Gene therapy for in-stent restenosis: Targets and delivery system
- *Corresponding Author:
- Dr Devendra K Agrawal
Center for Clinical & Translational Science, Creighton University School of Medicine, CRISS II Room 510, 2500 California Plaza, Omaha, Nebraska 68178-0405, USA.
Tel:402-280-2938
Fax:402-280-1421
E-mail:dkagr@creighton.edu
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
Gene therapy; Intimal hyperplasia; Nanotechnology; Re-endothelialization; Restenosis; Stem cell
Despite the increased use of drug-eluting stents (DES), the incidence of in-stent restenosis (ISR) requiring target vessel revascularization remains unacceptably high [1,2]. The incidence rate of ISR requiring target vessel revascularization (ie, ‘DES failure’) in the United States alone was conservatively estimated to be 10%, and may be more common in patients with diabetes mellitus [3]. Although the pathophysiology of DES failure is complex, histopathological changes of in-stent neointima following directional atherectomy at the time of reintervention have been shown to be remarkably similar between bare-metal stents (BMS) and DES, suggesting that a process mediated by the endothelial injury, inflammation, proliferation and migration of vascular smooth muscle cells (VSMCs), and thrombosis plays the same important role in the progress of ISR of DES [4,5]. In addition, antiproliferative drugs in DES, primarily aimed at preventing VSMC proliferation (which is central to the pathogenesis of ISR), also perturb endothelial recovery [6], which consequently increases the risk for subacute in-stent thrombosis and results in late stent thrombosis (ST) [7]. Furthermore, polymer coatings on DES induce a distinct inflammatory reaction compared with bare metal surfaces [8]. These results raise serious questions regarding the long-term durability and efficacy of DES, thereby resulting in an urgent need to develop new therapies to prevent restenosis. The present review focuses on the progress made to date in the use of gene therapy, including catheter-based gene delivery and gene-eluting stents (GES), to prevent ISR. We will also discuss the current and promising application of magnetic targeting gene delivery systems for antirestenosis therapy.
Evolution of antirestenosis therapy
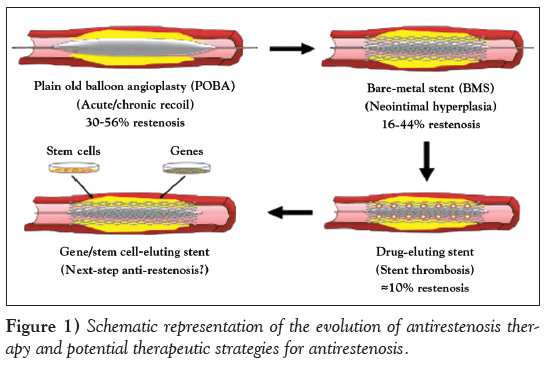
In the past several decades, percutaneous transluminal coronary angioplasties have revolutionized the treatment of atherosclerosis-related cardiovascular disease. At the same time, tremendous efforts have also been made to reduce the incidence of restenosis after percutaneous transluminal coronary angioplasties [9]. During the time in which ‘plain old balloon angioplasty’ was prevalent, rates of acute and chronic vessel occlusion were higher (30% to 56%), secondary to acute/chronic recoil and constrictive remodelling [10]. These problems led to the development of a second revolutionary treatment, BMS, which were first implanted in 1986 [11]. Although this ‘bailout’ scaffold significantly reduced the issue of acute and chronic recoil, its application was ultimately hampered by the risk for subacute thrombotic coronary artery occlusion and neointimal hyperplasia [12]. The restenosis rates with BMS were reported to be between 16% and 44%, with higher rates of stenosis attributable to several risk factors, particularly long lesion length and small vessel calibre [1,13].
Considerable progress to reduce the incidence of restenosis was made by the advent of DES, which are composed of a metallic stent, a polymer-based drug delivery platform and a pharmacological agent (typically an immunosuppressant and/or antiproliferative compounds, including sirolimus and paclitaxel [PTX]) [14]. Several classic studies unequivocally demonstrated that DES markedly reduced rates of ISR to 5% to 8% [15,16]. However, long-term follow-up studies have raised concerns about the long-term safety of DES, although DES definitely improves short- to midterm ISR [17]. The application of DES comes with an increased risk for ST because the pathophysiology of ISR and ST appear to be diametrically opposed. Briefly, ISR is related to an overzealous response of the vessel to injury, whereas ST occurs due to impaired or delayed endothelium healing, resulting in acute and subacute thrombotic events [14]. There is an urgent need, therefore, to develop novel and efficacious methods to deal with the problems observed in current antirestenosis therapy (Figure 1). Recently, Deuse et al [18] reported the identification of pyruvate dehydrogenase kinase isoform 2 as a key regulatory protein in the development of myointimal hyperplasia in human arteries for its regulated effect on hyperpolarization of mitochondrial membrane potential and resistance to apoptosis in smooth muscle cells (SMCs) during ISR. Normally, proliferation and apoptosis are very low and balanced in healthy vessels. However, vessel injury induces inflammatory response and temporary mitochondrial membrane potential hyperpolarization, which results in the temporarily acquired apoptosis resistance in SMCs and promotes myointima formation. Dichloroacetate, a pharmacological pyruvate dehydrogenase kinase isoform 2 blockade, counteracts temporarily acquired apoptosis resistance and reduces myointima formation in injured coronary arteries of human and various animal models [18]. Most importantly, dichloroacetate did not affect vessel re-endothelialization, suggesting a novel strategy for the prevention of proliferative vascular diseases, especially ISR. Moreover, magnetic nanotechnology and genetic engineering of human stem cells have also emerged as exciting alternatives to eliminate the underlying mechanisms of ISR, suggesting their possible roles as the next generation of antirestenosis therapy.
Therapeutic Gene targets
re-endothelialization
Stent implantation for coronary artery disease causes a marked vascular injury, inducing de-endothelialization, which is associated with thrombus formation and abnormal responses to endothelium-dependent agonists [19]. In recent years, several studies have focused on the use of vascular gene therapy targeting multiple pathways to promote reendothelialization [20-26]. The potential of vascular endothelial growth factors (VEGFs), a group of potent endothelial mitogens, to treat postangioplasty restenosis was first investigated in animal models. Several VEGF family members, including VEGF165, VEGF-2, VEGF-C and VEGF-D, have been found to inhibit neointimal thickening, reduce thrombogenicity and restore the endothelium-dependent vasomotor reactivity [27-29]. Yang et al [23] developed a stent coated with bilayered poly-lactide-coglycolide (PLGA) nanoparticles (NPs) containing VEGF plasmid in the outer layer and PTX in the inner core and found that it significantly promotes early endothelium healing while inhibiting SMC proliferation through sequential release of the VEGF gene and PTX [23].
Another interesting target is nitric oxide (NO), which is mainly synthesized by NO synthase (NOS). Most studies have focused on two main types of NOS, inducible NOS and endothelial NOS (eNOS), to reduce neointimal hyperplasia and accelerate re-endothelialization [30- 33]. Both catheter- and stent-based delivery techniques have verified eNOS and inducible NOS as potential therapeutic genes for accelerating re-endothelialization and reducing neointimal hyperplasia (Tables 1 and 2) [31-33]. Sharif et al [31] found that eNOS gene delivery can result in significantly enhanced re-endothelialization and decreased neointimal formation using an adenovirus GES. The same laboratory reported that nonviral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization as well as prolonged and localized eNOS expression in the blood vessel wall in a hypercholesterolemic rabbit model [22]. Cyclooxygenase-1 (COX-1) is the rate-limiting component in the synthesis of prostacyclin (PGI2), a potent vasodilator and antithrombotic molecule. In balloon-injured, atherosclerotic porcine and hypercholesterolemic rabbit models, COX-1 gene transduction induced an early increase in the production of PGI2, prostaglandin E2 and prostaglandin E1, resulting in long-lasting vasodilation [34].
| Author (reference) | Vector(s) | Gene(s) | Model/patients | Main outcome(s) |
|---|---|---|---|---|
| Taniyama et al (67) | Naked plasmid DNA | p53 | Rat carotid artery after balloon injury | Transgene expression of naked p53 plasmid DNA using ultrasound transfection method decreased the intimal-to-medial area ratio |
| Van Belle et al (69) | Naked plasmid DNA | VEGF 165 | Rabbit artery stent restenosis model | Transgene expression of naked VEGF165 plasmid DNA decreased mural thrombus and intimal thickening |
| Fuchs et al (64) | Naked plasmid DNA | C-type natriuretic peptide | Porcine restenosis model | Transgene expression of naked C-type natriuretic peptide plasmid DNA resulted in significant inhibition of neointimal formation without compromising endothelial repair |
| Zhang et al (62) | Naked plasmid DNA and anti-DNA antibody- immobilized stent | iNOS | Porcine coronary stent model | Tethering of pcDNA3.1-iNOS on the stent showed long-term therapeutic effects for antirestenosis |
| Chang et al (99) | Protamine sulfate plasmid DNA | Hepatocyte growth factor | Rat carotid artery injury model | The protamine sulfate/hepatocyte growth factor-pDNA multilayer coated stent can reduce in-stent restenosis in vivo |
| Brito et al (33) | Liposomes plasmid DNA | eNOS | Rabbit artery stent restenosis model | Efficient transfection of eNOS locally in the arterial lumen suppressed SMC proliferation and promoted re-endothelialization of the artery showing a significant reduction of restenosis |
| Deiner et al (100) | Liposomes plasmid DNA | VEGF-165 | Balloon-injured porcine coronary arteries model | Local delivery of VEGF-165 gene into the outer compartments of balloon-injured porcine coronary arteries reduced lumen area loss due to distinct positive remodelling |
| Yang et al (63) | PLGA/NPs plasmid DNA | Antisense for MCP-1 | Rabbit carotid artery injury model | Local infusion of anti-MCP-1 NPs significantly lowered the intimal hyperplasia |
| Walter et al (65) | Phosphorylcholine polymer plasmid DNA | VEGF-2 | Rabbit artery stent restenosis model | Transgene expression of VEGF-2 in the vessel wall improved functional recovery of stented segments |
| Hedman et al (66) | Liposomes plasmid DNA | VEGF | Patients with coronary artery disease | Transgene expression of VEGF cannot improve the ISR in human after the 6-month follow-up |
| Egashira et al (56) | ODN | Antisense NF-κB ODN | Patients with coronary artery disease | NF-κB ODN Transfection of NF-κB decoy ODN can obviously suppress the ISR in human |
| Matsushita et al (101) | ODN | Antisense p53 ODN | Rat intact carotid artery model | Loss of p53 by antisense p53 ODN resulted in an abnormal VSMC growth in vitro and in vivo |
| Nikol et al (68) | Liposomes plasmid DNA | Cecropin A | Porcine arterial injury model | Transfection of cecropins A prevented restenosis by limiting SMC proliferation In vivo |
| Chapman et al (102) | Liposomes plasmid DNA | Luciferase | Mongrel dogs model | The first study to show the efficiency of nonviral gene transfection using percutaneous catheter |
eNOS Endothelial nitric oxide synthase (NOS); iNOS Inducible NOS; ISR In-stent restenosis; MCP-1 Monocyte chemoattractant protein-1; NPs Nanoparticles; ODNs Oligodeoxynucleotides; PLGA Poly-lactide-coglycolide; SMC Smooth muscle cell; VEGF Vascular endothelial growth factor; VSCM Vascular smooth muscle cells
Table 1: Summary of major studies using nonviral vectors for antirestenosis gene therapy
| Author (reference) | Vector(s) | Gene(s) | Model/patients | Main outcome(s) |
|---|---|---|---|---|
| Li et al (44) | Adenovirus | miR-663 | Mice carotid artery injury model | Adeno-miR-663 markedly suppressed the neointimal lesion formation in mice |
| Merlet et al (43) | Adenovirus | miR-424/322 Magnetic nanoparticles | Rat carotid artery injury model | Adeno-miR-424/322 markedly suppressed the neointimal lesion formation in rats |
| Chorny et al (77) | Adenovirus | Rat carotid artery injury model | Magnetically targeted adenovirus-loaded magnetic nanoparticles represent a novel delivery system for efficient, vascular gene transfer in vivo | |
| Wu et al (80) | Adenovirus | Dominant-negative Skp2 | Rat carotid artery injury model | Knockdown of Skp2 inhibited VSMC proliferation, and the subsequent neointimal thickening in rat arteries |
| Fishbein et al (103) | PABT/PEI(PDT)/ HL-tethered adenoviral vectors | iNOS | Rat carotid artery stent model | Rat carotid stent delivery of iNOS resulted in significant inhibition of restenosis in rats |
| Johnson et al (48) | Adenovirus | TIMP-3 | Porcine coronary | Adenovirus-coated TIMP-3 stent significantly inhibited the in-stent neointimal formation in pigs |
| stent model | ||||
| Hedman et al (66) | Adenovirus | VEGF | Patients with coronary artery disease | Myocardial perfusion showed a significant improvement in the VEGF-adenovirus-treated patients after the six-month follow-up |
| Sinnaeve et al (104) | Adenovirus | PKG mutant | Porcine coronary stent model | Expression of a constitutively active PKG reduces neointimal formation after balloon injury in rats and reduces coronary in-stent restenosis in pigs |
| Liu et al (34) | Adenovirus | COX-1 | Rabbits carotid artery balloon injury model | Local gene transduction of COX-1 increases blood flow in injured atherosclerotic rabbit arteries |
| Lompré et al (50) | AAV2.5 | Sarco/endoplasmic reticulum Ca2+ ATPase isoform 2a | Rat carotid artery balloon injury model | AAV2.5 vector can be considered as a promising safe and effective vector for antirestenosis gene therapy in vivo |
| Sharif et al (79) | AAV2 | DNase-resistant particles | Rabbits iliac artery stent model | AAV2-coated stents can be used to deliver genes to the blood vessel wall for up to 28 days in vivo |
| Pankajakshan et al (82) | AAV2/9 | SM22α promoter | Swine coronary and peripheral arteries | AAV2/9 vector can be considered as a promising safe and effective vector for antirestenosis gene therapy in vivo |
| Ramirez Correa et al (78) | Recombinant AAV | TIMP-1 | Rat carotid injury arteries | Local AAV-TIMP-1 gene transfer represents an efficient strategy to prevent restenosis in rats |
| Squadrito et al (105) | Recombinant AAV | NF-κB inhibitory protein IκBα | Mice carotid artery injury model | Recombinant AAV-mediated gene transfer of IκBα inhibited the restenosis in mice |
| Yang et al (57) | Lentivirus | CBP | Rat carotid injury arteries | Lentivirus-mediated CBP silencing may represent a novel therapeutic approach for the prevention of restenosis after vascular interventions in rats |
| Bonta et al (76) | Lentivirus | Nurr1 | Rat carotid injury arteries | Lentivirus-mediated nuclear receptor Nurr1 is an attractive novel target for (local) intervention in restenosis in rats |
AAV Adeno-associated virus; CBP CREB-binding protein; COX Cyclooxygenase; iNOS Inducible nitric oxide synthase; Nurr1 Nuclear receptor-related 1; PKG Protein kinase G; TIMP Tissue inhibitor of metalloproteinase; VEGF Vascular endothelial growth factor; VSMC Vascular smooth muscle cell
Table 2: Summary of major studies using viral vector for antirestenosis gene therapy
The metabolic syndrome is defined as a cluster of numerous cardiovascular risk factors that encompass dyslipidemia and hypertension. Patients with the metabolic syndrome are more prone to developing ISR [35]. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a membrane protein that can bind and internalize oxidized low-density lipoprotein and advanced glycation end products, which further promote endothelial injury after percutaneous coronary interventions (PCI). Targeting the LOX-1 gene with gene silencer pyrrole-imidazole polyamide significantly decreased the expression of LOX-1 and promoted re-endothelialization, thus inhibiting neointimal hyperplasia after arterial injury [24]. Gene transfer of PGI2 synthase has also been shown to accelerate re-endothelialization and prevent neointimal formation in balloon-injured arteries. In a rabbit model, PGI2 synthase gene delivery by the lipotransfection method via dispatch catheter can accelerate re-endothelialization and attenuate neointimal formation [36]. Douglas et al [20] found that endothelial-specific overexpression of GTP-cyclohydrolase-1 can enhance endothelial cell function and increases NO production, thus promoting re-endothelialization after stent deployment.
