Evolution of type 2 diabetes mellitus in patients undergoing vertical sleeve gastrectomy or Roux-en-Y gastric bypass
Received: 04-Oct-2017 Accepted Date: Nov 02, 2017; Published: 10-Nov-2017
Citation: Martínez-Ortiz CA, Saldívar-Frías JB, Rodríguez-González AA, et al. Evolution of type 2 diabetes mellitus in patients undergoing vertical sleeve gastrectomy or Roux-en-Y gastric bypass. Appl Food Sci J. 2017;1(2):10-16.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: We have been given the task of studying the evolution of T2DM and alterations in glucose homeostasis in patients undergoing bariatric/metabolic surgery and determine if there is remission of T2DM in our patients undergoing vertical sleeve gastrectomy or Roux-en-Y gastric bypass and, if so, after which one is greater.
METHODS: Descriptive, observational, longitudinal and retrospective analysis of data from patients with diagnosis of obesity (BMI ≥ 30 kg/m2) and T2DM who underwent SG or RYGB at our obesity clinic, from March 2015 to March 2016. Diabetes remission was defined as glucose below the diagnostic thresholds for T2DM (HbA1c<6%, fasting glucose<100 mg/dl if HbA1c was not available) and absence of active hypoglycemic medication.
RESULTS: Bariatric/metabolic surgery can reverse or improve T2DM in obese patients, and is more effective than medical interventions and lifestyle modifications for weight loss, glycemic control, remission of T2DM, improvements in other cardiovascular risk factors and the decrease ofcardiovascular diseases
Keywords
Bariatric/metabolic surgery; Diabetes mellitus; Obesity; Remission; Roux-en-Y gastric bypass; Sleeve gastrectomy
O besity and diabetes have reached epidemic proportions and constitute an important health and economic problem [1,2]. Diabetes is common in obese patients in Mexico, which is among the countries with highest rates of type 2 diabetes mellitus (T2DM) and obesity. Risk factors affecting the distribution and frequency of T2DM and their complications are well known. It is often associated with overweight or obesity, which in turn can cause insulin resistance and elevated blood glucose levels. The global prevalence of obesity-related diabetes in our population is not well known.
In Mexican adults, according to data from the National Health and Nutrition Survey (NHNS) [3] 2016, the combined prevalence of overweight and obesity was 71.2% (CI 95% 70.5, 72.1) in NHNS 2012 and 72.5% (CI 95% 70.8, 74.3) in NHNS 2016. It is observed that the combined prevalence of overweight and obesity (BMI ≥ 25 kg/m2) is higher in women (75.6%, CI 95% 73.5, 77.5) than in men (69.4%, CI 95% 65.9, 72.6); and that the prevalence of obesity (BMI ≥ 30 kg/m2) is also higher in females (38.6%, CI 95% 36.1, 41.2) than in males (27.7% CI 95% 23.7, 32.1). Likewise, the category of morbid obesity (BMI ≥ 40.0 kg/m2) is 2.4 times higher in women than in men.
Obesity and T2DM are closely related and sometimes are difficult to control through current medical treatment, including diet, drug therapy, and behavioral modification. There is strong evidence that bariatric operations, primarily Roux-en-Y gastric bypass (RYGB), may favor the remission of T2DM associated with morbid obesity in most cases.
Some gastrointestinal operations, including partial gastrectomies, such as the sleeve gastrectomy (SG), and bariatric procedures, promote a dramatic and lasting improvement of T2DM. Given the magnitude and rapidity of the effect of bariatric surgery on hyperglycemia, together with experimental evidence that the rearrangement of the gastrointestinal anatomy performed in some procedures directly affects glucose homeostasis, gastrointestinal interventions have been suggested as a treatment for T2DM [4-6].
Due to its role in metabolic regulation, the gastrointestinal tract constitutes a clinically and biologically significant target for the management of T2DM. Bariatric/metabolic surgery can achieve excellent control of hyperglycemia and reduce cardiovascular risk factors associated with T2DM. Nevertheless, existing diabetes management algorithms do not include bariatric/metabolic surgery as a treatment option, despite growing evidence that surgery improves T2DM.
Surgical approaches to the treatment of obesity have increased 10-fold in the last decade. Roux-en-Y gastric bypass is the most commonly performed procedure, closely followed by vertical sleeve gastrectomy. Better glycemic control has recently been demonstrated in patients undergoing bariatric/ metabolic surgery compared to intensive medical treatment, resulting in the ability to remove or reduce glucose-lowering medications. The rapid rate of glucose reduction, disproportionate in relation to weight loss, suggests that bariatric/metabolic surgery reverses the fundamental pathophysiological defects of T2DM.
Bariatric/metabolic surgery has proven to be the most effective treatment for patients with morbid obesity.
Methods
This research was registered in the Online Electronic Registration System of the Health Research Coordination (SIRELCIS, for its acronym in Spanish) for evaluation and authorization by the Local Health Research Committee (CLIEIS, for its acronym in Spanish).
Patients who met all the inclusion criteria were included and divided into 2 groups, depending on the performed surgery, and data from the preoperative period to 12 months follow-up was collected.
