Factors associated with prognosis of Guillain-Barre syndrome
Citation: El-Khayat NM, Nada MA, El-Sayed HH, et al. Factors associated with prognosis of Guillian-Barre syndrome. Curr Res Cardiol 2018;2(1):25-7.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
40 patients diagnosed with GBS recruited from neuropsychiatry department of Nasser Institute Hospital. They were assessed clinically using the Hughes scale at onset and 3-6 months later. In follow up patients were divided into GBS with good and those with poor prognosis according to their score, Good prognostic group has only 2 or less in the Hughes score while above two patients were considered having poor prognosis At presentation, some lab studies were done to them including CSF examination. Nerve conduction study was done to all the 40 patients. They were treated with plasma pharesis sessions with different number of sessions (6 or less versus more than 6). Reassessment of patients using Hughes GBS disability scale, and nerve conduction study; done 3-6 months after onset.
Keywords
Gullian-Barre syndrome; AIDP; CSF; Neuromuscular disorder; Prognostic factors
Introduction
Despite the use of plasma exchanges and intravenous immunoglobulins, Guillain-Barré syndrome (GBS) still carries non-negligible morbidity and mortality. Furthermore, the psychosocial consequences of GBS may persist longer than expected. Various aetiological, clinical, electrophysiological and immunological factors may carry prognostic predictive value the prognosis of GBS is generally considered favourable. Despite the demonstrated efficacy of plasma exchange (PE) and intravenous immunoglobulins (IVIg), GBS however remains a disabling disease in a significant proportion of patients, and these treatments have not improved mortality. Long-term function is compromised in a significant proportion of subjects. Prognosis and potential determinants of clinical outcome in the disorder have been studied by several investigators in more recent years. Although several review articles have considered the various aspects of GBS, a synthesis of the important question of prognosis and its determinants has not, to date, to our knowledge, been performed. In this article, we review the literature on the specific issue of outcome and its predictors in adequately treated, adult-onset GBS [1].
Patients and Methods
This prospective observational study included 40 patients who are diagnosed with GBS and recruited from neuropsychiatry department of Nasser Institute Hospital.
Patients criteria
Inclusion criteria
• Both sexes are included.
• Age: any age.
• All patients met diagnosis of GBS [2]
Exclusion criteria
• Patients with severe organ failure
• Thyroid dysfunction
• Adrenal dysfunction
• Drug induced polyneuropathy
All patients were subjected to
Interviewing Structured Questionnaire: Full medical and neurological history including:
• Personal Data.
• Complaint.
• Past History
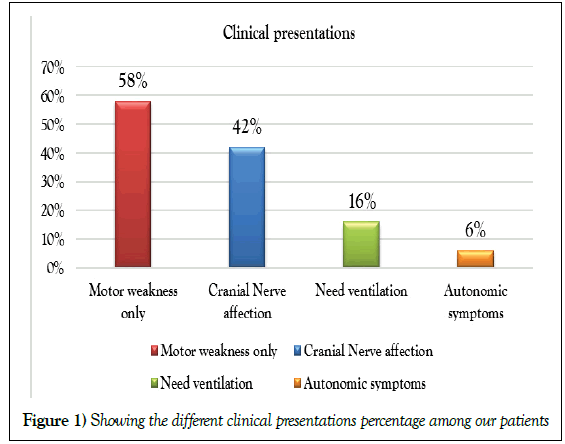
Clinical examination: Full neurological examination stressing on peripheral nervous system with Hughe’s scale [3,4] done in the first assessment and in follow up 3-6 months from onset (Table 1 and Figure 1).
| Variables | Improved group (33) |
Same or Worsened group (7) |
Mann-Whitney's U test |
|
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | p value | ||
| Disability score (on admission) |
3.4 (3 – 4) | 4.4 (4 – 5) | = 0.0016** | |
| Variable | Improved group (33) |
Same or Worsened  group (7) |
Chi square test |
|
| p value | ||||
| DM | No | 31 (93.9) | 6 (85.7%) | = 0.968 |
| Yes | 2 (6.1%) | 1 (14.3%) | ||
| HTN | No | 29 (87.9%) | 7 (100%) | = 0.781 |
| Yes | 4 (12.1%) | 0 (0%) | ||
| Disease onset |
Acute | 10 (30.3%) | 3 (42.9%) | = 0.841 |
| Subacute | 23 (69.7%) | 4 (57.1%) | ||
| Type of antecedent infection |
No infection | 19 (57.6%) | 0 (0%) | = 0.0004** |
| GIT infection | 7 (21.2%) | 7 (100%) | ||
| URT infection | 7 (21.2%) | 0 (0%) | ||
| Clinical presentations |
Motor weakness | 22 (66.7%) | 0 (0%) | <0.0001** |
| Cranial N. affection | 10 (30.3%) | 2 (28.6%) | ||
| Need ventilation | 0 (0%) | 5 (71.4%) | ||
| Autonomic symp. | 1 (3%) | 0 (0%) | ||
Table 1: Showing Comparison between the 2 groups as regards baseline clinical data using Mann-Whitney's U and Chi square tests
Investigations:
Laboratory
• CBC
• CRP
• Serum Na level
• Serum albumin level on admission and after treatment
• Random Blood Sugar
• HbA1C
• CSF examination on admission
• Nerve conduction study (NCS) on both ulnar, peroneal nerves from 0_3 weeks from symptoms and comment on MCV, SCV, F wave, amplitude.
