Evaluation of CHA2DS2-VASc risk stratification tool in pregnant women with atrial fibrillation or atrial flutter
2 Student Athlete, University of Lynchburg, USA
3 Maternal-Fetal Medicine, Washington University School of Medicine in St. Louis, USA
4 Department of Women’s Health, University of Texas at Austin, Dell Medical School, USA
5 Cardiovascular Division, Washington University School of Medicine in St. Louis, USA, Email: Kathryn.lindley@wustl.edu
Received: 14-Mar-2020 Accepted Date: Jun 02, 2020; Published: 09-Jun-2020
Citation: Ashai K, LoSapio D, Conner SN, et al.. Evaluation of CHA2DS2-VASc risk stratification tool in pregnant women with atrial fibrillation or atrial flutter. Clin Cardiol J 2020;4(2):4-7.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: The CHA2DS2-VASc score has not been validated as a risk stratification tool to determine need for anticoagulation therapy in pregnant women with atrial fibrillation (AF) and atrial flutter (AFL).
HYPOTHESIS: The CHA2DS2-VASc score will underestimate thromboembolic event (TE) risk in the hypercoagulable setting of pregnancy. existing or newly diagnosed AF or AFL delivering at a tertiary care institution from 2004-2017. AF or AFL was confirmed by electrocardiogram, holter monitor or electrophysiology study. The primary outcome was systemic TE up to six months post-partum (stroke, transient ischemic event, left atrial appendage [LAA] thrombus, other systemic TE); secondary outcome was bleeding events (post-partum hemorrhage or transfusion requirement).
METHODS: This is a restrospective cohort study of women with pre-existing or newly diagnosed AF or AFL delivering at a tertiary care institution from 2004-2017. AF or AFL was confirmed by electrocardiogram, holter monitor or electrophysiology study. The primary outcome was systemic TE up to six months post-partum (stroke, transient ischemic event, left atrial appendage [LAA] thrombus, other systemic TE); secondary outcome was bleeding events (post-partum hemorrhage or transfusion requirement).
RESULTS: AF was diagnosed in 42/53 (79%) women; 7/53 (13%) had AFL and 4/53 (8%) had both. Therapeutic anticoagulation was prescribed to 11 (21%) women during pregnancy and 13 (25%) post-partum; aspirin was prescribed to 13 (25%) during pregnancy and 11 (21%) post-partum. TE occurred in 3/53 (5.7%) women; TE occurred in 2/35 (5.7%) of women with CHA2DS2-VASc scores of 1 (predicted risk 0.5%), and 1/13 (7.7%) with CHA2DS2-VASc scores of 2 (predicted risk 1.0%). There was no increased bleeding risk for women on therapeutic anticoagulation during pregnancy (18% vs. 14%, p=0.7), but there was a trend towards more bleeding in women on therapeutic anticoagulation post-partum (31% vs. 10%, p=.09).
CONCLUSIONS: The CHA2DS2-VASc score underestimates the risk of systemic TE events in pregnant women with AF/AFL. Pregnancy is a hypercoagulable state which may increase the risk of TE in women with AF/AFL, even postpartum.
Keywords
Atrial fibrillation; Pregnancy; Thromboembolism; Risk stratification; Stroke
Introduction
Atrial fibrillation is the most commonly reported arrhythmia in pregnancy [1], with a 111% increase noted between 2000 and 2012 [2]. The CHA2DS2-VASc classification, commonly used to assess the benefit of anticoagulation, has not been validated in pregnancy, a hypercoagulable state [3-5]. Thromboembolic risk and optimal management of anticoagulation in this population is unknown [6,7]. We sought to analyze the risk of thromboembolic complications in pregnant and postpartum women with atrial fibrillation (AF) and atrial flutter (AFL), and compare these rates to those predicted by the CHA2DS2-VASc risk assessment tool.
Research Methodology
This is a retrospective cohort study performed at Barnes Jewish Hospital from 2004 to 2017. Fifty-three pregnant women with AF/AFL were identified by chart review, AF or AFL was confirmed by electrocardiogram, Holter monitor or electrophysiology study. Patients were included if they delivered beyond 20 weeks gestation between 2004 and 2017 and had a prior history of or developed de novo AF/AFL up to six months postpartum. Patientthromboembolic risk was estimated using the CHA2DS2- VASc scoring system.We also collected information about patient demographics, obstetric and medical history, cardiac medications up to six months post-partum, thromboembolic complications, and maternal and fetal outcomes. The primary outcome was systemic thromboembolic events (TE) up to six months post-partum (stroke, transient ischemic event, left atrial appendage [LAA] thrombus, other systemic TE); secondary outcome was bleeding events (post-partum hemorrhage or transfusion requirement).We usedthe student ’ s t-test to calculate the probability distribution and Fisher’s exact test to determine the statistical significance. The mean age of the studied population was 29.3 years with a relatively equal distribution of white and African American females at 51% vs. 47% (Table 1). The predominant pre-existing medical condition was hypertension with a prevalence of 14/53 (26%).