Antineointimal hyperplasia
Following injury of coronary arteries during interventional procedures, including balloon angioplasty and/or stent placement, medial SMCs change their phenotype from contractile to synthetic, proliferative and migrative in nature, initiating the process of neointimal hyperplasia [37]. Therefore, proliferation and migration of SMCs are crucial targets for the inhibition of intimal hyperplasia and restenosis. A number of studies have shown that transfer of cytotoxic antiproliferative genes, including thymidine kinase, cytosine deaminase and Fas ligand, which selectively destroy SMCs as they enter the S phase of the cell cycle, are able to significantly inhibit neointimal hyperplasia in balloon-injured vessels [38-40]. In addition, the transfection of antisense oligodeoxynucleotides (ODNs) targeting DNA binding proteins and transcription factors – including CDC2 and cyclin G1, which encode for regulatory proteins that are involved in the cell cycle – have also been shown to inhibit neointimal formation [41,42]. MicroRNA, a 21 to 24 nucleotide RNA molecule, inhibits their messenger RNA target by binding to complementary ‘seed’ sequences, leading to post-transcriptional repression or target messenger RNA degradation. Several microRNAs have been found to significantly reduce injury-induced neointima formation by inhibiting VSMC proliferation [43,44]. Merlet et al [43] found an upregulation of miR-424/322 after vascular injury. Overexpression of miR-424/322 in injured rat carotid arteries using an adenovirus has been demonstrated to inhibit restenosis.
Apart from the antiproliferative approach, inhibition of the migration of SMCs can reduce neointimal hyperplasia. Extracellular matrix degradation has been shown to play a crucial role in SMC migration, which is mainly regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) [45]. Although early studies using marimastat, a broad-spectrum MMP inhibitor, showed significantly reduced neointimal formation in cultured human saphenous vein graft segments, the potential of marimastat as an antimetastatic agent was halted at phase III due to a lack of efficacy and dose-limiting toxicity [46]. The potency of TIMP-1 as a therapeutic gene for antineointimal hyperplasia has been verified in various animal models [47,48]. Johnson et al [48] found that stent-based delivery of TIMP-3 adenovirus inhibits neointimal formation in porcine coronary arteries [48]. Downregulation of platelet-derived growth factor-β receptor expression and upregulation of plasminogen activator inhibitor-1 and light-type caldesmon, a potent cytostatic and antiangiogenic protein, using a gene targeting and adenoviral gene transfer approach, has also been found to inhibit neointima formation [49]. Lompré et al [50] investigated the functional effects of adeno-associated virus (AAV) 2/5-mediated sarco/endoplasmic reticulum Ca2+ATPase isoform 2a (SERCA2a) – a human gene known to inhibit VSMC proliferation – in a rat model of carotid artery balloon injury and found that AAV2/5-mediated SERCA2a gene transfection significantly decreases the neointimal hyperplasia without inducing obvious inflammation in vivo.
Activation of the tyrosine kinase family JAK and subsequent activation of signal transducer and activator of transcription (STAT) proteins are key events in signal transduction of different cytokines and growth factors. These proteins regulate different biological processes including SMC proliferation [51]. Suppressor of cytokine signalling-3 (SOCS3) protein decreases the JAK/STAT signalling by blocking the JAK tyrosine kinase activity and the activation of STAT factors, suggesting that it is a novel antineointimal hyperplasia target. Recently, our laboratory has found a significant reduction in SOCS3 expression in the neointimal lesion after balloon angioplasty in coronary arteries of atherosclerotic swine [52]. In addition, our laboratory demonstrated that hypermethylation of the SOCS3 gene in SMCs could be an underlying mechanism of intimal hyperplasia and restenosis, suggesting that inhibition of SOCS3 promoter methylation is a potential anti-neointimal hyperplasia target [53].
Anti-inflammation
Inflammation is a protective attempt by the organism to remove the injurious stimuli. However, stent implantation, including BMS and polymer-coated DES implantation, induces acute and chronic inflammation that stimulates accumulation of inflammatory cells, such as macrophages and T-lymphocytes, and inflammatory cytokine secretion [8]. Inhibition of inflammatory gene expression may have the therapeutic effect of reducing neointimal hyperplasia. Transforming growth factor-beta (TGF-β) is a crucial inflammatory cytokine that controls immunity/inflammation and cell proliferation. Delivery of the gene silencer pyrrole-imidazole polyamide, which targets the TGF-β1 promoter, can suppress neointimal hyperplasia after arterial injury by downregulating TGF-β1 expression and inhibiting restenosis [25]. Monocyte chemoattractant protein-1 (MCP-1) is a proinflammatory chemokine specific for monocytes and is shown to be present at increased levels after vascular injury. The peptide from the C-terminal domain of MCP-1 (ingramon) has been shown to inhibit monocyte migration and possess anti-inflammatory activity while at the same time inhibiting the postangioplasty restenosis in animal models [54]. In rabbit and monkey models, the same laboratory found that the local delivery of the C-terminal domain of MCP-1 gene (7ND) by a GES system attenuates in-stent stenosis [55]. In cynomolgus monkeys, a catheter-based anti-MCP-1 gene delivery system has also been shown to attenuate in-stent neointima formation [55].
As an essential transcriptional factor for inflammation, nuclear factor-kappa B (NF-κB) has been found to play a pivotal role for restenosis after PCI. In an open-label phase I/fgIIa clinical trial, Egashira et al [56] found that NF-κB decoy ODN transfection can suppress the inflammatory response and prevent restenosis. CREBbinding protein is a powerful transcriptional coactivator that regulates inflammation, cell proliferation and apoptosis in vascular endothelial and SMCs. Knockdown of CREB-binding protein using small interfering RNA demonstrated a potent inhibitory effect on balloon injury-induced NF-κB acetylation and attenuated neointimal formation in balloon-injured rat carotid artery [57]. Activator protein-1 is another crucial transcription factor regulating gene expression in response to a variety of proinflammatory stimuli. Using decoy ODN transfection against the activator protein-1 binding site has recently been shown to effectively increase re-endothelialization and inhibit neointimal proliferation [21].
Antithrombosis
Stent implantation-induced thrombosis is a classical example of device-induced, platelet-mediated arterial thrombosis [58]. In a porcine model of coronary restenosis, anti-CD34 antibody-coated sirolimus-eluting stents were associated with greater endothelialization, thus inhibiting platelet activation. However, a clear clinical benefit of stents coated with this technology has not been shown [58,59]. ADP, a key agonist required to trigger platelet aggregation, has received much attention as a potential antithrombosis therapeutic target. Takemoto et al [60] found that transfection of the human pE-NTPDase gene, encoding an enzyme that rapidly hydrolyzes ATP and ADP to AMP via cationic gelatin-coated stents, inhibits subacute in-stent thrombosis and suppresses neointimal hyperplasia and inflammation without antiplatelet drugs. More established antithrombotic genes, including PGI2 synthase, COX-1 and tissue factor pathway inhibitor, have been investigated in recent years for their potential in postangioplasty intervention, and have demonstrated successful suppression of thrombosis and/or restenosis in various animal models [34,36,58,61].
Gene Delivery systems
Gene vectors
Viral vector and nonviral vector are the classic gene delivery vectors, both of which have the ability to introduce therapeutic genes into vascular tissue [6]. For greater safety and less immunogenicity, nonviral vectors have held more promise for clinical application than viral vectors. However, the complex process of migration through the vascular endothelium and the extracellular matrix makes nonviral vectors inefficient in the areas of transfection and rapid intracellular degradation [9], which hinders the application of vectors of this kind. Several forms of nonviral vectors, including naked plasmid DNA, DNAcontaining cationic liposomes, DNA-polycation complexes, DNAphosphorylcholine polymer complexes, DNA-PLGA NPs and ODN, have been investigated as potential gene vectors for antirestenosis therapy [33,62-69] (Table 1). Chemical formulations, such as poloxamers, inhibit the endogenous DNAses, thereby protecting plasmid DNA from degradation in the extracellular matrix [70]. Furthermore, dodecylated chitosan-plasmid DNA complexes formed stable, positively charged nanospheres with small vector size and increased EGFP-C1 gene expression in stents but not in adjacent arterial segments or distal organs, suggesting an interesting method for sustained and site-specific nonviral vector transfection [71]. In addition, novel dendrimer-based poly (L-glutamic acid) derivatives have also been found to be an efficient and biocompatible gene delivery vector to inhibit restenosis in balloon-injured rabbit carotid arteries [72]. Furthermore, recent studies have shown that small vector sizes in nanoscale-range particles (ie, NPs) have many advantages in DNA transfection including increased cellular uptake, decreased inflammatory response and increased cellular incorporation. This suggests NPs as a novel platform for nonviral gene vectors [73,74].
Compared with nonviral vectors, viral vectors have evolutionary advantages in their extra- and intracellular interactions. However, viral vectors can be subjected to a host of immune responses that not only negate the vector efficacy but also result in inflammatory responses in vivo [75]. Several forms of viral vectors, including adenovirus, AAV and lentivirus, have been applied in preclinical studies and clinical trials (Table 2) [34,43,44,50,57,76-80]. Adenoviruses are commonly used viral vectors because they have many features that make them well suited for gene therapy. As shown in Table 2, adenovirus- coated genes, including micro-RNA, TIMP-3 and VEGF, can have significant inhibitory effects on in-stent neointimal formation in multiple artery stent/injury restenosis animal models [34,43,44,77,80]. AAV vectors have emerged as a versatile vehicle for gene delivery due to their efficient infection of dividing and nondividing cells in the presence of a helper virus [50,79]. Compared with native AAVs, vascular cell-specific peptide-modified AAVs have been shown to target gene delivery to vascular tissues, such as vascular endothelial cells (ECs), in vivo [81]. Our group has successfully transfected a novel recombinant AAV-2/9 vector with SM22α promoter to the medial layer SMCs of swine coronary and peripheral arteries [82]. It warrants mention that AAV-2/9 viral transduction in our study has no significant effect on serum amylase, fibrinogen and C-reactive protein levels, suggesting that AAV-2/9 is a safe and effective gene therapeutic vector for antirestenosis [82]. Lentiviral vectors also provide a promising strategy for the treatment of cardiovascular diseases due to their durable gene transfer and their ability to govern efficiently. Several groups have verified that lentivirus-mediated gene transfection may represent a novel therapeutic approach for the prevention of restenosis after vascular interventions [57,76] (Table 2).
