Gastroesophageal fat pad has no clinical significance in patients with GERD, giant hiatus hernia and in repeat surgery
2 Department of Medicine, University of Ljubljana, Slovenia, Email: miha.sok@kclj.si
Received: 15-Jun-2018 Accepted Date: Jul 20, 2018; Published: 25-Jul-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: The objective of the study was to assess the clinical significance of evident gastroesophageal fat pad (EGFP) in patients operated on for gastroesophageal reflux disease (GERD), giant hiatal hernia (HH) types III and IV and repeat surgery for recurrent hernia.
METHODS: Data about EGFP were prospectively evidenced at operation. An EGFP was defined as a well encapsulated lipoma-like lipidic tissue more than 2 cm in its small diameter, not covered with peritoneum. A group of 119 patients receiving surgery for either GERD and HH. Patients with repeat operations were also included in the study. The preferred operations were laparoscopic Toupet and Nissen fundoplication.
RESULTS: In total 49% of patients presented with an EGFP, 46% from the GERD group and 53% from the HH group. The presence of EGFP did not affect the symptoms of GERD. Number of stiches as indirect evaluation of hernia size was practically equal in patients with and without EGFP. A positive correlation was observed between EGFP and BMI of the patients. Of 119 patients, 12 patients received repeat surgery. In this group an EGFP was found in six cases (50%).
CONCLUSION: An EGFP was present in 49% of all studied patients and in 50% of the patients receiving reoperation. Patients with higher BMI had significantly more EGFP (p=0.001). An EGFP were not associated with the clinical manifestation of GERD and the size of HH. An EGFP resected from mediastinum and left intraabdominally is not associated with incidence of hernia recurrence.
Keywords
Gastroesophageal fat pad; Giant HH; GERD; Hiatal hernia recurrence; Repeat operation
On unconventional discussion, surgeons believe that the presence of a gastroesophageal fat pad (GFP) at the gastroesophageal junction (GEJ) is pretty much universal and varies in size, but in medical literature the incidence and possible significance in patients with gastroesophageal reflux disease (GERD) and hiatus hernia (HH) was not yet reported. It was not reported either at repeat surgery for hernia recurrence, dysphagia or reflux recurrence [1]. The purpose of the study was to retrospectively analyse recent operations for GERD, HH and reoperations with respect to GFP presentation.
Materials and Methods
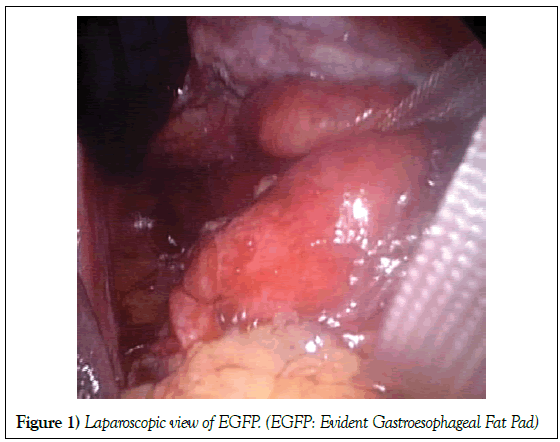
Data about gastroesophageal fat pad were prospectively evidenced at operation. An evident gastroesophageal fat pad (EGFP) was arbitrary defined as a lipoma-like lipidic tissue in the hiatus, tending to be encapsulated, roundish in shape, variable, more than 2 cm in its small diameter and not covered with peritoneum (Figure 1).
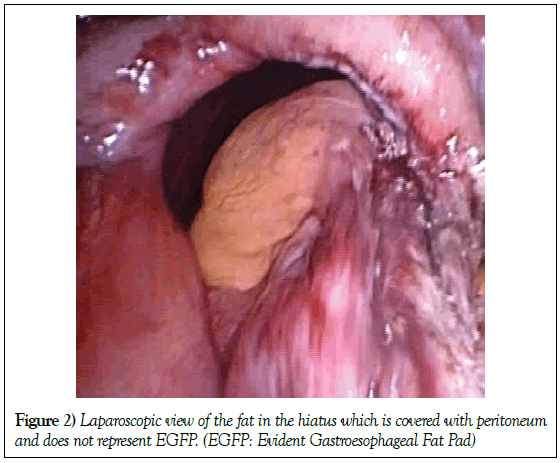
In some cases thick and hard pale yellow fat, probably from the lesser or large omentum, covered with peritoneum is found in the hiatal sac and is not registered as a GFP case (Figure 2).