We made a descriptive, observational, longitudinal and retrospective analysis of data from patients with diagnosis of obesity (BMI ≥ 30kg/m2) and T2DM who underwent SG or RYGB at our obesity clinic, from March 2015 to March 2016. Non-probabilistic sampling for convenience was done. All analyses were performed with SPSS software, version 22.0 for Windows (SPSS Inc.). The data was processed through a comparison analysis of measures repeated by ANOVA for quantitative variables, Turkey test for differences between measures, and chi square test for nominal qualitative data. The test was considered significant when the p value was less than 0.05 in any of the tests.
Diabetes remission was defined as glucose below the diagnostic thresholds for T2DM (HbA1c<6%, fasting glucose<100 mg/dl if HbA1c was not available) and absence of active hypoglycemic medication (Table 1).
| N=33 | SG | RYGB | p |
|---|---|---|---|
| Gender (n [%]) | |||
| Male | 1 (3.03) | 6 (18.18) | 0.444 |
| Female | 7 (21.21) | 19 (57.50) | |
| Age (años) [M (DE)] | 48.50 (7.010) | 44.60 (7.842) | 0.658 |
| Height (m) [M (DE)] | 1.59 (0.097) | 1.61 (0.097) | 0.960 |
| IBW (kg) [M (DE)] | 55.12 (8.26) | 57.37 (8.76) | 0.913 |
| Min W (kg) [M (DE)] | 46.92 (5.90) | 48.43 (6.19) | 0.967 |
| Max-W (kg) [M (DE)] | 63.15 (7.94) | 65.18 (8.27) | 0.967 |
| IBW Ideal body weight; M Mean; m meters; Max W Maximum weight; Min W Minimum weight; n Number of patients; N Total number of patients; RYGB Roux-en-Y gastric bypass; SD Standard deviation; SG Vertical sleeve gastrectomy; % Percentage | |||
IBW Ideal body weight; M Mean; m meters; Max W Maximum weight; Min W Minimum weight; n Number of patients; N Total number of patients; RYGB Rouxen- Y gastric bypass; SD Standard deviation; SG Vertical sleeve gastrectomy; % Percentage
Table 1: Demographics by group
Sleeve gastrectomy
Position of the patient and the surgical team: The operation is performed with the patient in the supine position, with the legs open and 45 degrees of inclination. Fixation of the patient on the operating table is done by placing bandages on the thighs and feet, with the placement of bags of crystalloid solution in popliteal spaces and ankles. The surgeon stood between his legs, with the first assistant, who handled the camera and the assistant clamp, to his left, and the second assistant to his right. Bladder catheter is used for collection and quantification of urine. Prophylactic antibiotic is given. The prevention of thromboembolic disease is done with the use of graduated compression stockings. A 36 Fr gastric tube is placed.
Neumoperitoneum and placement of the trocars: The pneumoperitoneum is performed with a Veress needle through an incision in the mesogastrium 12-15 cm below the xiphoid process, 2-3 cm to the left of the midline, where the first trocar, 12 mm, is placed for the 30 degree optics. The pressure is set at 17 mm Hg. the second trocar, 5 mm, for the liver separator, is placed near the xiphoid process, to the right of the midline; the third trocar, 12 mm, for the left hand of the surgeon, is placed on the right side of the patient in an intermediate position between the two anterior, 3-5 cm lateral to the midline; the fourth trocar, 12 mm, for the surgeon’s right hand, is placed adjacent to the left costal margin at the level of the middle clavicular line; and the fifth trocar, 5 mm, for the second assistant, is placed under the left costal border at the level of the anterior axillary line.
Gastric sleeve: Displacement of the left lobe of the liver with the spacer provides adequate visualization of the entire stomach during gastrectomy. The pylorus is identified and the major curve of the stomach is dissected. Medtronic Sonicision is used to enter the major sac through division of the major omentum and the greater curvature of the stomach is released from the omentum and short gastric vessels. The dissection begins at 5 cm from the pylorus and proceeds towards the angle of His. The 38 Fr gastric tubes is then passed under direct vision through the esophagus and stomach to the first portion of the duodenum, aligned along the minor curvature of the stomach and is used as a guide to perform vertical gastrectomy in a sleeve beginning 2 cm proximal to the pylorus and extending towards the angle of His. Medtronic Endo GIA Ultra linear cutter is used, starting with two 60 mm purple cartridges and then with 60 mm blue cartridges, always remaining to the left and lateral to the gastric tube. The severed stomach, which includes the major curvature, is completely released and withdrawn through the incision on the left flank side.
Hemostasis is checked on the staple line and, after a test with approximately 250 ml of 10% methylene blue-stained solution introduced through the 36 Fr gastric probes, the absence of leakage is also verified. Penrose drainage is placed through the site of the fifth trocar. Finally, the trocars are removed under direct visualization and the skin is closed with single points separated with non-absorbable suture 3-0.
Roux-en-Y gastric bypass: Position of the patient and the surgical team, and pneumoperitoneum and trocar placement were performed in the same fashion as for sleeve gastrectomy.