Vector delivery strategies
Percutaneous catheter-based delivery: Percutaneous catheter-based gene delivery, currently the most frequently used strategy for antirestenosis therapy, has several major theoretical advantages: concomitant performance with other percutaneous procedures, such as combining gene therapy with mesenchymal stem cell therapy; utilization of smaller quantities to reduce potentially harmful effects of systemic delivery [83]; and easy preparation, characterization and reproducibility. However, several major limitations have also limited the application of this strategy. First, most catheters require prolonged total occlusion of the target vessel for effective vector delivery, which may increase the risk for myocardial ischemia. Second, there is lack of efficiency in site-specific gene delivery, although current vector delivery systems have focused on modified balloon catheters that can trap a fluid within a short segment of vasculature [84]. Finally, the delivered viral or nonviral vectors inevitably disperse from side branches of the coronary vasculature, which carries the potential risk for distal spread.
Another promising method of transluminal gene delivery involves using vascular tissue-specific targeted systemic treatments. White et al [81] isolated human venous EC-targeting peptides and genetically incorporated them into AAV capsids. Intravenous infusion of engineered AAVs into mice caused reduced vector accumulation in the liver while enhancing uptake of virions in the vena cava [81]. Deglau et al [85] investigated a site-specific delivery system. First, they injured rabbit femoral arteries using biotin molecular-loaded balloons, then intravenously administered avidin-coated microspheres, which has a high affinity for biotin. They found that these microspheres attached to the biotin on the arterial wall, suggesting the potential to locally deliver a systemically injected antirestenotic agent [85]. In addition, genetic engineering of human stem cells with VEGF holds great potential as a platform for cellbased therapy to promote vascularization and tissue regeneration [86]. Nonviral, biodegradable polymeric NPs were recently developed to deliver genes to human MSCs [87], and their surface can be easily manipulated with the addition of special ligands. This may be responsible for enhancing vascular injury site-specific NP permeability, suggesting that stem cell-based delivery of gene-loaded NPs offers an interesting option to overcome such deficiency in transluminal gene delivery.
Stent-based delivery: As with DES, GES use stents as permanent scaffolds to deliver biologically active agents locally for a prolonged duration to the site of vascular disease [65]. Dichek et al [88] seeded stents with genetically engineered endothelial cells using retroviralmediated gene transfer – the first progress in the field of GES therapy to prevent ISR. In a pig coronary angioplasty model, Klugherz et al [89] reported the first successful transfection in vivo using a DNA controlled-release stent and a collagen-coated stent with monoclonal antibodies covalently bonded to adenovirus [90]. Much later, Walter et al [65] improved the efficiency of local delivery of naked plasmid DNA encoding for human VEGF-2 via GES in hypercholesterolemic rabbits. Swanson et al [91] also observed the effect of VEGF-coated stents on restenosis in hypercholesterolemic rabbits. Currently, stent backbones designed for gene loading include biodegradable, bioabsorbable, nanoporous metal stents coated with an outer layer of polymer, which may be bioabsorbable or nonbioabsorbable material, and can be loaded with therapeutic genes, thus providing more controlled and sustained gene delivery and allowing for more optimal gene-tissue interactions [26]. Non-biodegradable polymers (BPs) are currently the most frequently used method of coating the stents. However, considering that stents, similar to any other foreign material inside the human body, can cause infection and inflammation, BPs including poly-L-lactide, polyvinyl pyrrolidone, poly-lactide-cocaprolactone and PLGA, offer greater advantages. Bioresorbable stents, often composed of polylactides such as poly-L-lactide or poly-lactidecocaprolactone, are typically completely metabolized in approximately 12 to 18 months [92]. In a porcine model, Lincoff et al [26] have found that stents composed of high-molecular-weight poly-Llactide produced minimal inflammation and durable results compared with uncoated stents. Although BP-DES have yet to receive approval in the USA, they are widely used worldwide, including in Asia and Europe. In a five-year follow-up study, Kuramitsu et al [93] investigated the long-term coronary arterial response to BP-biolimus-eluting stents (BES) compared with durable polymer sirolimus-eluting stents and BMS, and found that BP-BES shows a favourable coronary arterial response compared with sirolimus-eluting stents [93]. However, in a network meta-analysis that included 113 trials involving 90,584 patients performed to compare the safety and efficacy of BPs, DES, BMS and durable-polymer DES in patients undergoing coronary revascularization [94], BP-BES was found to be associated with a higher risk for definite or probable ST compared with cobalt-chromium everolimus-eluting stents. These findings suggest the benefits of biodegradable polymer coating over a nonbiodegradable one such as durable polymer [26]. However, additional studies are warranted to confirm these conclusions.
Compared with a catheter-based delivery strategy, GES represents a more appealing method for gene delivery to atherosclerotic coronary vessels. However, there are a number of technical and biological challenges with a stent-based delivery strategy. First, the limited surface area of the stent does not always guarantee the abundant loading of vectors, which is necessary for sustained transgene expression. Second, there are difficulties with regard to preservation of the mechanical properties of stents after coating the material. Third, the interaction between the biological coating material and the tissue limits the cellular uptake and intracellular stability of the gene construct, as well as the efficient transcription and translation of the encoded protein. In addition, larger-sized particles loaded in GES may be taken up by macrophages rather than the target SMCs or ECs [6].
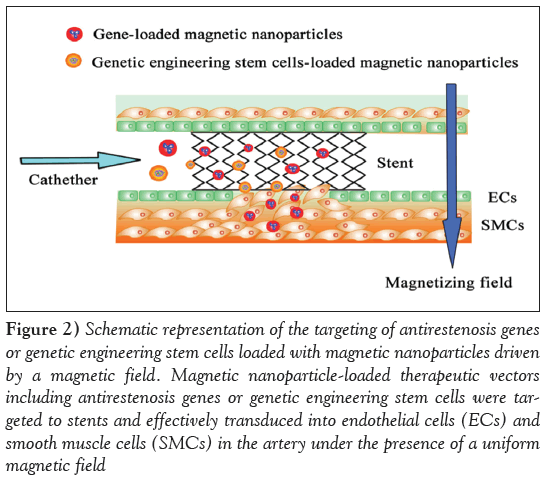
Magnetically guided delivery: Magnetic guidance is a physical targeting strategy with the potential to improve the safety and efficacy of a variety of therapeutic vectors [95]. Magnetically guided delivery systems loaded with drug and cells to stented blood vessels has recently been proposed as a potential therapeutic strategy for in-stent restenosis [96,97]. Magnetically targeted delivery systems are essentially composed of two primary components: magnetic particle-loaded therapeutic vectors and a magnetic field source generating a force attracting the vectors (Figure 2). Polyak et al [97] were the first to demonstrate high-magnetic-field gradient targeting of magnetic NP (MNP)-loaded endothelial cells to the surfaces of steel stents. At first, the investigators preloaded bovine aortic endothelial cells with biodegradable polymeric MNPs, thereby rendering the cells magnetically responsive. Then, the cells were injected via a catheter to the site advanced beyond the stent to the aortic arch. Under an external homogeneous magnetic field, the MNP-loaded bovine aortic endothelial cells were successfully targeted to the stent wires [97]. Recently, Chorny et al [77] reported a novel site-specific gene delivery to stented arteries using magnetically guided delivery. In their study, zinc oleate-based MNPs were loaded with replication-deficient adenovirus, which is limited in its clinical applicability by rapid inactivation. Under magnetic conditions, adenovirus-loaded MNPs were effectively transduced into cultured ECs and SMCs. In addition, localized arterial gene expression in the magnetically guided MNP delivery group were significantly stronger than in control groups in a rat stent model, confirming the feasibility of using adenovirus-loaded MNPs to achieve site-specific transduction in stented blood vessels [77]. Considering that recombinant vectors, whether viral or nonviral, must migrate through various biological barriers in one or more compartments to subsequently transfect target cells such as SMCs, an MNPs-based delivery system under a magnetic field may be seen as an exciting alternative for improving targeted antiproliferation gene release under catheter-based or stent-based delivery strategies (Figure 2).
Figure 2: Schematic representation of the targeting of antirestenosis genes or genetic engineering stem cells loaded with magnetic nanoparticles driven by a magnetic field. Magnetic nanoparticle-loaded therapeutic vectors including antirestenosis genes or genetic engineering stem cells were targeted to stents and effectively transduced into endothelial cells (ECs) and smooth muscle cells (SMCs) in the artery under the presence of a uniform magnetic field
Conclusion
The design of new antineointimal hyperplasia and re-endothelialization systems for the treatment of ISR was the major purpose of developing next-generation PCI technologies, which more safely and effectively prevent the progress of atherosclerosis. Vascular gene therapy offers the potential to overcome the limitations of pharmacological interventions such as the long-term use of dual antiplatelet therapy and the side effects of antiproliferation drugs loaded in DES. Many candidate genes have the potential to prevent restenosis in various artery stent/injury animal models. However, to date, few clinical studies have demonstrated similar success rates, which may be related to the species-specific differences between human and animal models of the cardiovascular system and host-immune response. Swine coronary restenosis highly resembles restenosis in humans; thus, the swine is widely regarded as an accurate model for mimicking the proliferative component of human restenosis [98]. There is a growing understanding that the physiological barriers to efficient, targeted gene transfer by gene vectors must be taken into consideration during the design of optimized therapeutic strategies. At present, viral vectors, including AAV and lentivirus, have the most potential to maximize transduction efficiency, although the toxicity, inflammation and interaction with tissue components remains to be fully evaluated. A GES-based delivery strategy is superior with regard to site-specific and sustained transgene expression. However, the stent design and material technology must be improved. The novel MNP formulation designed for magnetically guided adenoviral gene transfer to cells of the blood vessel wall addresses several prerequisites for a safe and effective gene transfection, especially targeting antiproliferation genes to SMCs. Genetic engineering of human mesenchymal stem cells using biodegradable polymeric NPs has recently been shown to enhance angiogenesis, offering an interesting option for enhancing endothelialization. These results suggest that gene-loaded NP systems and mesenchymal stem cells may be a next step for antirestenosis therapy, although the exact effect remains to be elucidated in preclinical studies and clinical trials.
Acknowledgement
This work was supported by the NIHNHLBI research grants R01HL104516, R01HL112597 and R01HL120659 to DK Agrawal. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have no financial disclosures or conflicts of interest to declare.
References
- Farooq V, Gogas BD, Serruys PW. Restenosis: Delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv 2011;4:195-205.
- Garg S, Serruys PW. Coronary stents: Current status. J Am Coll Cardiol 2010;56:S1-42.
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: Modulation by nutrients and inflammation. J Clin Invest 2008;118:2992-3002.
- Deng DX, Spin JM, Tsalenko A, et al. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: Distinct responses to stimuli. Arterioscler Thromb Vasc Biol 2006;26:1058-65.
- Goel SA, Guo LW, Liu B, Kent KC: Mechanisms of post-intervention arterial remodelling. Cardiovasc Res 2012;96:363-71.
- Goh D, Tan A, Farhatnia Y, Rajadas J, Alavijeh MS, Seifalian AM. Nanotechnology-based gene-eluting stents. Mol Pharm 2013;10:1279-98.
- Brodie B, Pokharel Y, Garg A, et al. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2012;5:1043-51.
- Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv 2010;3:174-83.
- Robertson KE, McDonald RA, Oldroyd KG, Nicklin SA, Baker AH. Prevention of coronary in-stent restenosis and vein graft failure: Does vascular gene therapy have a role? Pharmacol Ther 2012;136:23-34.
- Holmes DR Jr, Vlietstra RE, Smith HC, et al. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): A report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol 1984;53:77C-81C.
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701-6.
- Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med 1991;324:13-7.
- van Domburg RT, Foley DP, de Jaegere PP, et al. Long term outcome after coronary stent implantation: A 10 year single centre experience of 1000 patients. Heart 1999;(82 Suppl 2):II27-34.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: A brief review. Can J Cardiol 2014;30:35-45.
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23.
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221-31.
- Suzuki S, Ishii H, Matsudaira K, et al. Long-term outcome of drug-eluting vs. bare-metal stents in patients with acute myocardial infarction. Subgroup analysis of the nagoya acute myocardial infarction study (NAMIS). Circ J 2013;77:2024-31.
- Deuse T, Hua X, Wang D, et al. Dichloroacetate prevents restenosis in preclinical animal models of vessel injury. Nature 2014;509:641-4.
- Van Belle E, Bauters C, Asahara T, Isner JM: Endothelial regrowth after arterial injury: From vascular repair to therapeutics. Cardiovasc Res 1998;38:54-68.
- Douglas G, Van Kampen E, Hale AB, et al. Endothelial cell repopulation after stenting determines in-stent neointima formation: Effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur Heart J 2013;34:3378-88.
- Lin K, Zhao Z, Chen L. Stent-based delivery of decoy oligodeoxynucleotides against activator protein-1 binding site decreased restenosis in rabbits. Pharmazie 2013;68:661-7.
- Sharif F, Hynes SO, McCullagh KJ, et al. Gene-eluting stents: Non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther 2012;19:321-8.
- Yang J, Zeng Y, Zhang C, et al. The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent. Biomaterials 2013;34:1635-43.
- Yao EH, Fukuda N, Ueno T, et al. Novel gene silencer pyrrole-imidazole polyamide targeting lectin-like oxidized low-density lipoprotein receptor-1 attenuates restenosis of the artery after injury. Hypertension 2008;52:86-92.
- Yao EH, Fukuda N, Ueno T, et al. A pyrrole-imidazole polyamide targeting transforming growth factor-beta1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc Res 2009;81:797-804.
- Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 2014;4:175-200.
- Rutanen J, Turunen AM, Teittinen M, et al. Gene transfer using the mature form of VEGF-D reduces neointimal thickening through nitric oxide-dependent mechanism. Gene Ther 2005;12:980-7.