The term lipoma seems to be appropriate as we believe that the term “lipoma” itself is not a misnomer and is rather a benign neoplasm and is used in other abdominal hernia reports [2]. Retrospective review of 136 consecutive operations for GERD and HH performed at the tertiary Thoracic Centre between January 2012 and December 2014 is reported. Relevant clinical data were collected from medical records and operative notes where data for presence and size of lipoma were prospectively registered. Indications for anti-reflux fundoplication were symptomatic and documented gastroesophageal reflux that persists despite maximal medical therapy, extra-oesophageal manifestations and GERD related oesophageal injury. The indication for HH repair was clinical and when barium x-ray studies gave evidence of giant type III and IV HH. Hiatus hernia was defined according to Landrenau classification [3]. Giant HH was defined as more than 50% of the stomach herniated [4]. The intraoperative size of hernia was not assigned in cm but in number of stiches for hiatus closure suggesting that more stiches were required to repair a larger hernia. Number of stiches represented an approximation of the HH size. The preoperative evaluation consisted of clinical examination, endoscopy and barium upper GI study and, in some GERD cases, manometry and 24-hour pH-metry. The type of HH was identified by a barium x-ray. Some data, e.g. clinical manifestation, barium contrast studies, manometry, and pH-metry were not available for all patients. The data was collected from our database to assess clinical characteristics and postoperative outcome. Number of stiches for diaphragmatic crural closure as indirect measure of hernia size was detected. Some patients who underwent reoperation for reflux recurrence, dysphagia and hernia recurrence were also included in the study. Any symptomatic hernia seen on postoperative radiological contrast imaging was classified as a recurrence [5]. Operations were performed laparoscopically. A transthoracic approach was used in some cases of recurrent hiatus hernia. Patient’s obesity was classified by body mass index (BMI). Follow-up consisted of postoperative visits whenever necessary in addition to a regular 3 month and 1 year postop visits. A barium study was made on the first postoperative day and as required later on in symptomatic patients. Patients were followed up until January 2017 between 2 and 5 years in total.
Surgical Technique
Basic principles for HH and GERD repair were used. Gastroesophageal junction with or without EGFP was dissected from hiatal muscles. Extensive mobilisation of the intrathoracic oesophagus was strictly performed in case of short oesophagus. Nonabsorbables Ethibond 0 sutures with Teflon pledgets were used for crural closure. Some anterior sutures of the hiatus were also performed if necessary. No prosthetic mesh was used. The GFP usually lay within the hernia sac in the hiatus, around and posterior to the distal oesophagus and cardia in the posterior mediastinum, close to the crura and anterior to the aorta (Figure 1). Tailored Nissen or Toupet fundoplication was made. Bougies were only used in select cases. Dissection of the hiatus with EGFP is more difficult and time consuming.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences version 16.0 for Windows (SPSS, Chicago, IL) software according to the following relevant specifications: categorical variables were tested for association using the χ2 test; continuous data were expressed as mean ± standard deviation; for normally distributed variables the t-test was used; the Spearman Rho association test was used to measure the relationship between two variables; a p-value of <0.05 was considered statistically significant.
Results
Of 119 patients all but three were operated on laparoscopically. Three conversions to the open procedure were made in the HH group because of unmanageable bleeding. There were 73 female and 46 male patients. Preoperatively no patient was diagnosed with dysphagia or peptic stricture. No preoperative exams suggested the presence of GFP. The BMI of patients with and without the EGFP was 27.9 kg/m2 and 26.8 kg/m2, respectively. Older patients were generally more obese. A correlation calculation was performed to determine whether hiatal EGFP is associated with the BMI and the age of the patients. The association was considered statistically significant for BMI (p=0.001) but not for age (p=0.053) (Tables 1-3).