TABLE 1: Baseline demographics.
| Demographic Variables | Number (%) (n=53) |
|---|---|
| Age (mean, SD) | 29.3 (6) |
| Race | |
| White | 27 (51) |
| African American | 25 (47) |
| Body Mass Index (mean, SD) | 34.6 (8) |
| Tobacco Use | 7 (13) |
| Pre-existing Medical Conditions | |
| Hypertension | 14 (26) |
| Diabetes | 3 (6) |
| Stroke/TIA | 2 (4) |
| Heart Failure | 4 (8) |
| Myocardial Infarction | 1 (2) |
Results
AF was diagnosed in 42/53 (79%); AFL was found in 7/53 (13%), and 4/53 (8%) had both arrhythmias (Table 2). Of the women in the study, 33/53 (62%) had lone AF, while the arrhythmia was associated with other conditions including valvular disease, thyroid disorder, infections, cardiomyopathy, or congenital heart disease in the remainder of the cohort.
TABLE 2: Arrhythmia characteristics.
| Arrhythmia Characteristics | Pre-existing (n=22) | New Diagnosis (n=31) |
|---|---|---|
| Arrhythmia Type | ||
| Atrial Fibrillation | 13 (59) | 29 (94) |
| Atrial Flutter | 7 (32) | 0 (0) |
| Both | 2 (9) | 2 (7) |
| Lone AF vs. associated with other disease | ||
| Lone | 10 (46) | 23 (74) |
| Cardiomyopathy | 0 (0) | 1 (5) |
| Congenital Heart Disease | 9 (41) | 1 (3) |
| Valvular Disease | 2 (9) | 3 (10) |
| Thyroid Disorder | 0 (0) | 1 (3) |
| Infection | 0 (0) | 3 (10) |
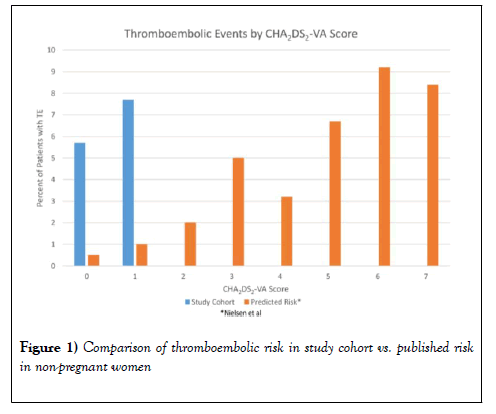
Thromboembolic events occurred in 3/53 (5.7%) women, all of whom had clinical evidence of AF during the pregnancy (Table 3). Of these women, 2/35 (5.7%) had CHA2DS2-VASc scores of 1 (predicted risk 0.7%), and 1/13 (7.7%) had a CHA2DS2-VASc score of 2 (predicted risk 1.9%). Therapeutic anticoagulation was prescribed to 11 (21%) women during pregnancy and 13 (25%) women post-partum; aspirin was prescribed to 13 (25%) of the women during pregnancy and 11 (21%) post-partum. The secondary outcome of bleeding complications was noted in 8/53 (15%) of women with CHA2DS2-VASc scores of either 1 or 2, (transfusion in 8/8 women, postpartum hemorrhage in 5 of the 8 women). There was no significant increased risk for bleeding complications for women on therapeutic anticoagulation during pregnancy (18% vs. 14%, p=0.7), but there was a trend towards more bleeding in women on therapeutic anticoagulation post-partum (31% vs. 10%, p=0.09).
TABLE 3: Stratification by CHA2DS2-VASc Score.
| CHA2DS2-VASc Score | 1 (n=35) | 2 (n=13) | 3 (n=4) | 4 (n=1) |
|---|---|---|---|---|
| Arrhythmia During Pregnancy | 33 (94) | 9 (69) | 2 (67) | 1 (100) |
| Anticoagulation During Pregnancy | 18 (53) | 4 (31) | 2 (50) | 0 (0) |
| Aspirin | 9 (27) | 2 (15) | 2 (50) | 0 (0) |
| Therapeutic | 8 (23) | 3 (23) | 0 (0) | 0 (0) |
| Anticoagulation Post-partum | 18 (53) | 4 (31) | 1 (25) | 0 (0) |
| Aspirin | 7 (21) | 3 (23) | 1 (25) | 0 (0) |
| Therapeutic | 10 (29) | 2 (15) | 1 (25) | 0 (0) |
| TE | 2 (6) | 1 (8) | 0 (0) | 0 (0) |
| On Aspirin | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| On No Anticoagulation | 1 (3) | 1 (8) | 0 (0) | 0 (0) |
| Bleeding Complication | 6 (17) | 2 (15) | 0 (0) | 0 (0) |
Of the women with TE, one was diagnosed with AF at the time of the stroke, one stroke occurred in a woman non-adherent with enoxaparin with a known history of paroxysmal AF, and one patient developed LAA thrombus while on aspirin therapy with persistent AF during her pregnancy (Figure 1 and Table 4).