- Hiltunen MO, Laitinen M, Turunen MP, et al. Intravascular adenovirus-mediated VEGF-C gene transfer reduces neointima formation in balloon-denuded rabbit aorta. Circulation 2000;102:2262-8.
- Asahara T, Chen D, Tsurumi Y, et al. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation 1996;94:3291-302.
- Waugh JM, Kattash M, Li J, et al. Gene therapy to promote thromboresistance: Local overexpression of tissue plasminogen activator to prevent arterial thrombosis in an in vivo rabbit model. Proc Natl Acad Sci U S A 1999;96:1065-70.
- Sharif F, Hynes SO, Cooney R, et al. Gene-eluting stents: Adenovirus-mediated delivery of eNOS to the blood vessel wall accelerates re-endothelialization and inhibits restenosis. Mol Ther 2008;16:1674-80.
- Muhs A, Heublein B, Schletter J, et al. Preclinical evaluation of inducible nitric oxide synthase lipoplex gene therapy for inhibition of stent-induced vascular neointimal lesion formation. Hum Gene Ther 2003;14:375-83.
- Brito LA, Chandrasekhar S, Little SR, Amiji MM. Non-viral eNOS gene delivery and transfection with stents for the treatment of restenosis. Biomed Eng Online 2010;9:56.
- Liu Q, Chen ZQ, Bobustuc GC, et al. Local gene transduction of cyclooxygenase-1 increases blood flow in injured atherosclerotic rabbit arteries. Circulation 2005;111:1833-40.
- Goyal SN, Bharti S, Krishnamurthy B, Agrawal Y, Ojha SK, Arya DS. Impact of metabolic syndrome on re-stenosis development: Role of drug-eluting stents. Diab Vasc Dis Res 2012;9:177-88.
- Numaguchi Y, Okumura K, Harada M, et al. Catheter-based prostacyclin synthase gene transfer prevents in-stent restenosis in rabbit atheromatous arteries. Cardiovasc Res 2004;61:177-85.
- Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: An underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol 2011;31:2391-6.
- Guzman RJ, Hirschowitz EA, Brody SL, Crystal RG, Epstein SE, Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A 1994;91:10732-6.
- Ohno T, Gordon D, San H, et al. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science 1994;265:781-4.
- Simari RD, San H, Rekhter M, et al. Regulation of cellular proliferation and intimal formation following balloon injury in atherosclerotic rabbit arteries. J Clin Invest 1996;98:225-35.
- Morishita R, Gibbons GH, Ellison KE, et al. Single intraluminal delivery of antisense cdc2 kinase and proliferating-cell nuclear antigen oligonucleotides results in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci U S A 1993;90:8474-8.
- Zhu NL, Wu L, Liu PX, et al. Downregulation of cyclin G1 expression by retrovirus-mediated antisense gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation. Circulation 1997;96:628-35.
- Merlet E, Atassi F, Motiani RK, et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res 2013;98:458-68.
- Li P, Zhu N, Yi B, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res 2013;113:1117-27.
- Osherov AB, Gotha L, Cheema AN, Qiang B, Strauss BH. Proteins mediating collagen biosynthesis and accumulation in arterial repair: Novel targets for anti-restenosis therapy. Cardiovasc Res 2011;91:16-26.
- Sparano JA, Bernardo P, Stephenson P, et al. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J Clin Oncol 2004;22:4683-90.
- Forough R, Koyama N, Hasenstab D, et al. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res 1996;79:812-20.
- Johnson TW, Wu YX, Herdeg C, et al. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol 2005;25:754-9.
- Yokouchi K, Numaguchi Y, Kubota R, et al. l-Caldesmon regulates proliferation and migration of vascular smooth muscle cells and inhibits neointimal formation after angioplasty. Arterioscler Thromb Vasc Biol 2006;26:2231-7.
- Lompré AM, Hadri L, Merlet E, et al. Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: A new perspective for in-stent restenosis gene therapy. Gene Ther 2013;20:901-12.
- Madamanchi NR, Moon SK, et al. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol 2005;25:950-6.
- Gupta GK, Dhar K, Del Core MG, Hunter WJ III, Hatzoudis GI, Agrawal DK. Suppressor of cytokine signaling-3 and intimal hyperplasia in porcine coronary arteries following coronary intervention. Exp Mol Pathol 2011;91:346-52.
- Dhar K, Rakesh K, Pankajakshan D, Agrawal DK. SOCS3 promotor hypermethylation and STAT3-NF-kappaB interaction downregulate SOCS3 expression in human coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 2013;304:H776-85.
- Arefieva TI, Krasnikova TL, Potekhina AV, et al. Synthetic peptide fragment (65-76) of monocyte chemotactic protein-1 (MCP-1) inhibits MCP-1 binding to heparin and possesses anti-inflammatory activity in stable angina patients after coronary stenting. Inflamm Res 2011;60:955-64.
- Egashira K, Nakano K, Ohtani K, et al. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol 2007;27:2563-8.
- Egashira K, Suzuki J, Ito H, Aoki M, Isobe M, Morishita R. Long-term follow up of initial clinical cases with NF-kappaB decoy oligodeoxynucleotide transfection at the site of coronary stenting. J Gene Med 2008;10:805-9.
- Yang J, Jiang H, Chen SS, et al. Lentivirus-mediated RNAi targeting CREB binding protein attenuates neointimal formation and promotes re-endothelialization in balloon injured rat carotid artery. Cell Physiol Biochem 2010;26:441-8.
- Granada JF, Price MJ, French PA, et al. Platelet-mediated thrombosis and drug-eluting stents. Circ Cardiovasc Interv 2011;4:629-37.
- Nakazawa G, Granada JF, Alviar CL, et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv 2010;3:68-75.
- Takemoto Y, Kawata H, Soeda T, et al. Human placental ectonucleoside triphosphate diphosphohydrolase gene transfer via gelatin-coated stents prevents in-stent thrombosis. Arterioscler Thromb Vasc Biol 2009;29:857-62.
- Yin X, Yutani C, Ikeda Y, et al. Tissue factor pathway inhibitor gene delivery using HVJ-AVE liposomes markedly reduces restenosis in atherosclerotic arteries. Cardiovasc Res 2002;56:454-63.
- Zhang LH, Luo T, Zhang C, et al. Anti-DNA antibody modified coronary stent for plasmid gene delivery: Results obtained from a porcine coronary stent model. J Gene Med 2011;13:37-45.
- Yang J, Zeng Y, Li Y, et al. Intravascular site-specific delivery of a therapeutic antisense for the inhibition of restenosis. Eur J Pharm Sci 2008;35:427-34.
- Fuchs AT, Kuehnl A, Pelisek J, et al. Inhibition of restenosis formation without compromising reendothelialization as a potential solution to thrombosis following angioplasty? Endothelium 2008;15:85-92.
- Walter DH, Cejna M, Diaz-Sandoval L, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: An alternative strategy for inhibition of restenosis. Circulation 2004;110:36-45.
- Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: Phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 2003;107:2677-83.
- Taniyama Y, Tachibana K, Hiraoka K, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 2002;105:1233-9.
- Nikol S, Huehns TY, Krausz E, et al. Needle injection catheter delivery of the gene for an antibacterial agent inhibits neointimal formation. Gene Ther 1999;6:737-48.
- Van Belle E, Tio FO, Chen D, Maillard L, Kearney M, Isner JM. Passivation of metallic stents after arterial gene transfer of phVEGF165 inhibits thrombus formation and intimal thickening. J Am Coll Cardiol 1997;29:1371-9.
- Escoffre JM, Teissie J, Rols MP. Gene transfer: How can the biological barriers be overcome? J Membr Biol 2010;236:61-74.
- Zhu D, Jin X, Leng X, et al. Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int J Nanomedicine 2010;5:1095-102.
- Zeng X, Pan S, Li J, et al. A novel dendrimer based on poly (L-glutamic acid) derivatives as an efficient and biocompatible gene delivery vector. Nanotechnology 2011;22:375102.
- Yang D, Hartman MR, Derrien TL, et al. DNA materials. Bridging nanotechnology and biotechnology. Acc Chem Res 2014;47:1902-11.
- Alqawlaq S, Sivak JM, Huzil JT, et al. Preclinical development and ocular biodistribution of gemini-DNA nanoparticles after intravitreal and topical administration: Towards non-invasive glaucoma gene therapy. Nanomedicine 2014 [Epub ahead of print]
- Eckhouse SR, Jones JA, Spinale FG. Gene targeting in ischemic heart disease and failure: Translational and clinical studies. Biochem Pharmacol 2013;85:1-11.
- Bonta PI, Pols TW, van Tiel CM, et al. Nuclear receptor Nurr1 is expressed in and is associated with human restenosis and inhibits vascular lesion formation in mice involving inhibition of smooth muscle cell proliferation and inflammation. Circulation 2010;121:2023-32.
- Chorny M, Fishbein I, Tengood JE, Adamo RF, Alferiev IS, Levy RJ. Site-specific gene delivery to stented arteries using magnetically guided zinc oleate-based nanoparticles loaded with adenoviral vectors. FASEB J 2013;27:2198-206.
- Ramirez Correa GA, Zacchigna S, Arsic N, et al. Potent inhibition of arterial intimal hyperplasia by TIMP1 gene transfer using AAV vectors. Mol Ther 2004;9:876-84.
- Sharif F, Hynes SO, McMahon J, et al. Gene-eluting stents: Comparison of adenoviral and adeno- associated viral gene delivery to the blood vessel wall in vivo. Hum Gene Ther 2006;17:741-50.
- Wu YJ, Sala-Newby GB, Shu KT, et al. S-phase kinase-associated protein-2 (Skp2) promotes vascular smooth muscle cell proliferation and neointima formation in vivo. J Vasc Surg 2009;50:1135-42.
- White SJ, Nicklin SA, Buning H, et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 2004;109:513-9.
- Pankajakshan D, Makinde TO, Gaurav R, et al. Successful transfection of genes using AAV-2/9 vector in swine coronary and peripheral arteries. J Surg Res 2012;175:169-75.
- Opie SR, Dib N. Local endovascular delivery, gene therapy, and cell transplantation for peripheral arterial disease. J Endovasc Ther 2004;(11 Suppl 2):II151-62.
- Seedial SM, Ghosh S, Saunders RS. Local drug delivery to prevent restenosis. J Vasc Surg 2013;57:1403-14.
- Deglau TE, Maul TM, Villanueva FS, Wagner WR. In vivo PEG modification of vascular surfaces for targeted delivery. J Vasc Surg 2012;55:1087-95.
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One 2007;2:e156.
- Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A 2010;107:3317-22.
- Dichek DA, Neville RF, Zwiebel JA, Freeman SM, Leon MB, Anderson WF. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation 1989;80:1347-53.
- Klugherz BD, Jones PL, Cui X, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol 2000;18:1181-4.
- Klugherz BD, Song C, DeFelice S, et al. Gene delivery to pig coronary arteries from stents carrying antibody-tethered adenovirus. Hum Gene Ther 2002;13:443-54.
- Swanson N, Hogrefe K, Javed Q, Malik N, Gershlick AH. Vascular endothelial growth factor (VEGF)-eluting stents: In vivo effects on thrombosis, endothelialization and intimal hyperplasia. J Invasive Cardiol 2003;15:688-92.
- Lincoff AM, Furst JG, Ellis SG, Tuch RJ, Topol EJ. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol 1997;29:808-16.
- Kuramitsu S, Sonoda S, Yokoi H, et al. Long-term coronary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: Five-year follow-up optical coherence tomography study. Atherosclerosis 2014;237:23-9.
- Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: A systematic review and Bayesian approach network meta-analysis. Eur Heart J 2014;35:1147-58.
- Chorny M, Fishbein I, Adamo RF, Forbes SP, Folchman-Wagner Z, Alferiev IS. Magnetically targeted delivery of therapeutic agents to injured blood vessels for prevention of in-stent restenosis. Methodist Debakey Cardiovasc J 2012;8:23-7.
- Chorny M, Fishbein I, Yellen BB, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A 2010;107:8346-51.
- Polyak B, Fishbein I, Chorny M, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci U S A 2008;105:698-703.
- Gupta GK, Agrawal T, Del Core MG, Hunter WJ III, Agrawal DK. Decreased expression of vitamin D receptors in neointimal lesions following coronary artery angioplasty in atherosclerotic swine. PLoS One 2012;7:e42789.
- Chang H, Ren KF, Zhang H, Wang JL, Wang BL, Ji J. The (PrS/HGF-pDNA) multilayer films for gene-eluting stent coating: Gene-protecting, anticoagulation, antibacterial properties, and in vivo antirestenosis evaluation. J Biomed Mater Res B Appl Biomater 2014 [Epub ahead of print].
- Deiner C, Schwimmbeck PL, Koehler IS, et al. Adventitial VEGF165 gene transfer prevents lumen loss through induction of positive arterial remodeling after PTCA in porcine coronary arteries. Atherosclerosis 2006;189:123-32.
- Matsushita H, Morishita R, Aoki M, et al. Transfection of antisense p53 tumor suppressor gene oligodeoxynucleotides into rat carotid artery results in abnormal growth of vascular smooth muscle cells. Circulation 2000;101:1447-52.
- Chapman GD, Lim CS, Gammon RS, et al. Gene transfer into coronary arteries of intact animals with a percutaneous balloon catheter. Circ Res 1992;71:27-33.
- Fishbein I, Alferiev I, Bakay M, et al. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation 2008;117:2096-103.