| Variables | EGFP n=58 |
no EGFP n=61 |
|
|---|---|---|---|
| Female | 32 | 41 | |
| Male | 26 | 20 | |
| Age mean ± SD (range) | 54.8 ± 16.6 (23–89) | 52.7 ± 16.9 (16–87) | P=0.053 |
| GERD patients | 32 | 38 | |
| HH patients | 26 | 23 | |
| Repeat operations | 6 | 6 |  P=0.92 |
| BMI kg/m2 mean ± SD (range) | 27.9 ± 4.6 (17.3– 39.0) | 26.8 ±3.9 (17.7–37.0) | P=0.003 |
| Number of stiches mean ± SD (range) | 3.3 ± 1.3 (2-6) | 3.2± 1.2 (1-7) |
EGFP: Evident Gastroesophageal Fat Pad,
HH: Hiatal Hernia,
GERD: Gastroesophageal Reflux Disease,
BMI: Body Mass Index
Table 1: Effect of a-mangostin on body weight gain
| Variables | EGFP | No EGFP | |||
|---|---|---|---|---|---|
| n=32 | % | n=38 | % | ||
| Female/male | 19/13 | 23/15 | |||
| Age mean | 50 | 47 | |||
| Heartburn | 29 | 91 | 37 | 95 | |
| Extraesophageal manifestation | 19 | 59 | 20 | 53 | |
| Dysphagia | 0 | 0 | |||
| * DeMeester score mean ± SD | 26.4 ± 22.4 (4–127) | 28.8 ± 29.7 (3–166) | |||
| Manometry, LES decreased (<13 mm Hg) | 17 (out of 21) | 81 | 30 (out of 36) | 83 | |
| Resistance to medication | 15 (out of 26) | 58 | 24 (out of 34) | 71 | |
| Barrett’s | 3 (out of 25) | 12 | 1 (out of 38) | 3 | |
| # BMI mean ± SD (range) | 27.5 ± 4.6 (17.3–37) | 26.5 ± 4.0 (17.7–37) | |||
EGFP=evident gastroesophageal fat pad
BMI=body mass index
# P=0.026
*P=0.29
Table 2:Effect of a-mangostin on liver and fat weight
| Variables | ND | HFD | HFD+M |
|---|---|---|---|
| Serum TG (mg/dl) | 95.3 ± 8.6 | 92.9 ± 7.0 | 96.8 ± 4.1 |
| Serum TC (mg/dl) | 187.7 ± 36.5* | 223.2 ± 13.4 | 203.9 ± 17.9* |
| Hepatic TG (mg/g liver) | 66.86 ± 15.6 | 85.56 ± 14.2 | 44.53 ± 7.11* |
EGFP: Evident Gastroesophageal Fat Pad
BMI: Body Mass Index
Table 3: Characteristics of HH patients with different hiatal EGFP status (n=49)
Mean BMI in GERD and HH group were 27.5 and 28.5, respectively (p<0.05). Mean BMI of reoperated and non-reoperated patients were 28.5 and 27.8 respectively (p<0.05). A total of 119 patients were entered into the study group, as well as 12 reoperated patients. Basic characteristics of patients in the study with respect to EGFR presence are shown in Table 1. In total, 58 (49%) patients had an EGFP; 32 (46%) of the GERD group, 26 (53%) of the HH group and six (50%) in the reoperated group of patients. The GERD group included 70 patients. In 46% EGFR was found.
Table 2 shows the significance of EGFP presence in respect to clinical manifestation. Not all clinical data were available for every patient. There was no significant difference in the incidence of heartburn, extra-oesophageal manifestations, De Meester score or diminished lower oesophageal sphincter (LES) pressure values at manometry, (less than 13 mmHg) and resistance to medication. Patients with an EGFP presence had again statistically significantly higher BMI in comparison with patients without EGFP (p=0.026). Notably, more patients in the group of EGFP had histologically confirmed Barrett’s changes. The HH group comprised of 49 patients. All but 5 patients were operated on electively. In 53% EGFR was found. EGFR was not associated with the size of hernia. Patients operated on for HH required significantly more stiches in comparison to GERD patients e.g., mean 3.3 ± 1.31 (2-7) and 2.6 ± 1.14 (1-4) respectively (t=9.04; p=0.0001). Number of stiches in patients with or without EGFP were not different suggesting that EGFP is not associated with hernia size. In the reoperated group of patients the mean number of stiches at first operation was lower than in the entire HH group e.g., 3.3 ± 1.31 (2-6) and 3.3 ± 1.31 (2-7) respectively (p=0.12) (Tables 1-3).
Reoperated patients
A total of 12 patients (10%) received reoperations, 3 (4%) in the GERD group (one for dysphagia, one for HH recurrence and one for reflux recurrence) and 9 (18%) in the HH group (eight for symptomatic, radiographically confirmed hernia recurrence and one for dysphagia). The difference is statistically significant (p=0.029). Reoperated group of patients were older (55.9 years vs. 54.7 years) (p>0.05). An EGFP was found in six (50%) reoperated patients. The HH recurrence was not associated with EGFP presence at the first operation (p=0.104).