Results
The patients were divided into two groups:
• Improved group: 33 patients.
• Non-improved group: 7 patients.
In this study highly significant increase in disability score (on admission) was found in the worsened group; compared to the improved group; with highly significant statistical difference (p=0.0016).
Meanwhile there was a highly significant increase in the incidence of GIT infection in worsened group; compared to the improved group; with highly significant statistical difference (p=0.0004).
A highly significant increase in the presence of cranial nerve affection and the need for ventilation in the worsened group; compared to the improved group; with highly significant statistical difference (p< 0.0001).
There was a non-significant difference as regards DM, HTN and disease onset (p>0.05) between the 2 groups (Table 2).
| Variables | Improved group (33) |
Same or Worsened  group (7) |
Mann-Whitney's U test |
|---|---|---|---|
| Median (IQR) | Median (IQR) | p value | |
| Na (on admission) (mEq/L) |
138 (135 – 145) |
119 (117 – 121.75) |
= 0.000037** |
| Albumin (on admission) (g/dL) | 3.7 (3.4 – 3.9) |
2.6 (2.3 – 2.8) |
= 0.0001** |
| RBS (mg/dL) | 130 (109 – 166) |
75 (70.7 – 231.5) |
= 0.454 |
| HbA1C (mg/dL) | 5.1 (4.6 – 5.4) |
5.2 (4.4 – 8.1) |
= 0.544 |
Table 2: Shows a comparison between the 2 groups as regards baseline laboratory data using Mann-Whitney's U test
The results of this study showed highly significant decrease in Na and albumin levels (on admission) in worsened group; compared to improved group; with highly significant statistical difference (p<0.01 respectively). Also the study revealed non-significant difference as regards RBS and HbA1C (p>.05) between the two groups (Table 3).
| Variables | Improved group (33) |
Same or Worsened group (7) |
Mann-Whitney's U test |
|
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | p value | ||
| CSF Protein (gm/dL)* |
57 (16.5 – 91.5) |
163 (149.2 – 182) |
= 0.000072** | |
| Variable | Improved group (33) |
Same or Worsened  group (7) |
Chi square Test |
|
| p value | ||||
| Cyto-albuminous Dissociation* |
Present | 10 (41.7%) | 0 (0%) | = 0.106 |
| Absent | 14 (58.3%) | (100%) | ||
CSF: Cerebro-Spinal Fluid
Table 3: Shows a comparison between the 2 groups as regards baseline laboratory data using Mann-Whitney's U test
Our study revealed; a marked increase in the CSF protein in worsened group; compared to improved group; with highly significant statistical difference (p<0.01), and also marked increase in the cyto-albuminous dissociation in the worsened group; compared to the improved group; but not reaching statistical significant difference (p<0.05) (Table 4).
| Variables | Improved group (33) |
Same or Worsened group (7) |
Chi square Test |
|
|---|---|---|---|---|
| p value | ||||
| NCS (on admission) |
Normal | 0 (0%) | 0 (0%) | = 0.027* |
| AIDP | 19 (57.6%) | 0 (0%) | ||
| AMAN | 5 (15.2%) | 4 (57.1%) | ||
| AMSAN | 4 (12.1%) | 1 (14.3%) | ||
| Mixed | 5 (15.2%) | 2 (28.6%) | ||
AMAN: Acute Motor Axonal Neuropathy; AIDP: Acute Inflammatory Demyelinating Polyradiculoneuropathy; AMSAN: Acute Motor and Sensory Axonal Neuropathy
Table 4: Shows comparison between the 2 groups as regards baseline NCS data using Chi square test
The study showed a significant increase in the AMAN type at baseline NCS assessment; in worsened group; compared to improved group; with significant statistical difference (p=0.027).
Discussion
In this study, the presence of Antecedent diarrheal illness was found to be related to disease severity and poor outcome with high significant level. Most studies show Antecedent diarrhea is a predictor of poor outcome [5].
Cranial nerve involvement was found in 12 patients (30. 3%) and the most cranial nerve involved was facial nerve, most studies report cranial nerve involvement ranging from 30-60 % with facial and bulbar nerves being commonly involved, in a study by Sejvar and coauthors [6], 40% patients had cranial nerve affection. In another recent study by Khan and coauthors [7] one third of patients had cranial nerve involvement.
Ophthalmoparesis was noted in one case of Miller Fisher Syndrome (MFS _GBS), autonomic dysfunction was seen in 3% of our patients who had Cardiac arrest from the incidence of autonomic dysfunction has varied in previously reported studies from 17% by Ashok et al., to 66% in (8), 5(71.4%) patients in group II need ventilation and this is associated with disease severity and poor outcome. Compared to previous studies, our patients had a higher GBS disability scale at treatment initiation. Most of our patients had a GBS disability scale greater than 4 in group II before treatment initiation, this was similar to the study by Koningsveld and coauthors, a higher score on admission seen to predict a poor outcome (p 0.0016) [9].