- Sinnaeve P, Chiche JD, Gillijns H, et al. Overexpression of a constitutively active protein kinase G mutant reduces neointima formation and in-stent restenosis. Circulation 2002;105:2911-6.
- Squadrito F, Deodato B, Bova A, et al. Crucial role of nuclear factor-kappaB in neointimal hyperplasia of the mouse carotid artery after interruption of blood flow. Atherosclerosis 2003;166:233-42.
- *Corresponding Author:
- Dr Devendra K Agrawal
Center for Clinical & Translational Science, Creighton University School of Medicine, CRISS II Room 510, 2500 California Plaza, Omaha, Nebraska 68178-0405, USA.
Tel:402-280-2938
Fax:402-280-1421
E-mail:dkagr@creighton.edu
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
the advent of the drug-eluting stent, the incidence of in-stent restenosis remains unacceptably high. Gene therapeutic strategies, including catheter-based gene delivery and gene-eluting stents, offer novel treatment methods to promote re-endothelialization, and inhibit inflammation, neointimal hyperplasia and late stent thrombosis. The translation of gene therapy into clinical application must be safe and requires an effective, site-specific delivery system as well as the ability to provide sustained transgene expression. The progress of magnetic nanotechnology and genetic engineering of human stem cells can provide such elements. In the present review, the authors discuss the evolution of antirestenosis therapy, underlying mechanisms of restenosis and the applications of gene therapy to prevent in-stent restenosis. Current gene delivery methods, including gene vectors and delivery strategies, are critically reviewed.
-Keywords
Gene therapy; Intimal hyperplasia; Nanotechnology; Re-endothelialization; Restenosis; Stem cell
Despite the increased use of drug-eluting stents (DES), the incidence of in-stent restenosis (ISR) requiring target vessel revascularization remains unacceptably high [1,2]. The incidence rate of ISR requiring target vessel revascularization (ie, ‘DES failure’) in the United States alone was conservatively estimated to be 10%, and may be more common in patients with diabetes mellitus [3]. Although the pathophysiology of DES failure is complex, histopathological changes of in-stent neointima following directional atherectomy at the time of reintervention have been shown to be remarkably similar between bare-metal stents (BMS) and DES, suggesting that a process mediated by the endothelial injury, inflammation, proliferation and migration of vascular smooth muscle cells (VSMCs), and thrombosis plays the same important role in the progress of ISR of DES [4,5]. In addition, antiproliferative drugs in DES, primarily aimed at preventing VSMC proliferation (which is central to the pathogenesis of ISR), also perturb endothelial recovery [6], which consequently increases the risk for subacute in-stent thrombosis and results in late stent thrombosis (ST) [7]. Furthermore, polymer coatings on DES induce a distinct inflammatory reaction compared with bare metal surfaces [8]. These results raise serious questions regarding the long-term durability and efficacy of DES, thereby resulting in an urgent need to develop new therapies to prevent restenosis. The present review focuses on the progress made to date in the use of gene therapy, including catheter-based gene delivery and gene-eluting stents (GES), to prevent ISR. We will also discuss the current and promising application of magnetic targeting gene delivery systems for antirestenosis therapy.
Evolution of antirestenosis therapy
In the past several decades, percutaneous transluminal coronary angioplasties have revolutionized the treatment of atherosclerosis-related cardiovascular disease. At the same time, tremendous efforts have also been made to reduce the incidence of restenosis after percutaneous transluminal coronary angioplasties [9]. During the time in which ‘plain old balloon angioplasty’ was prevalent, rates of acute and chronic vessel occlusion were higher (30% to 56%), secondary to acute/chronic recoil and constrictive remodelling [10]. These problems led to the development of a second revolutionary treatment, BMS, which were first implanted in 1986 [11]. Although this ‘bailout’ scaffold significantly reduced the issue of acute and chronic recoil, its application was ultimately hampered by the risk for subacute thrombotic coronary artery occlusion and neointimal hyperplasia [12]. The restenosis rates with BMS were reported to be between 16% and 44%, with higher rates of stenosis attributable to several risk factors, particularly long lesion length and small vessel calibre [1,13].
Considerable progress to reduce the incidence of restenosis was made by the advent of DES, which are composed of a metallic stent, a polymer-based drug delivery platform and a pharmacological agent (typically an immunosuppressant and/or antiproliferative compounds, including sirolimus and paclitaxel [PTX]) [14]. Several classic studies unequivocally demonstrated that DES markedly reduced rates of ISR to 5% to 8% [15,16]. However, long-term follow-up studies have raised concerns about the long-term safety of DES, although DES definitely improves short- to midterm ISR [17]. The application of DES comes with an increased risk for ST because the pathophysiology of ISR and ST appear to be diametrically opposed. Briefly, ISR is related to an overzealous response of the vessel to injury, whereas ST occurs due to impaired or delayed endothelium healing, resulting in acute and subacute thrombotic events [14]. There is an urgent need, therefore, to develop novel and efficacious methods to deal with the problems observed in current antirestenosis therapy (Figure 1). Recently, Deuse et al [18] reported the identification of pyruvate dehydrogenase kinase isoform 2 as a key regulatory protein in the development of myointimal hyperplasia in human arteries for its regulated effect on hyperpolarization of mitochondrial membrane potential and resistance to apoptosis in smooth muscle cells (SMCs) during ISR. Normally, proliferation and apoptosis are very low and balanced in healthy vessels. However, vessel injury induces inflammatory response and temporary mitochondrial membrane potential hyperpolarization, which results in the temporarily acquired apoptosis resistance in SMCs and promotes myointima formation. Dichloroacetate, a pharmacological pyruvate dehydrogenase kinase isoform 2 blockade, counteracts temporarily acquired apoptosis resistance and reduces myointima formation in injured coronary arteries of human and various animal models [18]. Most importantly, dichloroacetate did not affect vessel re-endothelialization, suggesting a novel strategy for the prevention of proliferative vascular diseases, especially ISR. Moreover, magnetic nanotechnology and genetic engineering of human stem cells have also emerged as exciting alternatives to eliminate the underlying mechanisms of ISR, suggesting their possible roles as the next generation of antirestenosis therapy.
Figure 1: Schematic representation of the evolution of antirestenosis therapy and potential therapeutic strategies for antirestenosis.
Therapeutic Gene targets
re-endothelialization
Stent implantation for coronary artery disease causes a marked vascular injury, inducing de-endothelialization, which is associated with thrombus formation and abnormal responses to endothelium-dependent agonists [19]. In recent years, several studies have focused on the use of vascular gene therapy targeting multiple pathways to promote reendothelialization [20-26]. The potential of vascular endothelial growth factors (VEGFs), a group of potent endothelial mitogens, to treat postangioplasty restenosis was first investigated in animal models. Several VEGF family members, including VEGF165, VEGF-2, VEGF-C and VEGF-D, have been found to inhibit neointimal thickening, reduce thrombogenicity and restore the endothelium-dependent vasomotor reactivity [27-29]. Yang et al [23] developed a stent coated with bilayered poly-lactide-coglycolide (PLGA) nanoparticles (NPs) containing VEGF plasmid in the outer layer and PTX in the inner core and found that it significantly promotes early endothelium healing while inhibiting SMC proliferation through sequential release of the VEGF gene and PTX [23].
Another interesting target is nitric oxide (NO), which is mainly synthesized by NO synthase (NOS). Most studies have focused on two main types of NOS, inducible NOS and endothelial NOS (eNOS), to reduce neointimal hyperplasia and accelerate re-endothelialization [30- 33]. Both catheter- and stent-based delivery techniques have verified eNOS and inducible NOS as potential therapeutic genes for accelerating re-endothelialization and reducing neointimal hyperplasia (Tables 1 and 2) [31-33]. Sharif et al [31] found that eNOS gene delivery can result in significantly enhanced re-endothelialization and decreased neointimal formation using an adenovirus GES. The same laboratory reported that nonviral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization as well as prolonged and localized eNOS expression in the blood vessel wall in a hypercholesterolemic rabbit model [22]. Cyclooxygenase-1 (COX-1) is the rate-limiting component in the synthesis of prostacyclin (PGI2), a potent vasodilator and antithrombotic molecule. In balloon-injured, atherosclerotic porcine and hypercholesterolemic rabbit models, COX-1 gene transduction induced an early increase in the production of PGI2, prostaglandin E2 and prostaglandin E1, resulting in long-lasting vasodilation [34].
| Author (reference) | Vector(s) | Gene(s) | Model/patients | Main outcome(s) |
|---|---|---|---|---|
| Taniyama et al (67) | Naked plasmid DNA | p53 | Rat carotid artery after balloon injury | Transgene expression of naked p53 plasmid DNA using ultrasound transfection method decreased the intimal-to-medial area ratio |
| Van Belle et al (69) | Naked plasmid DNA | VEGF 165 | Rabbit artery stent restenosis model | Transgene expression of naked VEGF165 plasmid DNA decreased mural thrombus and intimal thickening |
| Fuchs et al (64) | Naked plasmid DNA | C-type natriuretic peptide | Porcine restenosis model | Transgene expression of naked C-type natriuretic peptide plasmid DNA resulted in significant inhibition of neointimal formation without compromising endothelial repair |
| Zhang et al (62) | Naked plasmid DNA and anti-DNA antibody- immobilized stent | iNOS | Porcine coronary stent model | Tethering of pcDNA3.1-iNOS on the stent showed long-term therapeutic effects for antirestenosis |
| Chang et al (99) | Protamine sulfate plasmid DNA | Hepatocyte growth factor | Rat carotid artery injury model | The protamine sulfate/hepatocyte growth factor-pDNA multilayer coated stent can reduce in-stent restenosis in vivo |
| Brito et al (33) | Liposomes plasmid DNA | eNOS | Rabbit artery stent restenosis model | Efficient transfection of eNOS locally in the arterial lumen suppressed SMC proliferation and promoted re-endothelialization of the artery showing a significant reduction of restenosis |
| Deiner et al (100) | Liposomes plasmid DNA | VEGF-165 | Balloon-injured porcine coronary arteries model | Local delivery of VEGF-165 gene into the outer compartments of balloon-injured porcine coronary arteries reduced lumen area loss due to distinct positive remodelling |
| Yang et al (63) | PLGA/NPs plasmid DNA | Antisense for MCP-1 | Rabbit carotid artery injury model | Local infusion of anti-MCP-1 NPs significantly lowered the intimal hyperplasia |
| Walter et al (65) | Phosphorylcholine polymer plasmid DNA | VEGF-2 | Rabbit artery stent restenosis model | Transgene expression of VEGF-2 in the vessel wall improved functional recovery of stented segments |
| Hedman et al (66) | Liposomes plasmid DNA | VEGF | Patients with coronary artery disease | Transgene expression of VEGF cannot improve the ISR in human after the 6-month follow-up |
| Egashira et al (56) | ODN | Antisense NF-κB ODN | Patients with coronary artery disease | NF-κB ODN Transfection of NF-κB decoy ODN can obviously suppress the ISR in human |
| Matsushita et al (101) | ODN | Antisense p53 ODN | Rat intact carotid artery model | Loss of p53 by antisense p53 ODN resulted in an abnormal VSMC growth in vitro and in vivo |
| Nikol et al (68) | Liposomes plasmid DNA | Cecropin A | Porcine arterial injury model | Transfection of cecropins A prevented restenosis by limiting SMC proliferation In vivo |
| Chapman et al (102) | Liposomes plasmid DNA | Luciferase | Mongrel dogs model | The first study to show the efficiency of nonviral gene transfection using percutaneous catheter |
eNOS Endothelial nitric oxide synthase (NOS); iNOS Inducible NOS; ISR In-stent restenosis; MCP-1 Monocyte chemoattractant protein-1; NPs Nanoparticles; ODNs Oligodeoxynucleotides; PLGA Poly-lactide-coglycolide; SMC Smooth muscle cell; VEGF Vascular endothelial growth factor; VSCM Vascular smooth muscle cells
Table 1: Summary of major studies using nonviral vectors for antirestenosis gene therapy
| Author (reference) | Vector(s) | Gene(s) | Model/patients | Main outcome(s) |
|---|---|---|---|---|
| Li et al (44) | Adenovirus | miR-663 | Mice carotid artery injury model | Adeno-miR-663 markedly suppressed the neointimal lesion formation in mice |
| Merlet et al (43) | Adenovirus | miR-424/322 Magnetic nanoparticles | Rat carotid artery injury model | Adeno-miR-424/322 markedly suppressed the neointimal lesion formation in rats |
| Chorny et al (77) | Adenovirus | Rat carotid artery injury model | Magnetically targeted adenovirus-loaded magnetic nanoparticles represent a novel delivery system for efficient, vascular gene transfer in vivo | |
| Wu et al (80) | Adenovirus | Dominant-negative Skp2 | Rat carotid artery injury model | Knockdown of Skp2 inhibited VSMC proliferation, and the subsequent neointimal thickening in rat arteries |
| Fishbein et al (103) | PABT/PEI(PDT)/ HL-tethered adenoviral vectors | iNOS | Rat carotid artery stent model | Rat carotid stent delivery of iNOS resulted in significant inhibition of restenosis in rats |
| Johnson et al (48) | Adenovirus | TIMP-3 | Porcine coronary | Adenovirus-coated TIMP-3 stent significantly inhibited the in-stent neointimal formation in pigs |
| stent model | ||||
| Hedman et al (66) | Adenovirus | VEGF | Patients with coronary artery disease | Myocardial perfusion showed a significant improvement in the VEGF-adenovirus-treated patients after the six-month follow-up |
| Sinnaeve et al (104) | Adenovirus | PKG mutant | Porcine coronary stent model | Expression of a constitutively active PKG reduces neointimal formation after balloon injury in rats and reduces coronary in-stent restenosis in pigs |
| Liu et al (34) | Adenovirus | COX-1 | Rabbits carotid artery balloon injury model | Local gene transduction of COX-1 increases blood flow in injured atherosclerotic rabbit arteries |
| Lompré et al (50) | AAV2.5 | Sarco/endoplasmic reticulum Ca2+ ATPase isoform 2a | Rat carotid artery balloon injury model | AAV2.5 vector can be considered as a promising safe and effective vector for antirestenosis gene therapy in vivo |
| Sharif et al (79) | AAV2 | DNase-resistant particles | Rabbits iliac artery stent model | AAV2-coated stents can be used to deliver genes to the blood vessel wall for up to 28 days in vivo |
| Pankajakshan et al (82) | AAV2/9 | SM22α promoter | Swine coronary and peripheral arteries | AAV2/9 vector can be considered as a promising safe and effective vector for antirestenosis gene therapy in vivo |
| Ramirez Correa et al (78) | Recombinant AAV | TIMP-1 | Rat carotid injury arteries | Local AAV-TIMP-1 gene transfer represents an efficient strategy to prevent restenosis in rats |
| Squadrito et al (105) | Recombinant AAV | NF-κB inhibitory protein IκBα | Mice carotid artery injury model | Recombinant AAV-mediated gene transfer of IκBα inhibited the restenosis in mice |
| Yang et al (57) | Lentivirus | CBP | Rat carotid injury arteries | Lentivirus-mediated CBP silencing may represent a novel therapeutic approach for the prevention of restenosis after vascular interventions in rats |
| Bonta et al (76) | Lentivirus | Nurr1 | Rat carotid injury arteries | Lentivirus-mediated nuclear receptor Nurr1 is an attractive novel target for (local) intervention in restenosis in rats |
AAV Adeno-associated virus; CBP CREB-binding protein; COX Cyclooxygenase; iNOS Inducible nitric oxide synthase; Nurr1 Nuclear receptor-related 1; PKG Protein kinase G; TIMP Tissue inhibitor of metalloproteinase; VEGF Vascular endothelial growth factor; VSMC Vascular smooth muscle cell
Table 2: Summary of major studies using viral vector for antirestenosis gene therapy
The metabolic syndrome is defined as a cluster of numerous cardiovascular risk factors that encompass dyslipidemia and hypertension. Patients with the metabolic syndrome are more prone to developing ISR [35]. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a membrane protein that can bind and internalize oxidized low-density lipoprotein and advanced glycation end products, which further promote endothelial injury after percutaneous coronary interventions (PCI). Targeting the LOX-1 gene with gene silencer pyrrole-imidazole polyamide significantly decreased the expression of LOX-1 and promoted re-endothelialization, thus inhibiting neointimal hyperplasia after arterial injury [24]. Gene transfer of PGI2 synthase has also been shown to accelerate re-endothelialization and prevent neointimal formation in balloon-injured arteries. In a rabbit model, PGI2 synthase gene delivery by the lipotransfection method via dispatch catheter can accelerate re-endothelialization and attenuate neointimal formation [36]. Douglas et al [20] found that endothelial-specific overexpression of GTP-cyclohydrolase-1 can enhance endothelial cell function and increases NO production, thus promoting re-endothelialization after stent deployment.
