southeast Spain). Appl Geochem. 2011;26:405-14.
- Charlesworth S, De Miguel E, Ordonez A. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ Geochem Health. 2011;33: 103- 23.
- Harikumar PS, Jisha TS. Distribution pattern of trace metal pollutants in the sediments of an urban wetland in the southwest coast of India. Int J Environ Engr Sci Tech 2010;2(5): 840-50.
- Al-Momani IF. Assessment of trace metal distribution and contamination in surface soils of Amman, Jordan. Jordan J Chem 2009;4(1):77-87.
- Chatterjee A, Banerjee RN. Science of total environment. 1999;227:175.