In this study, the most common Nerve Conduction Study pattern was axonal, and this was different from the previous studies by Tavee and coauthors. They found that demyelinating pattern was most commonly seen followed by axonal. Higher prevalence of Acute Motor Axonal Neuropathy (AMAN) has been reported from China [10]. This difference may be because study was done in tertiary center which most severe cases referred to.
Our study showed that Hyponatremia was related to disease severity and poor outcome with significant p value 0.000037, also correlation study done by multiple regression model found hyponatremia had an independent effect on increasing follow up disability score and thus was consistent with most results that found hyponatremia as an important predictor of outcome in GBS patients [11,12].
Also, hypoalbuminemia assessed by correlation study by multiple regression model found hypoalbuminemia had an independent effect on increasing disability score, also by logistic regression analysis, hypoalbuminemia had an independent effect on increasing of probability of NCS worsening. Samukawa found that serum albumin levels decreased in GBS patients in the subacute period and that there was a negative correlation between albumin levels and Hughes’ scores (admission/discharge) [10].
Chen found that impaired lab markers hyponatremia, hypoalbuminemia, hyperglycemia poor predictors of outcome and occur more in elder [13].
In this study, marked increase in CSF protein in group II with high significant value and this result consistent with Kuitwaard that found protein level in CSF was an independent prognostic factor. As regard cytoalbuminous dissociation, So far, no previous studies discussed this result before. No significant difference was found as regard RBS, HbA1C levels as predictors of outcome.
Khan, studied the relation between Fasting Blood glucose (FBG), HbA1C and disease severity and short-term prognosis [14], found that FBG positively correlated to disability score, no relation with HbA1C. As regard RBs, so far no previous studies discuss this result before.
Conclusion
Antecedent infection is common and presence of diarrheal illness associated with axonal subtype which is associated with poor outcome. The most common electrophysiological subtype was axonal followed by demyelinating. High GBS score on admission associated with poor outcome. Hyponatremia, hypoalbuminemia were associated with poor outcome. RBS, HbA1C level were not related to outcome. High CSF protein level is correlated with poor prognosis. CSF cytoalbuminous dissociation are independent predictors of poor outcome.
Recommendations
• Early identification of poor outcome predictors that negatively affect the functional outcome.
• Early intervention with appropriate treatment if possible to achieve better functional outcome, reduce level of disability.
REFERENCES
- Baranwal AK, Ravi RN, Singh R. Exchange transfusion: A low-cost alternative for severe childhood Guillain–Barré syndrome. J Child Neurol 2006;21:960-65.
- Fokke C, Van den Berg B, Drenthen J, et al. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain 2014; 137(1):33-43.
- Chen Y, Andary M, Buschbacher R, et al. Electrodiagnostic reference values for upper and lower limb nerve conduction studies in adult populations. Muscle Nerve 2016;54(3):371Y377.
- Hughes RA, Swan AV, Van Doorn PA. Intravenous immunoglobulin for Guillain-Barre syndrome. The Cochrane database of systematic reviews 2014;9:CD002063.
- Khan F1, Pallant JF, Amatya B, et al. Outcomes of high- and low-intensity rehabilitation programme for persons in chronic phase after Guillain–Barré syndrome: A randomized controlled trial. J Rehabil Med 2011;43:638-46 .
- Khan F, Amatya B. Rehabilitation interventions in patients with acute demyelinating inflammatory polyneuropathy: A systematic review. Eur J Phys Rehabil Med 2012;48:507–22.
- Korinthenberg R, Schessl J, Kirschner J. Clinical presentation and course of childhood Gullian-Barre syndrome: A prospective multicentre study. Neuropediatrics 2007;38:10-17.
- Kuwabara S, Mori M, Ogawara K, et al. Indicators of rapid clinical recovery in Guillain-Barre´syndrome. J Neurol Neurosurg Psychiatry 2001;70: 560e2.
- Saifudheen K, Jose J, Abdul Gafoor V, et al. Guillain-Barre´ syndrome and SIADH. Neurology 2011; 76:701e4.
- Samukawa M, Hamada Y, Kuwahara M, et al. Clinical features in Guillain–Barré syndrome with anti-Gal C antibody. J Neurol Sci 2014;337;55-60.
- Sejvar JJ, Baughman AL, Wise M, et al. Population incidence of Guillain-Barre´ syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011;36:123e33.
- Tavee JO, Polston D, Zhou L, et al. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564Y569.
- Uncini A, Manzoli C, Notturno F, et al. Pitfalls in electrodiagnosis of Gullian-Barre syndrome subtypes. J Neurol Neurosurg Psychiatry 2010; 81:1157-63.
- Walgaard C, Lingsma HF, Ruts L, et al. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology 2011;76:968-75. (2011).