Antineointimal hyperplasia
Following injury of coronary arteries during interventional procedures, including balloon angioplasty and/or stent placement, medial SMCs change their phenotype from contractile to synthetic, proliferative and migrative in nature, initiating the process of neointimal hyperplasia [37]. Therefore, proliferation and migration of SMCs are crucial targets for the inhibition of intimal hyperplasia and restenosis. A number of studies have shown that transfer of cytotoxic antiproliferative genes, including thymidine kinase, cytosine deaminase and Fas ligand, which selectively destroy SMCs as they enter the S phase of the cell cycle, are able to significantly inhibit neointimal hyperplasia in balloon-injured vessels [38-40]. In addition, the transfection of antisense oligodeoxynucleotides (ODNs) targeting DNA binding proteins and transcription factors – including CDC2 and cyclin G1, which encode for regulatory proteins that are involved in the cell cycle – have also been shown to inhibit neointimal formation [41,42]. MicroRNA, a 21 to 24 nucleotide RNA molecule, inhibits their messenger RNA target by binding to complementary ‘seed’ sequences, leading to post-transcriptional repression or target messenger RNA degradation. Several microRNAs have been found to significantly reduce injury-induced neointima formation by inhibiting VSMC proliferation [43,44]. Merlet et al [43] found an upregulation of miR-424/322 after vascular injury. Overexpression of miR-424/322 in injured rat carotid arteries using an adenovirus has been demonstrated to inhibit restenosis.
Apart from the antiproliferative approach, inhibition of the migration of SMCs can reduce neointimal hyperplasia. Extracellular matrix degradation has been shown to play a crucial role in SMC migration, which is mainly regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) [45]. Although early studies using marimastat, a broad-spectrum MMP inhibitor, showed significantly reduced neointimal formation in cultured human saphenous vein graft segments, the potential of marimastat as an antimetastatic agent was halted at phase III due to a lack of efficacy and dose-limiting toxicity [46]. The potency of TIMP-1 as a therapeutic gene for antineointimal hyperplasia has been verified in various animal models [47,48]. Johnson et al [48] found that stent-based delivery of TIMP-3 adenovirus inhibits neointimal formation in porcine coronary arteries [48]. Downregulation of platelet-derived growth factor-β receptor expression and upregulation of plasminogen activator inhibitor-1 and light-type caldesmon, a potent cytostatic and antiangiogenic protein, using a gene targeting and adenoviral gene transfer approach, has also been found to inhibit neointima formation [49]. Lompré et al [50] investigated the functional effects of adeno-associated virus (AAV) 2/5-mediated sarco/endoplasmic reticulum Ca2+ATPase isoform 2a (SERCA2a) – a human gene known to inhibit VSMC proliferation – in a rat model of carotid artery balloon injury and found that AAV2/5-mediated SERCA2a gene transfection significantly decreases the neointimal hyperplasia without inducing obvious inflammation in vivo.
Activation of the tyrosine kinase family JAK and subsequent activation of signal transducer and activator of transcription (STAT) proteins are key events in signal transduction of different cytokines and growth factors. These proteins regulate different biological processes including SMC proliferation [51]. Suppressor of cytokine signalling-3 (SOCS3) protein decreases the JAK/STAT signalling by blocking the JAK tyrosine kinase activity and the activation of STAT factors, suggesting that it is a novel antineointimal hyperplasia target. Recently, our laboratory has found a significant reduction in SOCS3 expression in the neointimal lesion after balloon angioplasty in coronary arteries of atherosclerotic swine [52]. In addition, our laboratory demonstrated that hypermethylation of the SOCS3 gene in SMCs could be an underlying mechanism of intimal hyperplasia and restenosis, suggesting that inhibition of SOCS3 promoter methylation is a potential anti-neointimal hyperplasia target [53].
Anti-inflammation
Inflammation is a protective attempt by the organism to remove the injurious stimuli. However, stent implantation, including BMS and polymer-coated DES implantation, induces acute and chronic inflammation that stimulates accumulation of inflammatory cells, such as macrophages and T-lymphocytes, and inflammatory cytokine secretion [8]. Inhibition of inflammatory gene expression may have the therapeutic effect of reducing neointimal hyperplasia. Transforming growth factor-beta (TGF-β) is a crucial inflammatory cytokine that controls immunity/inflammation and cell proliferation. Delivery of the gene silencer pyrrole-imidazole polyamide, which targets the TGF-β1 promoter, can suppress neointimal hyperplasia after arterial injury by downregulating TGF-β1 expression and inhibiting restenosis [25]. Monocyte chemoattractant protein-1 (MCP-1) is a proinflammatory chemokine specific for monocytes and is shown to be present at increased levels after vascular injury. The peptide from the C-terminal domain of MCP-1 (ingramon) has been shown to inhibit monocyte migration and possess anti-inflammatory activity while at the same time inhibiting the postangioplasty restenosis in animal models [54]. In rabbit and monkey models, the same laboratory found that the local delivery of the C-terminal domain of MCP-1 gene (7ND) by a GES system attenuates in-stent stenosis [55]. In cynomolgus monkeys, a catheter-based anti-MCP-1 gene delivery system has also been shown to attenuate in-stent neointima formation [55].
As an essential transcriptional factor for inflammation, nuclear factor-kappa B (NF-κB) has been found to play a pivotal role for restenosis after PCI. In an open-label phase I/fgIIa clinical trial, Egashira et al [56] found that NF-κB decoy ODN transfection can suppress the inflammatory response and prevent restenosis. CREBbinding protein is a powerful transcriptional coactivator that regulates inflammation, cell proliferation and apoptosis in vascular endothelial and SMCs. Knockdown of CREB-binding protein using small interfering RNA demonstrated a potent inhibitory effect on balloon injury-induced NF-κB acetylation and attenuated neointimal formation in balloon-injured rat carotid artery [57]. Activator protein-1 is another crucial transcription factor regulating gene expression in response to a variety of proinflammatory stimuli. Using decoy ODN transfection against the activator protein-1 binding site has recently been shown to effectively increase re-endothelialization and inhibit neointimal proliferation [21].
Antithrombosis
Stent implantation-induced thrombosis is a classical example of device-induced, platelet-mediated arterial thrombosis [58]. In a porcine model of coronary restenosis, anti-CD34 antibody-coated sirolimus-eluting stents were associated with greater endothelialization, thus inhibiting platelet activation. However, a clear clinical benefit of stents coated with this technology has not been shown [58,59]. ADP, a key agonist required to trigger platelet aggregation, has received much attention as a potential antithrombosis therapeutic target. Takemoto et al [60] found that transfection of the human pE-NTPDase gene, encoding an enzyme that rapidly hydrolyzes ATP and ADP to AMP via cationic gelatin-coated stents, inhibits subacute in-stent thrombosis and suppresses neointimal hyperplasia and inflammation without antiplatelet drugs. More established antithrombotic genes, including PGI2 synthase, COX-1 and tissue factor pathway inhibitor, have been investigated in recent years for their potential in postangioplasty intervention, and have demonstrated successful suppression of thrombosis and/or restenosis in various animal models [34,36,58,61].
Gene Delivery systems
Gene vectors
Viral vector and nonviral vector are the classic gene delivery vectors, both of which have the ability to introduce therapeutic genes into vascular tissue [6]. For greater safety and less immunogenicity, nonviral vectors have held more promise for clinical application than viral vectors. However, the complex process of migration through the vascular endothelium and the extracellular matrix makes nonviral vectors inefficient in the areas of transfection and rapid intracellular degradation [9], which hinders the application of vectors of this kind. Several forms of nonviral vectors, including naked plasmid DNA, DNAcontaining cationic liposomes, DNA-polycation complexes, DNAphosphorylcholine polymer complexes, DNA-PLGA NPs and ODN, have been investigated as potential gene vectors for antirestenosis therapy [33,62-69] (Table 1). Chemical formulations, such as poloxamers, inhibit the endogenous DNAses, thereby protecting plasmid DNA from degradation in the extracellular matrix [70]. Furthermore, dodecylated chitosan-plasmid DNA complexes formed stable, positively charged nanospheres with small vector size and increased EGFP-C1 gene expression in stents but not in adjacent arterial segments or distal organs, suggesting an interesting method for sustained and site-specific nonviral vector transfection [71]. In addition, novel dendrimer-based poly (L-glutamic acid) derivatives have also been found to be an efficient and biocompatible gene delivery vector to inhibit restenosis in balloon-injured rabbit carotid arteries [72]. Furthermore, recent studies have shown that small vector sizes in nanoscale-range particles (ie, NPs) have many advantages in DNA transfection including increased cellular uptake, decreased inflammatory response and increased cellular incorporation. This suggests NPs as a novel platform for nonviral gene vectors [73,74].
Compared with nonviral vectors, viral vectors have evolutionary advantages in their extra- and intracellular interactions. However, viral vectors can be subjected to a host of immune responses that not only negate the vector efficacy but also result in inflammatory responses in vivo [75]. Several forms of viral vectors, including adenovirus, AAV and lentivirus, have been applied in preclinical studies and clinical trials (Table 2) [34,43,44,50,57,76-80]. Adenoviruses are commonly used viral vectors because they have many features that make them well suited for gene therapy. As shown in Table 2, adenovirus- coated genes, including micro-RNA, TIMP-3 and VEGF, can have significant inhibitory effects on in-stent neointimal formation in multiple artery stent/injury restenosis animal models [34,43,44,77,80]. AAV vectors have emerged as a versatile vehicle for gene delivery due to their efficient infection of dividing and nondividing cells in the presence of a helper virus [50,79]. Compared with native AAVs, vascular cell-specific peptide-modified AAVs have been shown to target gene delivery to vascular tissues, such as vascular endothelial cells (ECs), in vivo [81]. Our group has successfully transfected a novel recombinant AAV-2/9 vector with SM22α promoter to the medial layer SMCs of swine coronary and peripheral arteries [82]. It warrants mention that AAV-2/9 viral transduction in our study has no significant effect on serum amylase, fibrinogen and C-reactive protein levels, suggesting that AAV-2/9 is a safe and effective gene therapeutic vector for antirestenosis [82]. Lentiviral vectors also provide a promising strategy for the treatment of cardiovascular diseases due to their durable gene transfer and their ability to govern efficiently. Several groups have verified that lentivirus-mediated gene transfection may represent a novel therapeutic approach for the prevention of restenosis after vascular interventions [57,76] (Table 2).