Gastric pouch: Preparation to create the gastric pouch begins with dissection of the His angle and opening of the left gastrophrenic ligament with Medtronic Sonicision to expose the lateral aspect of the left diaphragmatic pillar. Then, the fat pad of the Belsey gastroesophageal junction is made. Dissection of the minor curvature is performed between the second and third blood vessels, obtaining access to the posterior gastric wall. Medtronic Endo GIA Ultra linear cutter is used. The first shot is carried out in the horizontal direction with a 45 mm blue cartridge. The dissection of the retrogastric space is then initiated until a complete visualization of the left diaphragmatic pillar is performed, whereupon cranial stapling is performed with 60 mm blue cartridges, finishing the gastric bag. Both the staple line of the excluded stomach and that of the gastric bag are reinforced with continuous simple surgeon with 2-0 nonabsorbable suture.
Gastroenterostomy: The major omentum is mobilized to the upper abdomen, open mid-way in the longitudinal direction with Medtronic Sonicision®, to bring the jejunal loop to the supramesocolic area for the anastomosis. This maneuver allows easy access to the Treitz angle and the initial segment of the jejunum, to define a length of 100 mm biliopancreatic loop. Keeping the proximal part of the jejunum always to the right side of the surgeon, the handle mobilizes to the upper abdomen without being sectioned, similar to the Billroth II isoperistaltic reconstruction. After making two small holes with Medtronic Sonicision, one on the posterior surface of the gastric bag and the bull in the jejunal loop, a 20 mm gastroenterostomy with a 45 mm white cartridge is performed. The aperture was sutured with a simple seromuscular continuous artery with absorbable 2-0 thread, using the 36 Fr gastric probes for calibration. After the suture is completed, the catheter is removed allowing a better fit for the final caliber of the anastomosis. Seromuscular stitches are separated with non-absorbable suture 2-0 at the apex of the anastomosis.
Close up of Petersen space: With superior traction of the mesocolon, near the medial part of the transverse colon towards the liver, the Petersen space is closed with 2-0 absorbable continuous suture, from the distal angle between the mesocolon and mesenterium of the alimentary loop to the edge of the colon transverse and jejunum.
Enteroenterostomy: From the gastroenterostomy, in the direction of the distal jejunum and according to the surgeon’s preference, a jejunum segment of 150 mm is measured to anastomose with the biliopancreatic loop in the afferent portion of the gastroenterostomy, thus determining the length of the alimentary handle. The anastomosis is performed in an isoperistaltic manner, with a white cartridge of 45 mm. After reviewing hemostasis on the staple line, the incision was sutured with a simple seromuscular continuous surgeon with absorbable 2-0 thread. The mesenteric defect is closed with continuous surgeon with absorbable suture 2-0.
After a test with approximately 250 ml of 10% methylene blue-stained solution introduced through the 36 Fr gastric probes, it is possible to verify the integrity of the two anastomosis (which are connected up to now as in a procedure Billroth II). Next, the corresponding segment of the biliopancreatic loop is sectioned along the gastroenterostomy to convert the initial arrangement of double omega into a Roux-en-Y.
Penrose drainage is placed through the site of the fifth trocar. The trocars are then removed under direct visualization and the skin is closed with single stitches separated with non-absorbable suture 3-0.
Results
Thirty-three patients with diagnosis of obesity (Table 2) and type 2 diabetes mellitus were divided into two groups, depending on the performed surgery: 8 patients, 1 man (3.03%) and 7 women (21.21%), SG; and 25 patients, 6 men (18.18%) and 19 women (57.50%), RYGB. After 1 year of follow-up, there was remission in 7 (21.21%) of 8 patients in the SG group and in 24 (72.72%) in 25 (93.93%) patients in the RYGB group (Tables 3 and 4).
| Diagnosis(n [%]) | SG | RYGB |
|---|---|---|
| Obesity class I | 0 (0.00) | 0 (0.00) |
| Obesity class II | 0 (0.00) | 3 (9.09) |
| Obesity class III | 8 (24.32) | 22 (66.66) |
n number of patients; N Total number of patients; RYGB: Roux-en-Y gastric bypass; SG Vertical sleeve gastrectomy; % Percentage
Table 2: Diagnosis by group
| Group | N | M | SD | |
|---|---|---|---|---|
| Weight (kg) Month 0 | SG | 8 | 153.22 | 40.604 |
| RYGB | 25 | 120.71 | 17.640 | |
| BMI (kg/m2) Month 0 | SG | 8 | 60.03 | 11.202 |
| RYGB | 25 | 46.27 | 5.612 | |
| EBW (kg) Month 0 | SG | 8 | 98.09 | 34.636 |
| RYGB | 25 | 63.33 | 14.078 |
BMI Body mass index; EBW Excess body weight; M Mean; N Number of patients; RYGB Roux-en-Y gastric bypass; SD Standard deviation; SG Vertical sleeve gastrectomy
Table 3: Somatometry by group 1
| F | Sig. | |
|---|---|---|
| Weight (kg) Month 0 | 8.122 | 0.008 |
| BMI (kg/m2) Month 0 | 14.813 | 0.001 |
| EBW (kg) Month 0 | 14.830 | 0.001 |
| T2DM | 6.464 | 0.016 |
BMI Body mass index; EBW Excess body weight; N Number of patients; RYGB Roux-en-Y gastric bypass; SG Vertical sleeve gastrectomy; T2DM Type 2 diabetes mellitus
Table 4: Somatometry by group 2
The mean age was 48.50 years (7,010) in the SG group and 44.60 years (7,842) in the RYGB group (p=0.658); the mean preoperative weight (month 0) was 153.22 kg (40.604) in the SG group and 120.71 kg (17.640) in the RYGB group (p=0.008); the mean preoperative body mass index (BMI) was 60.03 kg/m2 (11.202) in the SG group and 46.27 kg/m2 (5,612) in the RYGB group (p=0.001); and the mean preoperative excess body weight (EBW) was 98.09 kg (34.636) in the SG group, and 63.33 kg (14,078) in the RYGB group (p=0.001).