Discussion
Anatomically, there should always be some GFP of variable size in the hiatus region around the EG junction. We defined an EGFP arbitrarily as lipoma like tissue more than 2 cm in its small diameter and not covered with peritoneum. Protrusion of the fat from the omentum minus or majus which is covered with peritoneum was not classified as an EGFP. More patients with EGFP had Barrett’s changes and confirm the data reported [6,7]. No patient had dysphagia before operation. We believe that some space occupying tissue, as EGFP might be, can cause problems but as a fat pad grows slowly, tissue around it accommodates as observed in intramucosal oesophageal lipomas which only begin to cause dysphagia once huge in size [8,9]. Results show that presence of an EGFP has no impact on clinical manifestations of the GERD and pH –metry and manometry results. It has been reported that obese and morbidly obese patients have significantly higher incidence of recurrent hiatal hernia [8-10]. In our study however the group of reoperated patients had slightly nonsignificantly higher mean BMI (28.5 kg/m2) in comparison to other non-reoperated patients (27.8 kg/m2) (data not shown). Patients with EGFP didn’t have more repeat operations (Table 1). EGFP presence at first operation didn’t favour later hernia recurrence. The percentage of EGFP positive patients in hernia recurrence and in patients operated for HH was 50% and 53% respectively. It is obvious that all patients in HH group had a hernia sac with or without GFP resected from the mediastinum but not from the abdomen. The vast majority of experts advocate that the mediastinal hernia sac should be dissected completely from the mediastinum [11,12] but there is controversy as to whether or not the hernia sac, once mobilized from its intra-thoracic position, has to be respected, i.e., excised from abdominal cavity as well [11]. In the extensive article about HH repair by Luketich et al. [13] the GFP is mentioned but the emphasis is on the hernia sac, suggesting it should not only be dissected but excised and removed. In another article from the same centre dissection and not removal of the hernia sac is described [14]. Guidelines for the management of hiatal hernia strongly recommend hernia dissection and excision from mediastinal structures, but recommendation of excision from abdominal cavity is not stated. In our practice we follow Watson et al. [15] argument where importance of resection of the hernia sac from mediastinum was pointed out and where the authors declared that excision of the redundant hernia sac from the abdominal cavity is unnecessary because it can be pulled downward, so that it lies below the completed fundoplication and is still attached to the gastric cardia. Our approach is also based on the experience that laparoscopic complete removal of hernia sac especially with EGFP from the abdomen through 10 mm ports is problematic and time consuming with important risk for posterior vagus traumatisation. Our study shows no significant hernia recurrence in patients with EGFP left in the abdomen and we believe that could be an indirect confirmation that the hernia sac with EGFP can stay intraabdominally and should not be indiscriminately excised. However in the group of patients with hiatus hernia III and IV HH recurrence was significantly increased in comparison to GERD patients with or without sliding hernia. One important factor is crural muscle weakness in HH patients. However there is some suggestion that the hernia sac can also act as one of the factors of intraabdominal hernia recurrences. The relationship between HH and inguinal hernia (IH) was reported [5]. The presence of lipoma in both types of hernias was described, as well as lipoma in recurrent IH [16]. The authors speculate that a cord lipoma in IH could be a migrated lipoma and even the leading point in hernia pathogenesis and they recommend complete lipoma and hernia sac removal [17]. Authors suggest common aetiology for both IH in adults and HH and hypothesise “push” factors. They state that intra-abdominal pressure rises above normal, either intermittently or chronically, due to obesity or external compression.
As a result, the EG junction is pushed up, allowing HH recurrence to occur. On the basis of this speculation it makes sense that firm intraabdominal fixation of EG junction and obliteration of the hiatus region to prevent intraabdominal tissue to reenter this region might be a potential step for HH recurrence prevention. The size of HH was not estimated at endoscopy, radiology, high resolution manometry or at operation but with number of stiches for crural closure which is a speculation that more stiches are needed for larger hernia. Larger hernias are more prone to recur [18]. However reoperated patients in our study had a lower mean number of stiches at first operation. But we registered more stiches for hiatus closure in HH patients in comparison to GERD patients. Based on number of stiches as indirect evaluation of HH size the presence of EGFP was not associated with the size of HH. In our study with no oesophageal elongation procedure and without mesh cruroplasty we recorded 1% and 16% of hernia recurrence in GERD and HH patients respectively. The results are comparable to some other studies [19-21].