TABLE 4: Thromboembolic event characteristics.
| Patients | TE Timing | TE Type | CHA2DS2-VASc Score | Anticoagulation at time of TE | Details |
|---|---|---|---|---|---|
| 1 | 4 months post-partum | Occipital stroke | 2 | None | Known paroxysmal AF, was in sinus rhythm during pregnancy; was in AF at time of TE |
| 2 | 12 weeks gestation | Central retinal artery occlusion | 1 | None | Diagnosed with AF at time of TE |
| 3 | 4 months post-partum | LAA thrombus seen on TEE | 1 | Aspirin | Persistent AF during pregnancy |
*TEE - Transesophageal Echocardiogram
Discussion
This study identified an increased risk of TE in pregnant women with AF/ AFL. The risk identified in our study cohort is higher than would be expected based on the calculated CHA2DS2-VASc score, the tool commonly applied for the determination of anticoagulation. Additionally, therapeutic anticoagulation was not associated with excessive bleeding risk over the course of the pregnancy, though there was noted to be a trend towards increased post-partum bleeding. All patients had a CHA2DS2-VASc of 1 or higher simply by virtue of being female. Current guidelines do not recommend therapeutic anticoagulation for women unless they have two additional risk factors [8]. The predicted risk for a stroke/TIA/other event over our study timeframe is 0.5% for women with no additional risk factors, but the actual event rate was 5.7% in our study population, over 10 times higher [9]. In women with one non-sex risk factor, the predicted risk is 1.0% but was found to be 7.7% in our study population [9].
The currently accepted AHA/ACC/HRS guidelines for management of atrial fibrillation unfortunately do not address anticoagulation in pregnant women [8,10]. Current European Society of Cardiology Guidelines on the management of cardiovascular disease in pregnancy recommend applying the same rules for stroke risk stratification as used in non-pregnant patients [11]. Cumyn et al. surveyed general internists, obstetricians, and cardiologists from 11 countries about their approach to managing anticoagulation of AF in pregnancy. Of 61 respondents, 2/3 based their clinical decision making on the CHADS2 or CHA2DS2-VASc score [7]. The currently accepted risk stratification system of using the CHA2DS2-VASc score takes into account female sex as a risk modifier in the assessment of possible thromboembolic events as females are noted to show greater rates of TEs as compared to men, in the presence of additional thromboembolic risk factors [9,12]. In our pregnant population, 66% of patients carry a low risk score, implying a low risk of thromboembolic events. However, our study suggests this may be a significant underestimation of risk in this population, and research on the physiology of pregnancy and hypercoagulability may provide explanations for this discrepancy.
Some of the unique features of pregnancy include increased blood volume and venous stasis [13], increased estrogen and progesterone levels [14], and an increase in pro-coagulants such as fibrinogen/von Wille brand factor and decrease in anti-coagulant factors such as protein S, which can contribute to the higher risk of thromboembolism. Secondly, the risk of adverse events may be higher because pregnancy is itself a potentially arrhythmogenic state [5,15]. The increase in potential arrhythmias occurs in the setting of increased left ventricular mass and increased left atrial size to accommodate the increased blood volume precipitating additional myocardial stretch as well as physiologic tachycardia (up to 10-15 beats per minute higher than normal) which potentiates irregular atrial rhythms [1,14]. Third, women with underlying cardiovascular disease are at higher risk of complications and progression due to the aforementioned physiologic factors, thereby increasing their risk of thromboembolism [16]. Our study had a combination of women with lone AF as well as those with associated conditions. Those with lone AF may have been related to the physiologic changes previously mentioned.
Another important finding from our work is that the circumstances leading up to the observed complications were very diverse. One woman was adherent to the prescribed aspirin therapy after having been in persistent AF during her pregnancy and was noted to have a left atrial appendage thrombus four months postpartum. Another patient was known to have paroxysmal AF but had been non-adherent with her enoxaparin when she developed her occipital stroke four months postpartum. The third patient was pregnant at the time of her central retinal artery occlusion with no prior history of arrhythmia and was thus not on any anticoagulation or antiplatelet therapy. Complications also occurred at different temporal points, both during pregnancy as well as post-partum. The heterogeneity of factors preceding complications suggests that further research is necessary to develop appropriate risk stratification tools.