Vector delivery strategies
Percutaneous catheter-based delivery: Percutaneous catheter-based gene delivery, currently the most frequently used strategy for antirestenosis therapy, has several major theoretical advantages: concomitant performance with other percutaneous procedures, such as combining gene therapy with mesenchymal stem cell therapy; utilization of smaller quantities to reduce potentially harmful effects of systemic delivery [83]; and easy preparation, characterization and reproducibility. However, several major limitations have also limited the application of this strategy. First, most catheters require prolonged total occlusion of the target vessel for effective vector delivery, which may increase the risk for myocardial ischemia. Second, there is lack of efficiency in site-specific gene delivery, although current vector delivery systems have focused on modified balloon catheters that can trap a fluid within a short segment of vasculature [84]. Finally, the delivered viral or nonviral vectors inevitably disperse from side branches of the coronary vasculature, which carries the potential risk for distal spread.
Another promising method of transluminal gene delivery involves using vascular tissue-specific targeted systemic treatments. White et al [81] isolated human venous EC-targeting peptides and genetically incorporated them into AAV capsids. Intravenous infusion of engineered AAVs into mice caused reduced vector accumulation in the liver while enhancing uptake of virions in the vena cava [81]. Deglau et al [85] investigated a site-specific delivery system. First, they injured rabbit femoral arteries using biotin molecular-loaded balloons, then intravenously administered avidin-coated microspheres, which has a high affinity for biotin. They found that these microspheres attached to the biotin on the arterial wall, suggesting the potential to locally deliver a systemically injected antirestenotic agent [85]. In addition, genetic engineering of human stem cells with VEGF holds great potential as a platform for cellbased therapy to promote vascularization and tissue regeneration [86]. Nonviral, biodegradable polymeric NPs were recently developed to deliver genes to human MSCs [87], and their surface can be easily manipulated with the addition of special ligands. This may be responsible for enhancing vascular injury site-specific NP permeability, suggesting that stem cell-based delivery of gene-loaded NPs offers an interesting option to overcome such deficiency in transluminal gene delivery.
Stent-based delivery: As with DES, GES use stents as permanent scaffolds to deliver biologically active agents locally for a prolonged duration to the site of vascular disease [65]. Dichek et al [88] seeded stents with genetically engineered endothelial cells using retroviralmediated gene transfer – the first progress in the field of GES therapy to prevent ISR. In a pig coronary angioplasty model, Klugherz et al [89] reported the first successful transfection in vivo using a DNA controlled-release stent and a collagen-coated stent with monoclonal antibodies covalently bonded to adenovirus [90]. Much later, Walter et al [65] improved the efficiency of local delivery of naked plasmid DNA encoding for human VEGF-2 via GES in hypercholesterolemic rabbits. Swanson et al [91] also observed the effect of VEGF-coated stents on restenosis in hypercholesterolemic rabbits. Currently, stent backbones designed for gene loading include biodegradable, bioabsorbable, nanoporous metal stents coated with an outer layer of polymer, which may be bioabsorbable or nonbioabsorbable material, and can be loaded with therapeutic genes, thus providing more controlled and sustained gene delivery and allowing for more optimal gene-tissue interactions [26]. Non-biodegradable polymers (BPs) are currently the most frequently used method of coating the stents. However, considering that stents, similar to any other foreign material inside the human body, can cause infection and inflammation, BPs including poly-L-lactide, polyvinyl pyrrolidone, poly-lactide-cocaprolactone and PLGA, offer greater advantages. Bioresorbable stents, often composed of polylactides such as poly-L-lactide or poly-lactidecocaprolactone, are typically completely metabolized in approximately 12 to 18 months [92]. In a porcine model, Lincoff et al [26] have found that stents composed of high-molecular-weight poly-Llactide produced minimal inflammation and durable results compared with uncoated stents. Although BP-DES have yet to receive approval in the USA, they are widely used worldwide, including in Asia and Europe. In a five-year follow-up study, Kuramitsu et al [93] investigated the long-term coronary arterial response to BP-biolimus-eluting stents (BES) compared with durable polymer sirolimus-eluting stents and BMS, and found that BP-BES shows a favourable coronary arterial response compared with sirolimus-eluting stents [93]. However, in a network meta-analysis that included 113 trials involving 90,584 patients performed to compare the safety and efficacy of BPs, DES, BMS and durable-polymer DES in patients undergoing coronary revascularization [94], BP-BES was found to be associated with a higher risk for definite or probable ST compared with cobalt-chromium everolimus-eluting stents. These findings suggest the benefits of biodegradable polymer coating over a nonbiodegradable one such as durable polymer [26]. However, additional studies are warranted to confirm these conclusions.
Compared with a catheter-based delivery strategy, GES represents a more appealing method for gene delivery to atherosclerotic coronary vessels. However, there are a number of technical and biological challenges with a stent-based delivery strategy. First, the limited surface area of the stent does not always guarantee the abundant loading of vectors, which is necessary for sustained transgene expression. Second, there are difficulties with regard to preservation of the mechanical properties of stents after coating the material. Third, the interaction between the biological coating material and the tissue limits the cellular uptake and intracellular stability of the gene construct, as well as the efficient transcription and translation of the encoded protein. In addition, larger-sized particles loaded in GES may be taken up by macrophages rather than the target SMCs or ECs [6].
Magnetically guided delivery: Magnetic guidance is a physical targeting strategy with the potential to improve the safety and efficacy of a variety of therapeutic vectors [95]. Magnetically guided delivery systems loaded with drug and cells to stented blood vessels has recently been proposed as a potential therapeutic strategy for in-stent restenosis [96,97]. Magnetically targeted delivery systems are essentially composed of two primary components: magnetic particle-loaded therapeutic vectors and a magnetic field source generating a force attracting the vectors (Figure 2). Polyak et al [97] were the first to demonstrate high-magnetic-field gradient targeting of magnetic NP (MNP)-loaded endothelial cells to the surfaces of steel stents. At first, the investigators preloaded bovine aortic endothelial cells with biodegradable polymeric MNPs, thereby rendering the cells magnetically responsive. Then, the cells were injected via a catheter to the site advanced beyond the stent to the aortic arch. Under an external homogeneous magnetic field, the MNP-loaded bovine aortic endothelial cells were successfully targeted to the stent wires [97]. Recently, Chorny et al [77] reported a novel site-specific gene delivery to stented arteries using magnetically guided delivery. In their study, zinc oleate-based MNPs were loaded with replication-deficient adenovirus, which is limited in its clinical applicability by rapid inactivation. Under magnetic conditions, adenovirus-loaded MNPs were effectively transduced into cultured ECs and SMCs. In addition, localized arterial gene expression in the magnetically guided MNP delivery group were significantly stronger than in control groups in a rat stent model, confirming the feasibility of using adenovirus-loaded MNPs to achieve site-specific transduction in stented blood vessels [77]. Considering that recombinant vectors, whether viral or nonviral, must migrate through various biological barriers in one or more compartments to subsequently transfect target cells such as SMCs, an MNPs-based delivery system under a magnetic field may be seen as an exciting alternative for improving targeted antiproliferation gene release under catheter-based or stent-based delivery strategies (Figure 2).
Figure 2: Schematic representation of the targeting of antirestenosis genes or genetic engineering stem cells loaded with magnetic nanoparticles driven by a magnetic field. Magnetic nanoparticle-loaded therapeutic vectors including antirestenosis genes or genetic engineering stem cells were targeted to stents and effectively transduced into endothelial cells (ECs) and smooth muscle cells (SMCs) in the artery under the presence of a uniform magnetic field
Conclusion
The design of new antineointimal hyperplasia and re-endothelialization systems for the treatment of ISR was the major purpose of developing next-generation PCI technologies, which more safely and effectively prevent the progress of atherosclerosis. Vascular gene therapy offers the potential to overcome the limitations of pharmacological interventions such as the long-term use of dual antiplatelet therapy and the side effects of antiproliferation drugs loaded in DES. Many candidate genes have the potential to prevent restenosis in various artery stent/injury animal models. However, to date, few clinical studies have demonstrated similar success rates, which may be related to the species-specific differences between human and animal models of the cardiovascular system and host-immune response. Swine coronary restenosis highly resembles restenosis in humans; thus, the swine is widely regarded as an accurate model for mimicking the proliferative component of human restenosis [98]. There is a growing understanding that the physiological barriers to efficient, targeted gene transfer by gene vectors must be taken into consideration during the design of optimized therapeutic strategies. At present, viral vectors, including AAV and lentivirus, have the most potential to maximize transduction efficiency, although the toxicity, inflammation and interaction with tissue components remains to be fully evaluated. A GES-based delivery strategy is superior with regard to site-specific and sustained transgene expression. However, the stent design and material technology must be improved. The novel MNP formulation designed for magnetically guided adenoviral gene transfer to cells of the blood vessel wall addresses several prerequisites for a safe and effective gene transfection, especially targeting antiproliferation genes to SMCs. Genetic engineering of human mesenchymal stem cells using biodegradable polymeric NPs has recently been shown to enhance angiogenesis, offering an interesting option for enhancing endothelialization. These results suggest that gene-loaded NP systems and mesenchymal stem cells may be a next step for antirestenosis therapy, although the exact effect remains to be elucidated in preclinical studies and clinical trials.
Acknowledgement
This work was supported by the NIHNHLBI research grants R01HL104516, R01HL112597 and R01HL120659 to DK Agrawal. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have no financial disclosures or conflicts of interest to declare.
References
- Farooq V, Gogas BD, Serruys PW. Restenosis: Delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv 2011;4:195-205.
- Garg S, Serruys PW. Coronary stents: Current status. J Am Coll Cardiol 2010;56:S1-42.
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: Modulation by nutrients and inflammation. J Clin Invest 2008;118:2992-3002.
- Deng DX, Spin JM, Tsalenko A, et al. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: Distinct responses to stimuli. Arterioscler Thromb Vasc Biol 2006;26:1058-65.
- Goel SA, Guo LW, Liu B, Kent KC: Mechanisms of post-intervention arterial remodelling. Cardiovasc Res 2012;96:363-71.
- Goh D, Tan A, Farhatnia Y, Rajadas J, Alavijeh MS, Seifalian AM. Nanotechnology-based gene-eluting stents. Mol Pharm 2013;10:1279-98.
- Brodie B, Pokharel Y, Garg A, et al. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2012;5:1043-51.
- Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv 2010;3:174-83.
- Robertson KE, McDonald RA, Oldroyd KG, Nicklin SA, Baker AH. Prevention of coronary in-stent restenosis and vein graft failure: Does vascular gene therapy have a role? Pharmacol Ther 2012;136:23-34.
- Holmes DR Jr, Vlietstra RE, Smith HC, et al. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): A report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol 1984;53:77C-81C.
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701-6.
- Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med 1991;324:13-7.
- van Domburg RT, Foley DP, de Jaegere PP, et al. Long term outcome after coronary stent implantation: A 10 year single centre experience of 1000 patients. Heart 1999;(82 Suppl 2):II27-34.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: A brief review. Can J Cardiol 2014;30:35-45.
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23.
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221-31.
- Suzuki S, Ishii H, Matsudaira K, et al. Long-term outcome of drug-eluting vs. bare-metal stents in patients with acute myocardial infarction. Subgroup analysis of the nagoya acute myocardial infarction study (NAMIS). Circ J 2013;77:2024-31.
- Deuse T, Hua X, Wang D, et al. Dichloroacetate prevents restenosis in preclinical animal models of vessel injury. Nature 2014;509:641-4.
- Van Belle E, Bauters C, Asahara T, Isner JM: Endothelial regrowth after arterial injury: From vascular repair to therapeutics. Cardiovasc Res 1998;38:54-68.
- Douglas G, Van Kampen E, Hale AB, et al. Endothelial cell repopulation after stenting determines in-stent neointima formation: Effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur Heart J 2013;34:3378-88.
- Lin K, Zhao Z, Chen L. Stent-based delivery of decoy oligodeoxynucleotides against activator protein-1 binding site decreased restenosis in rabbits. Pharmazie 2013;68:661-7.
- Sharif F, Hynes SO, McCullagh KJ, et al. Gene-eluting stents: Non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther 2012;19:321-8.
- Yang J, Zeng Y, Zhang C, et al. The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent. Biomaterials 2013;34:1635-43.
- Yao EH, Fukuda N, Ueno T, et al. Novel gene silencer pyrrole-imidazole polyamide targeting lectin-like oxidized low-density lipoprotein receptor-1 attenuates restenosis of the artery after injury. Hypertension 2008;52:86-92.
- Yao EH, Fukuda N, Ueno T, et al. A pyrrole-imidazole polyamide targeting transforming growth factor-beta1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc Res 2009;81:797-804.
- Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 2014;4:175-200.
- Rutanen J, Turunen AM, Teittinen M, et al. Gene transfer using the mature form of VEGF-D reduces neointimal thickening through nitric oxide-dependent mechanism. Gene Ther 2005;12:980-7.
- Hiltunen MO, Laitinen M, Turunen MP, et al. Intravascular adenovirus-mediated VEGF-C gene transfer reduces neointima formation in balloon-denuded rabbit aorta. Circulation 2000;102:2262-8.
- Asahara T, Chen D, Tsurumi Y, et al. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation 1996;94:3291-302.