In the SG group, 8 (24.24%) patients had class III obesity; and in the RYGB group, 3 (0.09%) patients had class II obesity and 22 (66.66%) patients had class III obesity.
The mean preoperative glucose (month 0) was 133.75 g/dl (59,223) in the SG group, and 106.36 g/dl (27,741) in the RYGB group (p=0.013); and the mean HbA1c was 7.00% (1,323) in the SG group, and 5.94% (0.856) in the RYGB group (p=0.295).
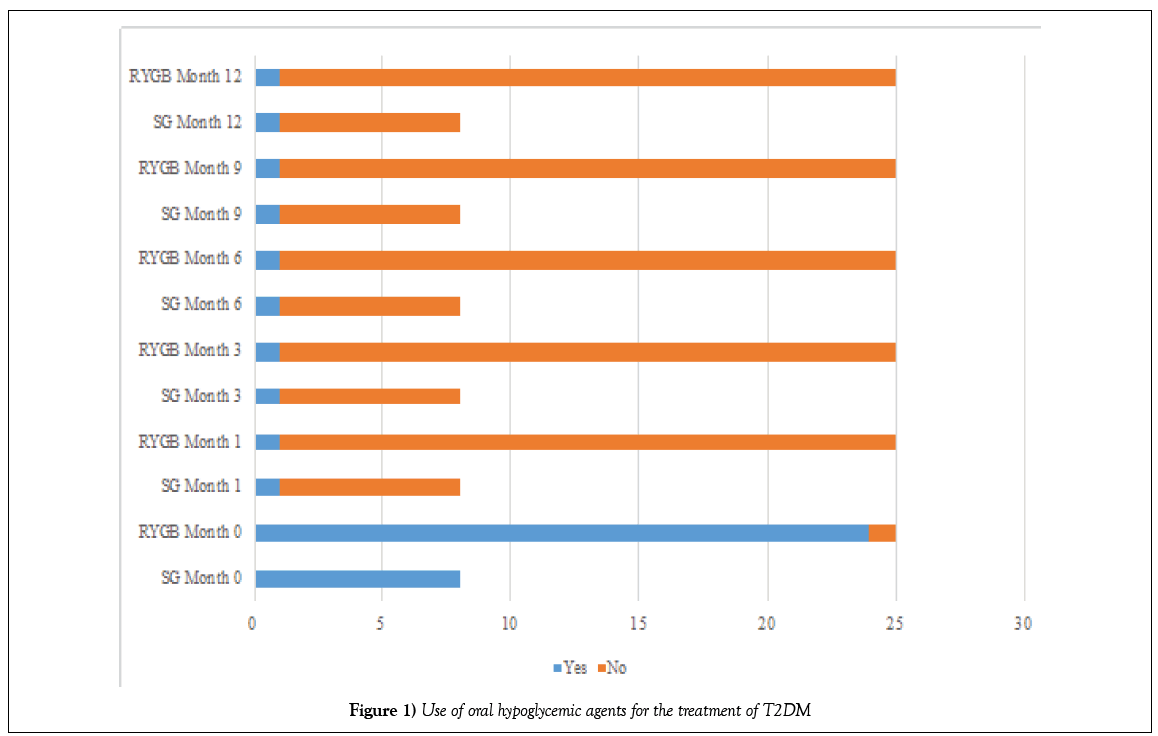
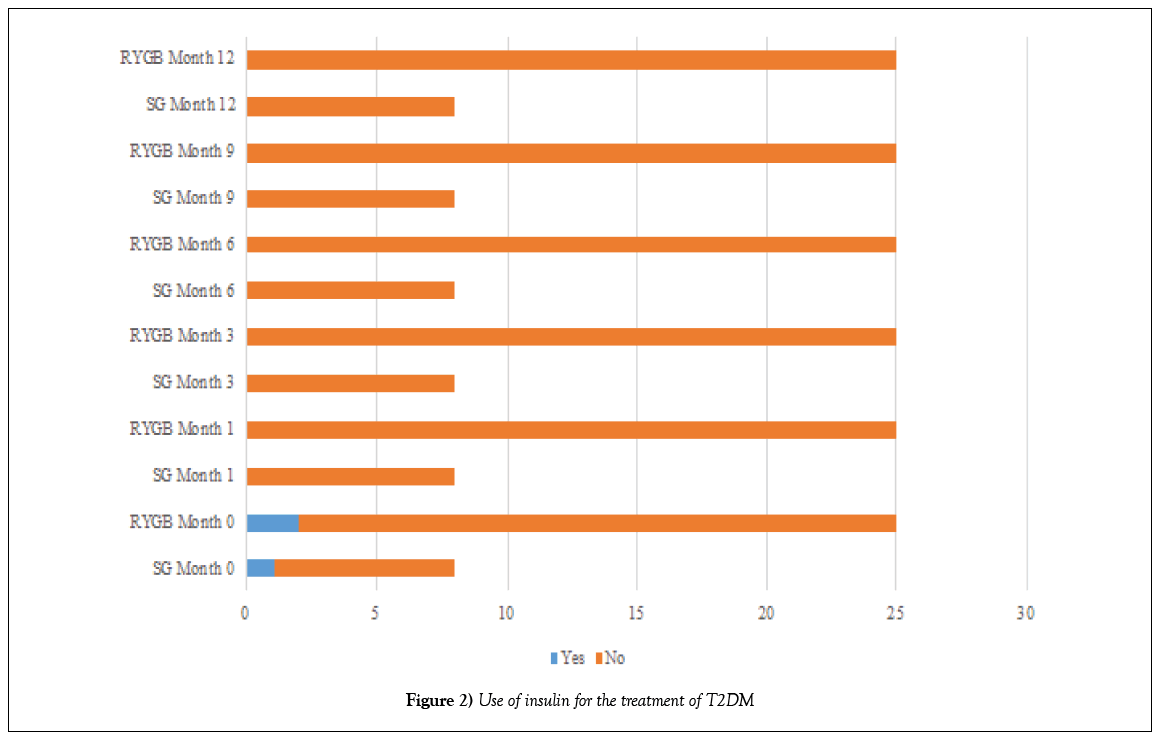
Before the operation, 8 (24.32%) patients in the SG group and 24 (72.72%) patients in the DGRY group (p=0.566) were treated with oral hypoglycemic agents for T2DM (Table 5 and Figure 1); and 1 (3.03%) patient in the SG group and 2 (6.06%) patients in the DGRY group (p=0.050) administered insulin as part of the treatment for T2DM (Tables 6 and 7 and Figure 2).
| Use of oral hypoglycemic agents (n [%]) | SG | RYGB | p |
|---|---|---|---|
| Month 0 | |||
| Yes | 8 (24.32) | 24 (72.72) | 0.556 |
| No | 0 (0.00) | 1 (3.03) | |
| Month 1, 3, 6, 9, 12 | |||
| Yes | 1 (3.03) | 1 (3.03) | 0.001 |
| No | 7 (21.21) | 24 (72.72) |
n number of patients; RYGB Roux-en-Y gastric bypass; SG Vertical sleeve gastrectomy
Table 5: Use of oral hypoglycemic agents for the treatment of T2DM
| Uso of insulin (n [%]) | SG | RYGB | p |
|---|---|---|---|
| Month 0 | |||
| Yes | 1 (3.03) | 2 (6.06) | 0.050 |
