Estimation of safety of weekly injection type of glucagon-like peptide-1 (GLP-1) analogue during fasting condition running head: Weekly injection type of GLP-1 analogue and risk of hypoglycemia
Received: 26-Mar-2018 Accepted Date: Apr 03, 2018; Published: 10-Apr-2018
Citation: Okada S, Muto S, Okada J, et al. Estimation of safety of weekly injection type of glucagon-like peptide-1 (GLP-1) analogue during fasting condition running head: Weekly injection type of GLP-1 analogue and risk of hypoglycemia. J Food Drug Res. 2018;1(1):21-22.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Letter to the Editor
Exenatide and dulaglutide is a weekly injection type of Glucagon-like peptide-1 (GLP-1) analogue to treat patients with type 2 diabetes mellitus. However, we were unable to identify any reported evidence for the safety of both exenatide and dulaglutide when people have a loss of appetite. To address this issue, the corresponding author elected to undergo a 72 h fasting condition subsequent to a single injection of either exenatide or dulaglutide on different day. Interstitial glucose levels were determined by Free Style Libre (Abbotto Diabetes Care Ltd. UK.) every 15 minutes. Hypoglycemia was experienced approximately 46 hours after the initiation of starvation GLP-1 analogue injections. Thus, it is speculated that weekly injection type of GLP-1 analogue is safe for up to approximately 46 hours when individuals do not ingest any calories.
Fasting condition requires increment of gluconeogenesis by counter regulatory hormones to avoid hypoglycemia. Exenatide and dulaglutide is a weekly injection type of Glucagon-like peptide-1 (GLP-1) analogue to treat patients with type 2 diabetes mellitus [1]. GLP-1 stimulates pancreatic cells to secrete insulin in a glucose-dependent manner and therefore is considered less likely to cause hypoglycemia compared to insulin injection or sulfonylurea therapy [2]. However, we were unable to identify any reported evidence for the safety of both exenatide and dulaglutide when patients have a loss of appetite. To address this issue, the corresponding author was elected to undergo a 72-h fasting condition. The corresponding author was 60 years old with a body mass index of 25.5 (kg/m2) and with normal glucose tolerance based on 75 g oral glucose tolerance test. During the 72-fasting condition, he was allowed to freely ingest water containing trace amount of sodium chloride. He was a naïve to exenatide and dulaglutide and was given a single either exenatide (2 mg) or dulaglutide injection (0.75 mg) at 9:00 pm and the fasting period began 48 hours later from either exenatide or dulaglutide injection. During this time frame, interstitial glucose levels were determined by FreeStyleLibre (Abbotto Diabetes Care Ltd. UK.) every 15 minutes and the fasting state was terminated when interstitial glucose levels were below 80 mg/dL and hypoglycemic symptoms became apparent.
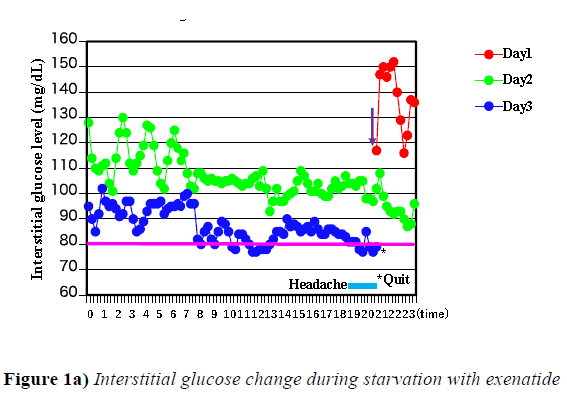
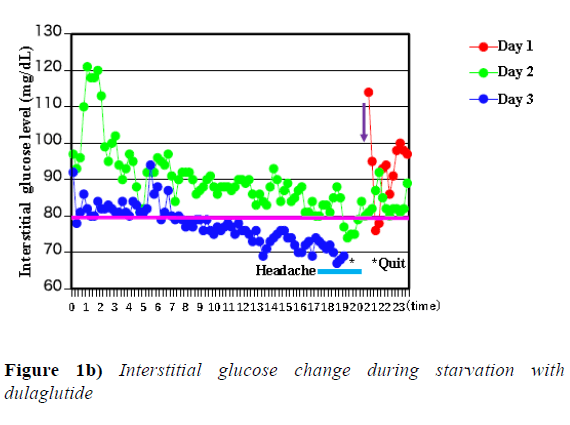
When weekly injection type of GLP-1 analogue was given under fasting condition, hypoglycemia was experienced approximately 46 hours after the injection of either exenatide or dulaglutide and the experiment was discontinued as shown by * mark in Figure 1a and 1b.
Y-axis shows interstitial glucose levels measured by FreeStyleLibre and interstitial glucose was measured every 15 minutes. X-axis shows times of day. * mark represents point that the clinical examination was stopped. Light blue line represents headache accompanied with hypoglycemia. Pink line represents 80 mg/dL interstitial glucose level. Red line represents day 1, green line represents day 2, and blue line represents day 3 after exenatide was injected which is shown by purple arrow.
Y-axis shows interstitial glucose levels measured by Free Style Libre and interstitial glucose was measured every 15 minutes. X-axis shows times of day. * mark represents point that the clinical examination was stopped. Light blue line represents headache accompanied with hypoglycemia. Pink line represents 80 mg/dL interstitial glucose level. Red line represents day 1, green line represents day 2, and blue line represents day 3 after dulaglutide was injected which is shown by purple arrow.
Brock et al. [3] reported that the counter-regulatory response during 48 h of subcutaneous GLP-1 infusion was preserved despite 48 h fasting with no apparent increased risk of hypoglycemic episodes although the GLP-1 analog in their study was daily injection type. Based on these observations, they concluded that use of long-acting GLP-1 analogues may not increase the risk of hypoglycemia [3]. Seino et al. [4] reported that under acute hypoglycemic condition, daily injection type of GLP-1 analogue does not affect glucagon response. However, our study design is different from that of their studies. Firstly, we used exenatide and dulaglutide to the same individual which is a weekly injection type. Secondly, weekly injection type of GLP-1 analogue produces higher concentration of active GLP-1 compared to daily injection type of GLP-1 [5]. Based on our observation, we newly discovered that both exenatide and dulaglutide is safe for up to approximately 46 hours when an individual does not ingest any calories. However, if an individual exhibits illnesses or other issues such as vomiting or diarrhea accompanied with loss of appetite, it is likely that the risk of hypoglycemia with either exenatide or dulaglitide mono therapy will be greatly increased.
REFERENCES
- Madsbad S, Kielgast U, Asmar M, et al. An overview of once-weekly glucagon-like peptide-1 receptor agonists available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13:394-407.
- Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19:524-36.
- Birgitte Brock B, Soendergaard L, Rungby J, et al. No increased risk of hypoglycaemic episodes during 48 h of subcutaneous glucagon-like-peptide-1 administration in fasting healthy subjects. Clinical Endocrinology. 2009;71:500-6.
- Seino Y, Eto T, Shiramoto M, et al. Effects of DPP-4 inhibitor linagliptin and GLP-1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: A randomized, open-label, 2-arm parallel comparative, exploratory trial. Diabetes Obes Metab. 2017;19:442-7.
- Geiser JS, Heathman MA, Cui X, et al. A Clinical pharmacokinetics of dulaglutide in patients with type 2 diabetes: analysis of data from clinical trials. Clin. Pharmacokinet. 2016;55:625-34.