Conclusion
We emphasise that GFP is a frequent finding in operations for HH and GERD. The incidence of GFP is higher in patients with an elevated BMI score. A hiatal GFP is not associated with clinical manifestations of GERD and is not directly associated with HH recurrence. EGFP can make some problems during operation. Fallings include lack of complete results and small group number.
Disclosures
Drs Mihael Sok, Miha Zavrl, and Tomaž Štupnik have no conflicts of interest or financial ties to disclose.
REFERENCES
- Kohn GP, Price RR, De Meester SR, et al. SAGES Guidelines Committee. Guidelines for the management of hiatal hernia. Surg Endosc. 2013;27(12):4409-4428.
- De Luca L, Di Giorgio P, Signoriello G, et al. Relationship between hiatal hernia and inguinal hernia. Dig Dis Sci. 2004;49(2):243-247.
- Landreneau RJ, Del Pino M, Santos R. Management of paraesophageal hernias. The Surgical clinics of North America. 2005;85(3):411-432.
- Awais O, Luketich JD. Management of giant paraesophageal hernia. Minerva Chir. 2009;64(2):159-168.
- Parameswaran R, Ali A, Velmurugan S, et al. Laparoscopic repair of large paraesophageal hiatus hernia: quality of life and durability. Surg Endosc. 2006; 20(8):1221-1224.
- Eusebi LH, Fuccio L, Bazzoli F. The role of obesity in gastroesophageal reflux disease and Barrett's esophagus. Dig Dis. 2012;30(2):154-157.
- Nelsen EM, Kirihara Y, Takahashi N, et al. Distribution of body fat and its influence on esophageal inflammation and dysplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2012;10(7):728-734.
- Medici JR, Gomez NL, Wright FG, et al. Giant esophageal lipoma. J Gastrointest Surg. 2016;20(2):473-475.
- Baldaque-Silva F, Marques M, Sanchez-Hernandez E, et al. Endoscopic submucosal dissection of a giant esophageal lipomaa. Am J Gastroenterol. 2016;111(12):1680.
- Akimoto S, Nandipati KC, Kapoor H, et al. Association of Body Mass Index (BMI) with patterns of fundoplication failure: insights gained. J Gastrointest Surg. 2015(11);19:1943-1948.
- Naunheim KS, Edwards M. Paraesophageal hiatal hernia. In: Shilds TW, LociceroIII J, Reed CE, (eds) General thoracic surgery. Philadelphia: Lippincott Williams&Wilkins. 2009;1951-1959.
- Morrow EH, Oelschlager BK. Laparoscopic paraesophageal hernia repair. Surgical laparoscopy, endoscopy & percutaneous techniques. 2013;23(5):446–448.
- Luketich JD, Raja S, Fernando HC, et al. Laparoscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg. 2000;232(4):608-618.
- Nason KS, Luketich JD, Witteman BP, et al. The laparoscopic approach to paraesophageal hernia repairs. J Gastrointest Surg. 2002;16(2):417-426.
- Watson DI, Davies N, Devitt PG, et al. Jamieson GG Importance of dissection of the hernial sac in laparoscopic surgery for large hiatal hernias. Arch Surg. 1999;134(10):1069–1073.
- Nasr AO, Tormey S, Walsh TN. Lipoma of the cord and round ligament: An overlooked diagnosis? Hernia. 2005;9(3):245-247.
- Lilly MC, Arregui ME. Lipomas of the cord and round ligament. Ann Surg. 2002;235(4):586-590.
- Le Page PA, Furtado R, Hayward M, et al. Durability of giant hiatus hernia repair in 455 patients over 20 years. Ann R Coll Surg Engl. 2015(3);97:188-193.
- Low DE, Unger T. Open repair of paraesophageal hernia: reassessment of subjective and objective outcomes. Ann Thorac Surg. 2005;80(1):287-294.
- Rathore MA, Andrabi SI, Bhatti MI, et al. A Metaanalysis of recurrence after laparoscopic repair of paraesophageal hernia. JSLS. 2007;11(4):456–460.
- Antiporda M, Veenstra B, Jackson C, et al. Laparoscopic repair of giant paraesophageal hernia: are there factors associated with anatomic recurrence? Surg Endosc. 2018;32(2):945-954.