| No | 7 (21.21) | 23 (69.60) | |
| Month 1 | |||
| Yes | 0 (0.00) | 0 (0.00) | 0.000 |
| No | 8 (24.24) | 25 (75.75) | |
| Month 3, 6, 9, 12 | |||
| Yes | 0 (0.00) | 0 (0.00) | - |
| No | 0/0.00 | 0/0.00 | - |
| n number of patients; RYGB Roux-en-Y gastric bypass; SG Vertical sleeve gastrectomy | |||
n number of patients; RYGB Roux-en-Y gastric bypass; SG Vertical sleeve gastrectomy
Table 6: Use of insulin for the treatment of T2DM
| Glucose (g/dl) | Group | M | SD | p |
|---|---|---|---|---|
| Month 0 | SG | 133.75 | 59.223 | 0.013 |
| RYGB | 106.36 | 27.741 | ||
| Month 1 | SG | 100.13 | 16.217 | 0.070 |
| RYGB | 94.42 | 8.895 | ||
| Month 3 | SG | 101.25 | 14.859 | 0.630 |
| RYGB | 90.54 | 12.518 | ||
| Month 6 | SG | 96.75 | 12.023 | 0.927 |
| RYGB | 88.16 | 11.273 | ||
| Month 9 | SG | 94.00 | 6.698 | 0.373 |
| RYGB | 84.71 | 11.277 | ||
| Month 12 | SG | 88.75 | 7.833 | 0.849 |
| RYGB | 84.25 | 8.593 |
M Mean; SD Standard deviation; SG Vertical sleeve gastrectomy; RYGB Roux-en-Y gastric bypass
Table 7: Evolution of serum glucose per group
One month after the surgical procedure, 7 (21.21%) patients in the SG group and 23 (69.69%) patients in the DGRY group (p=0.001) had discontinued treatment with oral hypoglycemic agents for T2DM; only 1 (3.03%) patient in each group continued treatment during the 12 months follow-up. All patients in both groups given insulin stopped (p=0.000).