- Waugh JM, Kattash M, Li J, et al. Gene therapy to promote thromboresistance: Local overexpression of tissue plasminogen activator to prevent arterial thrombosis in an in vivo rabbit model. Proc Natl Acad Sci U S A 1999;96:1065-70.
- Sharif F, Hynes SO, Cooney R, et al. Gene-eluting stents: Adenovirus-mediated delivery of eNOS to the blood vessel wall accelerates re-endothelialization and inhibits restenosis. Mol Ther 2008;16:1674-80.
- Muhs A, Heublein B, Schletter J, et al. Preclinical evaluation of inducible nitric oxide synthase lipoplex gene therapy for inhibition of stent-induced vascular neointimal lesion formation. Hum Gene Ther 2003;14:375-83.
- Brito LA, Chandrasekhar S, Little SR, Amiji MM. Non-viral eNOS gene delivery and transfection with stents for the treatment of restenosis. Biomed Eng Online 2010;9:56.
- Liu Q, Chen ZQ, Bobustuc GC, et al. Local gene transduction of cyclooxygenase-1 increases blood flow in injured atherosclerotic rabbit arteries. Circulation 2005;111:1833-40.
- Goyal SN, Bharti S, Krishnamurthy B, Agrawal Y, Ojha SK, Arya DS. Impact of metabolic syndrome on re-stenosis development: Role of drug-eluting stents. Diab Vasc Dis Res 2012;9:177-88.
- Numaguchi Y, Okumura K, Harada M, et al. Catheter-based prostacyclin synthase gene transfer prevents in-stent restenosis in rabbit atheromatous arteries. Cardiovasc Res 2004;61:177-85.
- Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: An underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol 2011;31:2391-6.
- Guzman RJ, Hirschowitz EA, Brody SL, Crystal RG, Epstein SE, Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A 1994;91:10732-6.
- Ohno T, Gordon D, San H, et al. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science 1994;265:781-4.
- Simari RD, San H, Rekhter M, et al. Regulation of cellular proliferation and intimal formation following balloon injury in atherosclerotic rabbit arteries. J Clin Invest 1996;98:225-35.
- Morishita R, Gibbons GH, Ellison KE, et al. Single intraluminal delivery of antisense cdc2 kinase and proliferating-cell nuclear antigen oligonucleotides results in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci U S A 1993;90:8474-8.
- Zhu NL, Wu L, Liu PX, et al. Downregulation of cyclin G1 expression by retrovirus-mediated antisense gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation. Circulation 1997;96:628-35.
- Merlet E, Atassi F, Motiani RK, et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res 2013;98:458-68.
- Li P, Zhu N, Yi B, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res 2013;113:1117-27.
- Osherov AB, Gotha L, Cheema AN, Qiang B, Strauss BH. Proteins mediating collagen biosynthesis and accumulation in arterial repair: Novel targets for anti-restenosis therapy. Cardiovasc Res 2011;91:16-26.
- Sparano JA, Bernardo P, Stephenson P, et al. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J Clin Oncol 2004;22:4683-90.
- Forough R, Koyama N, Hasenstab D, et al. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res 1996;79:812-20.
- Johnson TW, Wu YX, Herdeg C, et al. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol 2005;25:754-9.
- Yokouchi K, Numaguchi Y, Kubota R, et al. l-Caldesmon regulates proliferation and migration of vascular smooth muscle cells and inhibits neointimal formation after angioplasty. Arterioscler Thromb Vasc Biol 2006;26:2231-7.
- Lompré AM, Hadri L, Merlet E, et al. Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: A new perspective for in-stent restenosis gene therapy. Gene Ther 2013;20:901-12.
- Madamanchi NR, Moon SK, et al. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol 2005;25:950-6.
- Gupta GK, Dhar K, Del Core MG, Hunter WJ III, Hatzoudis GI, Agrawal DK. Suppressor of cytokine signaling-3 and intimal hyperplasia in porcine coronary arteries following coronary intervention. Exp Mol Pathol 2011;91:346-52.
- Dhar K, Rakesh K, Pankajakshan D, Agrawal DK. SOCS3 promotor hypermethylation and STAT3-NF-kappaB interaction downregulate SOCS3 expression in human coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 2013;304:H776-85.
- Arefieva TI, Krasnikova TL, Potekhina AV, et al. Synthetic peptide fragment (65-76) of monocyte chemotactic protein-1 (MCP-1) inhibits MCP-1 binding to heparin and possesses anti-inflammatory activity in stable angina patients after coronary stenting. Inflamm Res 2011;60:955-64.
- Egashira K, Nakano K, Ohtani K, et al. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol 2007;27:2563-8.
- Egashira K, Suzuki J, Ito H, Aoki M, Isobe M, Morishita R. Long-term follow up of initial clinical cases with NF-kappaB decoy oligodeoxynucleotide transfection at the site of coronary stenting. J Gene Med 2008;10:805-9.
- Yang J, Jiang H, Chen SS, et al. Lentivirus-mediated RNAi targeting CREB binding protein attenuates neointimal formation and promotes re-endothelialization in balloon injured rat carotid artery. Cell Physiol Biochem 2010;26:441-8.
- Granada JF, Price MJ, French PA, et al. Platelet-mediated thrombosis and drug-eluting stents. Circ Cardiovasc Interv 2011;4:629-37.
- Nakazawa G, Granada JF, Alviar CL, et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv 2010;3:68-75.
- Takemoto Y, Kawata H, Soeda T, et al. Human placental ectonucleoside triphosphate diphosphohydrolase gene transfer via gelatin-coated stents prevents in-stent thrombosis. Arterioscler Thromb Vasc Biol 2009;29:857-62.
- Yin X, Yutani C, Ikeda Y, et al. Tissue factor pathway inhibitor gene delivery using HVJ-AVE liposomes markedly reduces restenosis in atherosclerotic arteries. Cardiovasc Res 2002;56:454-63.
- Zhang LH, Luo T, Zhang C, et al. Anti-DNA antibody modified coronary stent for plasmid gene delivery: Results obtained from a porcine coronary stent model. J Gene Med 2011;13:37-45.
- Yang J, Zeng Y, Li Y, et al. Intravascular site-specific delivery of a therapeutic antisense for the inhibition of restenosis. Eur J Pharm Sci 2008;35:427-34.
- Fuchs AT, Kuehnl A, Pelisek J, et al. Inhibition of restenosis formation without compromising reendothelialization as a potential solution to thrombosis following angioplasty? Endothelium 2008;15:85-92.
- Walter DH, Cejna M, Diaz-Sandoval L, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: An alternative strategy for inhibition of restenosis. Circulation 2004;110:36-45.
- Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: Phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 2003;107:2677-83.
- Taniyama Y, Tachibana K, Hiraoka K, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 2002;105:1233-9.
- Nikol S, Huehns TY, Krausz E, et al. Needle injection catheter delivery of the gene for an antibacterial agent inhibits neointimal formation. Gene Ther 1999;6:737-48.
- Van Belle E, Tio FO, Chen D, Maillard L, Kearney M, Isner JM. Passivation of metallic stents after arterial gene transfer of phVEGF165 inhibits thrombus formation and intimal thickening. J Am Coll Cardiol 1997;29:1371-9.
- Escoffre JM, Teissie J, Rols MP. Gene transfer: How can the biological barriers be overcome? J Membr Biol 2010;236:61-74.
- Zhu D, Jin X, Leng X, et al. Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int J Nanomedicine 2010;5:1095-102.
- Zeng X, Pan S, Li J, et al. A novel dendrimer based on poly (L-glutamic acid) derivatives as an efficient and biocompatible gene delivery vector. Nanotechnology 2011;22:375102.
- Yang D, Hartman MR, Derrien TL, et al. DNA materials. Bridging nanotechnology and biotechnology. Acc Chem Res 2014;47:1902-11.
- Alqawlaq S, Sivak JM, Huzil JT, et al. Preclinical development and ocular biodistribution of gemini-DNA nanoparticles after intravitreal and topical administration: Towards non-invasive glaucoma gene therapy. Nanomedicine 2014 [Epub ahead of print]
- Eckhouse SR, Jones JA, Spinale FG. Gene targeting in ischemic heart disease and failure: Translational and clinical studies. Biochem Pharmacol 2013;85:1-11.
- Bonta PI, Pols TW, van Tiel CM, et al. Nuclear receptor Nurr1 is expressed in and is associated with human restenosis and inhibits vascular lesion formation in mice involving inhibition of smooth muscle cell proliferation and inflammation. Circulation 2010;121:2023-32.
- Chorny M, Fishbein I, Tengood JE, Adamo RF, Alferiev IS, Levy RJ. Site-specific gene delivery to stented arteries using magnetically guided zinc oleate-based nanoparticles loaded with adenoviral vectors. FASEB J 2013;27:2198-206.
- Ramirez Correa GA, Zacchigna S, Arsic N, et al. Potent inhibition of arterial intimal hyperplasia by TIMP1 gene transfer using AAV vectors. Mol Ther 2004;9:876-84.
- Sharif F, Hynes SO, McMahon J, et al. Gene-eluting stents: Comparison of adenoviral and adeno- associated viral gene delivery to the blood vessel wall in vivo. Hum Gene Ther 2006;17:741-50.
- Wu YJ, Sala-Newby GB, Shu KT, et al. S-phase kinase-associated protein-2 (Skp2) promotes vascular smooth muscle cell proliferation and neointima formation in vivo. J Vasc Surg 2009;50:1135-42.
- White SJ, Nicklin SA, Buning H, et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 2004;109:513-9.
- Pankajakshan D, Makinde TO, Gaurav R, et al. Successful transfection of genes using AAV-2/9 vector in swine coronary and peripheral arteries. J Surg Res 2012;175:169-75.
- Opie SR, Dib N. Local endovascular delivery, gene therapy, and cell transplantation for peripheral arterial disease. J Endovasc Ther 2004;(11 Suppl 2):II151-62.
- Seedial SM, Ghosh S, Saunders RS. Local drug delivery to prevent restenosis. J Vasc Surg 2013;57:1403-14.
- Deglau TE, Maul TM, Villanueva FS, Wagner WR. In vivo PEG modification of vascular surfaces for targeted delivery. J Vasc Surg 2012;55:1087-95.
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One 2007;2:e156.
- Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A 2010;107:3317-22.
- Dichek DA, Neville RF, Zwiebel JA, Freeman SM, Leon MB, Anderson WF. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation 1989;80:1347-53.
- Klugherz BD, Jones PL, Cui X, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol 2000;18:1181-4.
- Klugherz BD, Song C, DeFelice S, et al. Gene delivery to pig coronary arteries from stents carrying antibody-tethered adenovirus. Hum Gene Ther 2002;13:443-54.
- Swanson N, Hogrefe K, Javed Q, Malik N, Gershlick AH. Vascular endothelial growth factor (VEGF)-eluting stents: In vivo effects on thrombosis, endothelialization and intimal hyperplasia. J Invasive Cardiol 2003;15:688-92.
- Lincoff AM, Furst JG, Ellis SG, Tuch RJ, Topol EJ. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol 1997;29:808-16.
- Kuramitsu S, Sonoda S, Yokoi H, et al. Long-term coronary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: Five-year follow-up optical coherence tomography study. Atherosclerosis 2014;237:23-9.
- Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: A systematic review and Bayesian approach network meta-analysis. Eur Heart J 2014;35:1147-58.
- Chorny M, Fishbein I, Adamo RF, Forbes SP, Folchman-Wagner Z, Alferiev IS. Magnetically targeted delivery of therapeutic agents to injured blood vessels for prevention of in-stent restenosis. Methodist Debakey Cardiovasc J 2012;8:23-7.
- Chorny M, Fishbein I, Yellen BB, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A 2010;107:8346-51.
- Polyak B, Fishbein I, Chorny M, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci U S A 2008;105:698-703.
- Gupta GK, Agrawal T, Del Core MG, Hunter WJ III, Agrawal DK. Decreased expression of vitamin D receptors in neointimal lesions following coronary artery angioplasty in atherosclerotic swine. PLoS One 2012;7:e42789.
- Chang H, Ren KF, Zhang H, Wang JL, Wang BL, Ji J. The (PrS/HGF-pDNA) multilayer films for gene-eluting stent coating: Gene-protecting, anticoagulation, antibacterial properties, and in vivo antirestenosis evaluation. J Biomed Mater Res B Appl Biomater 2014 [Epub ahead of print].
- Deiner C, Schwimmbeck PL, Koehler IS, et al. Adventitial VEGF165 gene transfer prevents lumen loss through induction of positive arterial remodeling after PTCA in porcine coronary arteries. Atherosclerosis 2006;189:123-32.
- Matsushita H, Morishita R, Aoki M, et al. Transfection of antisense p53 tumor suppressor gene oligodeoxynucleotides into rat carotid artery results in abnormal growth of vascular smooth muscle cells. Circulation 2000;101:1447-52.
- Chapman GD, Lim CS, Gammon RS, et al. Gene transfer into coronary arteries of intact animals with a percutaneous balloon catheter. Circ Res 1992;71:27-33.
- Fishbein I, Alferiev I, Bakay M, et al. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation 2008;117:2096-103.
- Sinnaeve P, Chiche JD, Gillijns H, et al. Overexpression of a constitutively active protein kinase G mutant reduces neointima formation and in-stent restenosis. Circulation 2002;105:2911-6.
- Squadrito F, Deodato B, Bova A, et al. Crucial role of nuclear factor-kappaB in neointimal hyperplasia of the mouse carotid artery after interruption of blood flow. Atherosclerosis 2003;166:233-42.