An additional factor that complicates management of AF/AFL in pregnant women is AF burden, and the relatively brief duration of the hypercoagulable state of pregnancy (nine months of pregnancy + three months post-partum). One study looked at the AF burden over a 14-day period for patients not on anticoagulation. It found that those with the highest AF burden, noted at >11.4% of the monitored time period, had a3x higher risk of TE with a median time of 8 months to time of the TE event [17]. This raises the question of the utility of assessing AF burden in the pregnant/postpartum population which we have demonstrated may already be at higher risk of TE complications. Study of AF burden in interaction with CHA2DS2-VASc scoring in the setting of cardiovascular implantable electronic devices, allowing for longer continuous monitoring than 14 days, also showed that AF duration increases the annual risk of stroke and systemic emboli, the metric used in this particular study [18].
Limitations
Our analysis is retrospective and identifies only 53 cases. Larger studies and prospective trials are needed to further inform best practices for anticoagulation usefor AF in pregnancy. Additionally, our follow-up was limited to 6 months and two events occurred at 4 months. This short window may underestimate the true incidence of postpartum thromboembolism from AF/AFL. Due to the small sample size, lack of difference in bleeding complications in women on therapeutic anticoagulation could have resulted due to type 2 error.
Further Research
The higher than anticipated risk of TE events in our study supports the need for further research on optimal anticoagulation strategies for women with AF in pregnancy. This includes prospective, randomized studies on anticoagulation strategies as well as evaluation of pregnancy-specific risk assessment tools.
Conclusion
There are no data-driven guidelines regarding management of atrial fibrillation and atrial flutter during pregnancy at present. The CHA2DS2- VASc schema is commonly used as a risk assessment tool, but does not account for the hypercoagulable physiology and arrhythmogenic state of pregnancy. This study suggests CHA2DS2-VASc may under-estimate thromboembolic risk in this unique population. Given the lack of a validated risk assessment tool and the potentially high morbidity/mortality of thromboembolic events in this young population, further research is necessary to develop evidence-based guidelines for the growing population of pregnant women with a history of AF.
REFERENCES
- MacIntyre C, Iwuala C, Parkash R. Cardiac arrhythmias and pregnancy. Curr Treat Options Cardiovasc Med. 2018;20(8):1-63.
- Vaidya VR, Arora S, Patel N, et al. Burden of arrhythmia in pregnancy. Circulation. 2017;135(6):619-21.
- Yarrington CD, Valente AM, Economy KE. Cardiovascular management in pregnancy: Antithrombotic agents and antiplatelet agents. Circulation. 2015;132(14):1354-64.
- Chen GC, Gao H, Zhang L, et al. Evaluation of therapeutic efficacy of anticoagulant drugs for patients with venous thromboembolism during pregnancy: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2019;238:7-11.
- ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol. 2018;132(1):1-17.
- Lameijer H, Aalberts JJ, Veldhuisen DJ, et al. Efficacy and safety of direct oral anticoagulants during pregnancy; a systematic literature review. Thromb Res. 2018;169:123-127.
- Cumyn A, Sauve N, Rey E. Atrial fibrillation with a structurally normal heart in pregnancy: An international survey on current practice. Obstet Med. 2017;10(2):74-8.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-32.
- Nielsen PB, Skjoth F, Overvad TF, et al. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: Should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137(8):832-40.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):1-76.
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165-241.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-72.
- Scheres LJJ, Bistervels IM, Middeldorp S. Everything the clinician needs to know about evidence-based anticoagulation in pregnancy. Blood Rev, 2019. 33: p. 82-97.
- Katsi V. Atrial fibrillation in pregnancy: A growing challenge. Curr Med Res Opin. 2017;33(8):1497-504.
- Gowda RM, Khan IA, Mehta NJ, et al. Cardiac arrhythmias in pregnancy: Clinical and therapeutic considerations. Int J Cardiol. 2003;88(2):129-33.
- Sauve N, Rey E, Cumyn A. Atrial fibrillation in a structurally normal heart during pregnancy: A review of cases from a registry and from the literature. J Obstet Gynaecol Can. 2017;39(1):18-24.
- Go AS. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: The KP-RHYTHM Study. JAMA Cardiology. 2018;3(7):601-08.
- Kaplan RM, Koehler J, Ziegler PD, et al. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140:1639-46.