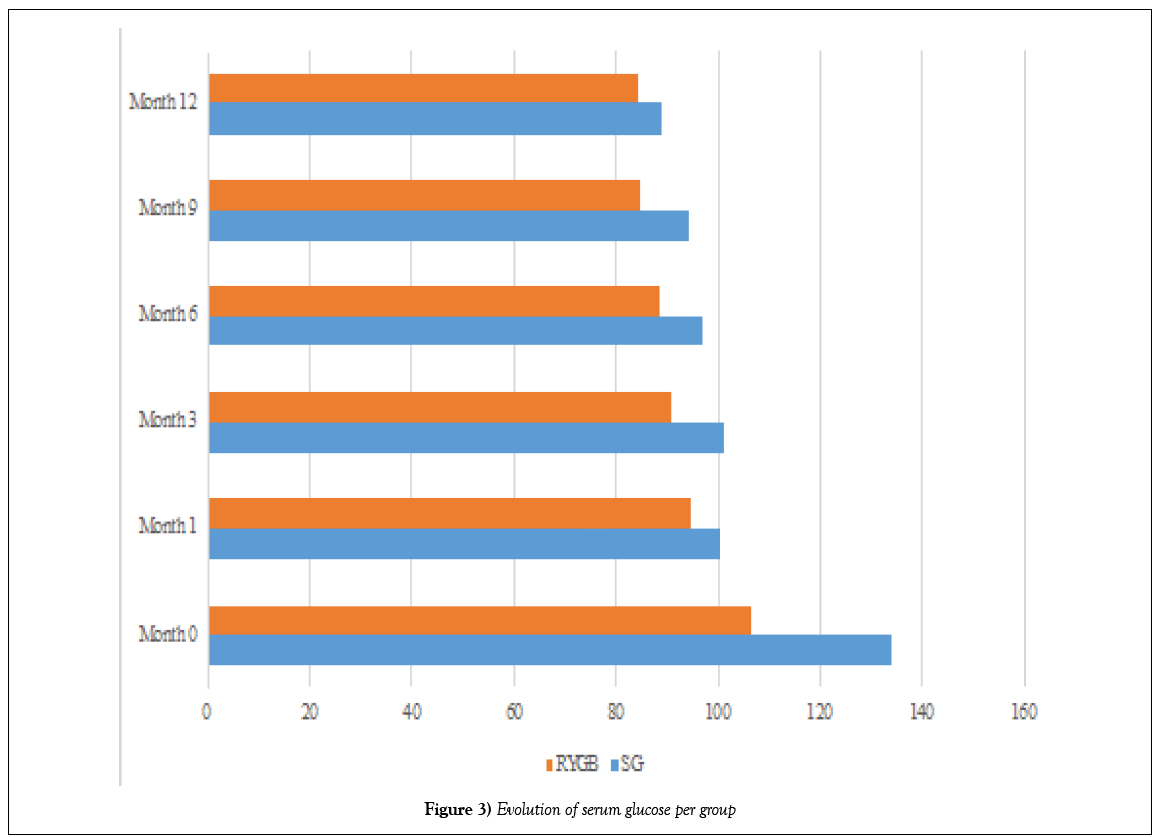
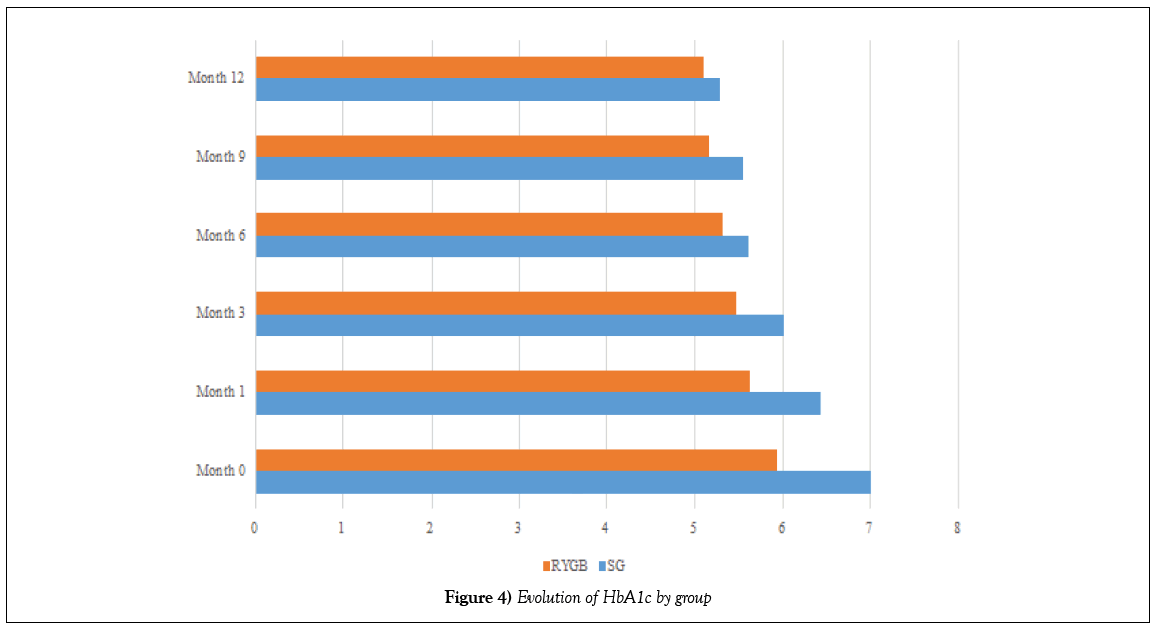
At the first month of follow-up, there was partial remission of T2DM in SG operated patients, and complete remission of T2DM in patients operated on RYGB. The mean glucose was 100.13 g/dl (16,217) in the SG group and 94.42 g/dl (8,895) in the RYGB group (p=0.070); and the mean HbA1c was 6.43% (0.656) in the SG group and 5.62% (0.559) in the RYGB group (p=0.448)(Table 8). There was complete remission of T2DM in patients in the first group until the sixth month of follow-up, when the mean glucose was 96.75 g/dl (12,023) (p=0.927) and the mean HbA1c was 5.61% (0.515) (p=0.430).
| HbA1c (%) | Group | M | SD | p |
|---|---|---|---|---|
| Month 0 | SG | 7.00 | 1.323 | 0.295 |
| RYGB | 5.94 | 0.856 | ||
| Month 1 | SG | 6.43 | 0.656 | 0.448 |
| RYGB | 5.62 | 0.559 | ||
| Month 3 | SG | 6.00 | 0.767 | 0.267 |
| RYGB | 5.47 | 0.480 | ||
| Month 6 | SG | 5.61 | 0.515 | 0.430 |
| RYGB | 5.32 | 0.415 | ||
| Month 9 | SG | 5.55 | 0.555 | 0.428 |
| RYGB | 5.16 | 0.433 | ||
| Month 12 | SG | 5.28 | 0.422 | 0.854 |
| RYGB | 5.09 | 0.390 |
M Mean; SD Standard deviation; SG Vertical sleeve gastrectomy; RYGB Roux-en-Y gastric bypass
Table 8: Evolution of HbA1c by group
At the end of follow-up, there were complete remissions in 7 (21.21%) of 8 patients in the SG group and in 24 (72.72%) in 25 patients in the RYGB group, 93.93% of patients included in our study (Figures 3 and 4).
Figure 3: Evolution of serum glucose per group
Figure 4: Evolution of HbA1c by group
Discussion
Overweight and obesity have become a global problem due to their incidence and prevalence, their comorbidity and it has a great negative economic and social impact. Their solution is not easy and demands great efforts.
The gastrointestinal tract contributes significantly to glucose homeostasis, and increasing evidence has shown benefits of bariatric/metabolic surgery to treat and prevent T2DM [4-21]. Beyond inducing metabolic improvements related to weight loss, some operations involve mechanisms that improve glucose homeostasis independent of weight loss, such as changes in the metabolism of intestinal hormones, in bile acid metabolism, in the microbiota, in the intestinal metabolism of glucose, and in the sensitivity to nutrients [4,22-29]. It has been hypothesized that changes in gastrointestinal hormone secretion favor the early improvement of T2DM, mainly after RYGB, a procedure in which the duodenum and proximal jejunum are excluded from contact with food (theory of the previous bowel). However, it has been proposed that weight loss obtained by simpler, and purely restrictive procedures, such as SG, also favors the remission of T2DM [30].
Data from an increasing number of recent randomized controlled trials (RCTs) in patients with T2DM [4-21], including mainly individuals with a BMI ≥ 35 kg/m2 (the threshold most commonly used for traditional bariatric surgery), as well as in some patients with BMI<35 kg/m2 (range 25-35 kg/ m2), consistently demonstrate a superior efficacy of bariatric/metabolic surgery in reducing weight and decreasing glycemia compared to a variety of modifications in lifestyle and medical interventions. Although the benefits of diabetes surgery often decrease over time, the relative superiority of surgery over lifestyle modifications and medical interventions in RCTs is similar over a range of 1-5 years. The analysis of RCTs shows a mean reduction of HbA1c of 2.0% for surgery compared to 0.5% for conventional therapies (p=0.001). In all of these trials, the final HbA1c in the surgical groups is about 6.0%, regardless of the baseline HbA1c level. However, most of these RCTs have only been examined for 1-2 years and only a few have examined the results for 3-5 years.
Bariatric operations cause remission of T2DM (defined as the achievement of non-diabetic levels of HbA1c without medications) in most cases. The remission is considered 1) partial, with glucose below the diagnostic thresholds for T2DM (HbA1c>6%<6.5%, fasting glucose 100-125 mg/dl), at least one year of duration, without active drug therapy or procedures in progress; 2) complete, with normal glucose (HbA1c<6%, fasting glucose<100 mg/dl), at least one year of duration, without active drug therapy or ongoing procedures; and 3) prolonged, when there is complete remission for at least five years [31]. Numerous RCTs with postoperative follow-up ranging from 1 to 5 years have consistently documented sustained remission of diabetes in 30-63% of patients [4-21]. Available data suggest an erosion of diabetes remission over time: 35-50% or more of patients who initially achieve remission of diabetes eventually experience recurrence. However, the mean disease-free period among individuals with DGY is 8.3 years [20,32]. With or without relapse of T2DM, the vast majority of patients undergoing surgery maintain a substantial improvement in baseline glycemic control of at least 5 to 15 years [14,20,32-36].
The expected duration of T2DM (eg>8 years) [13], insulin use, and poorer glycemic control are consistently associated with lower rates of remission of T2DM and/or an increased risk of relapse [18,25]. Basal visceral fat may also help predict postoperative outcomes[4].
Beyond the improvement in glycemia, it has been demonstrated in RCTs that bariatric/metabolic surgery confers additional health benefits, including major reductions compared to lifestyle modifications and medical interventions in other risk factors cardiovascular disease (CVD) [4-21] and improvement in the quality of life [9,13,14]. Improvements in other critical outcomes, such as microvascular and macrovascular complications of T2DM, CVD, cancer and death, have only been observed in nonrandomized studies [4,20,34,37,38].
In obese patients with T2DM, short-term studies show that bariatric/ metabolic surgery results in remission of T2DM in 60% to 90% of cases, and this is less common after treatment with changes in lifestyle, exercise alone, and medications for weight loss and/or anti-T2DM. However, although bariatric/metabolic surgery is associated with remission of T2DM and less complications more frequently than usual treatment, no long-term results are known [39,40].
Depending on the bariatric/metabolic procedure used, the diabetes remission rate can range from 45% to 97% of patients. This observation has raised the question of the surgical procedure of choice for treating T2DM. Bariatric/metabolic procedures are considered as an additional therapeutic option allowing improved diabetes control in most of patients. A more favorable diabetes remission is achievable in patients with a relatively short history of diabetes duration [41]. Patients with longer preoperative duration of diabetes, poorer glycemic control, and preoperative insulin use have a lower probability of diabetes remission after RYGB [42]. Both RYGB and SG are similarly efficient for T2DM remission at 1 year, with no significant difference according to the surgical procedure. The preoperative predictive factors of diabetes remission were baseline BMI ≤ 50 kg/m2, duration of T2DM ≤ 4 years, HbA1c ≤ 7.1%, fasting glucose<114 g/l and absence of insulin therapy. A short duration of diabetes and good preoperative glycaemic control increase the rate of T2DM remission 1 year after surgery. Preoperative metabolic data could be of greater importance than the choice of bariatric procedure [43]. Further investigations are needed to clarify the duration of the benefit of surgery in diabetes remission, the mechanism of the success of surgery, and the mechanism associated with diabetic recurrence [44].
REFERENCES
- Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-7.
- Maruthur NM. The growing prevalence of type 2 diabetes: increased incidence or improved survival? Curr Diab Rep. 2013;13(6):786-94.
- Instituto Nacional de Salud Pública. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016 (ENSANUT MC 2016). Ciudad de México. 2016.
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-77.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577-85.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567-76.
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs. intensive medical management for the control of type 2 diabetes, hypertension and hyperlipidemia: The Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240-9.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs. medical treatments for type 2 diabetes mellitus: A randomized clinical trial. JAMA. 2014;149(7):707-15.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: Feasibility and 1 year results of a randomized clinical trial. JAMA. 2014;149:716-26.
- Liang Z, Wu Q, Chen B, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: A randomized controlled trial. Diabetes Res Clin Pr. 2013;101(1):50-6.
- Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: A randomized controlled trial. Lancet Diabetes Endocrinol. 2014;2(7):545-52.
- Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260(4):617-22.
- Schauer PR, Bhatt DL, Kirwan JP et al. Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med. 2014;370:2002-13.
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964-73.
- Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2 year outcomes of a 5 year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;6:413-22.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs. intensive lifestyle and medical intervention for type 2 diabetes: The cross-roads randomised controlled trial. Diabetologia. 2016;59(5):945-53.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three year outcomes of bariatric surgery vs. lifestyle intervention for type 2 diabetes mellitus treatment: A randomized clinical trial. JAMA. 2015;10:931-40.
- Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934.
- Carlsson LMS, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695-704.
- Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-304
- Sjöholm K, Pajunen P, Jacobson P, et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia. 2015;58(7):1448-53.
- Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152-64.
- Salehi M, Woods SC, D’Alessio DA. Gastric bypass alters both glucose-dependent and glucose-independent regulation of islet hormone secretion. Obesity. 2015;23(10):2046-52.
- Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228-38.
- Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55(7):1890-901.
- Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950-5.
- Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183-8.
- Liou AP, Paziuk M, Luevano JM, et al. Conserved shifts in the gut microbiota due to gastric bypass reduces host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41.
- Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406-10.
- Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs. sleeve gastrectomy for type 2 diabetes mellitus. Arch Surg. 2011;146(2):143-8.
- Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42-55.
- Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23(1):93-102.
- Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35(7):1420-8.
- Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122-31.
- Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628-67.
- Hsu CC, Almulaifi A, Chen JC, et al. Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35: Five year outcome. JAMA. 2015;150(12):1-8.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56-65.
- Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62-701.
- Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374:113-23.
- Schauer PR, Mingrone G, Ikramuddin S, et al. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss and remission of diabetes. Diabetes Care. 2016;39(6):902-11.
- Raffaelli M, Sessa L, Mingrone G, et al. Assessing the obese diabetic patient for bariatric surgery: Which candidate do I choose? Diabetes Metab Syndr Obes. 2015;8:255-62.
- Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93-102.
- Robert M, Ferrand-Gaillard C, Disse E, et al. Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: Impact of surgical techniques. Obes Surg. 2013;23:770-5.
- Dixon JB, Zimmet P, Alberti KG, et al. International Diabetes Federation Taskforce on epidemiology and prevention bariatric surgery for diabetes: The International Diabetes Federation takes a position. J Diabetes. 2011;3:261-4.